Abstract
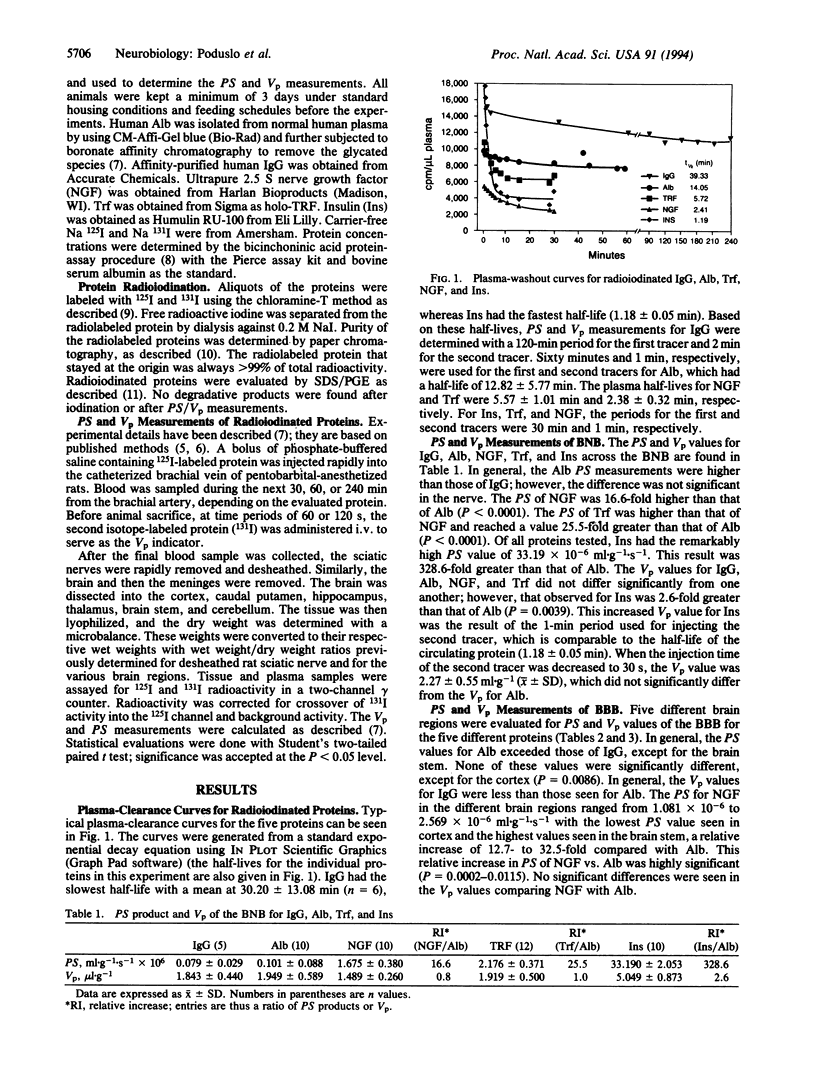
The permeability of insulin (Ins), nerve growth factor (NGF), albumin (Alb), transferrin (Trf), and IgG across the blood-nerve barrier (BNB) and blood-brain barrier (BBB) in normal adult rats was quantified by measuring the (permeability coefficient x surface area) product (PS) with the i.v. bolus-injection technique in the cannulated brachial vein and artery using radioiodinated proteins. The PS values of the BNB for IgG and Alb were low: 0.079 +/- 0.029 x 10(-6) and 0.101 +/- 0.088 x 10(-6) ml.g-1.s-1, (mean +/- SD, respectively). The PS values for NGF and Trf were 16.1-fold and 25.5-fold higher than for Alb. The PS for Ins across the BNB was 33.190 +/- 2.053 x 10(-6) ml.g-1.s-1--a remarkable 329-fold increase compared with Alb. The PS values of the BBB for IgG and Alb in different brain regions were all low, from 0.028 +/- 0.017 to 0.151 +/- 0.035 x 10(-6) ml.g-1.s-1 (mean +/- SD). NGF and Trf had comparable PS values from 13- to 32-fold higher than for Alb, except for the brain stem, where the PS for Trf was 66-fold higher than for Alb. The mean PS for Ins across the BBB ranged from 15.78 +/- 5.45 x 10(-6) ml.g-1.s-1 for the cortex to 22.62 +/- 7.50 x 10(-6) ml.g-1.s-1 for the brain stem--again a remarkable 105- to 390-fold increase relative to Alb. Because reliable PS measurements were obtained for all proteins tested, the BBB and BNB cannot be considered impermeable to proteins--a concept that has plagued brain- and nerve-barrier research. The low PS values for IgG and Alb indicate low rates of transfer; however, Alb, in particular, is the major protein of endoneurial and ventricular fluid, which suggests that these PS values may be significant. Ins had the highest PS values, which likely reflect the mechanism of transport across the barriers--that is, receptor-mediated transport. Because NGF and Trf had PS values 13- to 66-fold higher than for Alb, whether this reflects receptor-mediated uptake, adsorptive-mediated transcytosis, or some other mechanism is unclear. That the PS values for NGF and Trf differ from Alb and IgG clearly suggests, however, a different uptake mechanism. Finally, the remarkably high PS values for Ins across the BBB and BNB identify this protein and its putative receptor on capillary endothelial cells as a potential target for drug delivery into the central and peripheral nervous systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Burton L. E., Bennett G. L., Dugich-Djordjevic M. Recombinant human nerve growth factor is biologically active and labels novel high-affinity binding sites in rat brain. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):281–285. doi: 10.1073/pnas.88.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti R. H., Aneletti P. U., Levi-Montalcini R. Selective accumulation of ( 125 I) labelled nerve growth factor in sympathetic ganglia. Brain Res. 1972 Nov 13;46:421–425. doi: 10.1016/0006-8993(72)90033-9. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Audus K. L., Davis T. P. Permeability of the blood-brain barrier to peptides: an approach to the development of therapeutically useful analogs. Peptides. 1992 Nov-Dec;13(6):1289–1294. doi: 10.1016/0196-9781(92)90037-4. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. A brain-to-blood carrier-mediated transport system for small, N-tyrosinated peptides. Pharmacol Biochem Behav. 1984 Dec;21(6):943–946. doi: 10.1016/s0091-3057(84)80077-5. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Barrera C. M. Delivering peptides to the central nervous system: dilemmas and strategies. Pharm Res. 1991 Nov;8(11):1345–1350. doi: 10.1023/a:1015884603456. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Fasold M. B. Differential effect of aluminum on the blood-brain barrier transport of peptides, technetium and albumin. J Pharmacol Exp Ther. 1988 Feb;244(2):579–585. [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Horvath A., Michals E. A. Carrier-mediated transport of vasopressin across the blood-brain barrier of the mouse. J Neurosci Res. 1987;18(2):326–332. doi: 10.1002/jnr.490180209. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Michals E. A., Barrera C. M. Stereospecific transport of Tyr-MIF-1 across the blood-brain barrier by peptide transport system-1. Brain Res Bull. 1990 Oct;25(4):589–592. doi: 10.1016/0361-9230(90)90116-h. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Sandra A. Vascular transport of insulin to rat cardiac muscle. Central role of the capillary endothelium. J Clin Invest. 1988 Apr;81(4):1225–1233. doi: 10.1172/JCI113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera C. M., Kastin A. J., Banks W. A. D-[Ala1]-peptide T-amide is transported from blood to brain by a saturable system. Brain Res Bull. 1987 Dec;19(6):629–633. doi: 10.1016/0361-9230(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Bernd P., Martinez H. J., Dreyfus C. F., Black I. B. Localization of high-affinity and low-affinity nerve growth factor receptors in cultured rat basal forebrain. Neuroscience. 1988 Jul;26(1):121–129. doi: 10.1016/0306-4522(88)90131-5. [DOI] [PubMed] [Google Scholar]
- Bjerre B., Björklund A., Mobley W., Rosengren E. Short- and long-term effects of nerve growth factor on the sympathetic nervous system in the adult mouse. Brain Res. 1975 Aug 29;94(2):263–277. doi: 10.1016/0006-8993(75)90061-x. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D. Transcytosis of macromolecules through the blood-brain barrier: a cell biological perspective and critical appraisal. Acta Neuropathol. 1989;79(2):117–128. doi: 10.1007/BF00294368. [DOI] [PubMed] [Google Scholar]
- Chabrier P. E., Roubert P., Braquet P. Specific binding of atrial natriuretic factor in brain microvessels. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2078–2081. doi: 10.1073/pnas.84.7.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A., Morgan E. H. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992 Oct 2;592(1-2):8–16. doi: 10.1016/0006-8993(92)91652-u. [DOI] [PubMed] [Google Scholar]
- De Bruyn P. P., Michelson S., Bankston P. W. In-vivo endocytosis by bristle-coated pits and intracellular transport of endogenous albumin in the endothelium of the sinuses of liver and bone marrow. Cell Tissue Res. 1985;240(1):1–7. doi: 10.1007/BF00217551. [DOI] [PubMed] [Google Scholar]
- Duffy K. R., Pardridge W. M. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987 Sep 8;420(1):32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- Eskild W., Berg T. Endocytosis of formaldehyde-denatured serum albumin by nonparenchymal liver cells in vitro. Biochim Biophys Acta. 1984 Feb 17;803(1-2):63–70. doi: 10.1016/0167-4889(84)90055-7. [DOI] [PubMed] [Google Scholar]
- Fenstermacher J. D., Blasberg R. G., Patlak C. S. Methods for Quantifying the transport of drugs across brain barrier systems. Pharmacol Ther. 1981;14(2):217–248. doi: 10.1016/0163-7258(81)90062-0. [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Rubin J. B., Handrahan J. V., Connor J. R., Fine R. E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res. 1987;18(2):299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H. J., Pardridge W. M., Morris W. L., Rosenfeld R. G., Choi T. B. Binding and internalization of insulin and insulin-like growth factors by isolated brain microvessels. Diabetes. 1986 Jun;35(6):654–661. doi: 10.2337/diab.35.6.654. [DOI] [PubMed] [Google Scholar]
- Friden P. M., Walus L. R., Musso G. F., Taylor M. A., Malfroy B., Starzyk R. M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4771–4775. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried K., Risling M., Arvidsson U., Paulie S. Nerve growth factor receptor-like immunoreactivity in nerve fibers in the spinal and medullary dorsal horn of the adult monkey and cat: correlation with calcitonin gene-related peptide-like immunoreactivity. Brain Res. 1990 Dec 17;536(1-2):321–326. doi: 10.1016/0006-8993(90)90043-b. [DOI] [PubMed] [Google Scholar]
- Galina Z. H., Kastin A. J. MIF-1 antagonizes warm-, but not cold-water stress-induced analgesia: dissociation from immobility. Peptides. 1985 Nov-Dec;6(6):1109–1112. doi: 10.1016/0196-9781(85)90435-8. [DOI] [PubMed] [Google Scholar]
- Ghitescu L., Bendayan M. Transendothelial transport of serum albumin: a quantitative immunocytochemical study. J Cell Biol. 1992 May;117(4):745–755. doi: 10.1083/jcb.117.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Brandon M. R., Hunt S. V., Williams A. F., Gatter K. C., Mason D. Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984 Nov 8;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- King G. L., Johnson S. M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985 Mar 29;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- Koh S., Oyler G. A., Higgins G. A. Localization of nerve growth factor receptor messenger RNA and protein in the adult rat brain. Exp Neurol. 1989 Dec;106(3):209–221. doi: 10.1016/0014-4886(89)90154-4. [DOI] [PubMed] [Google Scholar]
- Kumagai A. K., Eisenberg J. B., Pardridge W. M. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J Biol Chem. 1987 Nov 5;262(31):15214–15219. [PubMed] [Google Scholar]
- Levi A., Alemà S. The mechanism of action of nerve growth factor. Annu Rev Pharmacol Toxicol. 1991;31:205–228. doi: 10.1146/annurev.pa.31.040191.001225. [DOI] [PubMed] [Google Scholar]
- Messina A., Bell C. Are genetically hypertensive rats deficient in nerve growth factor? Neuroreport. 1991 Jan;2(1):45–48. doi: 10.1097/00001756-199101000-00011. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Pettigrew K. D., Rapoport S. I. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol. 1978 Sep;235(3):H299–H307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Buciak J. L., Friden P. M. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther. 1991 Oct;259(1):66–70. [PubMed] [Google Scholar]
- Pardridge W. M., Eisenberg J., Cefalu W. T. Absence of albumin receptor on brain capillaries in vivo or in vitro. Am J Physiol. 1985 Sep;249(3 Pt 1):E264–E267. doi: 10.1152/ajpendo.1985.249.3.E264. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Eisenberg J., Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985 Jun;44(6):1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Eisenberg J., Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987 Sep;36(9):892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Kumagai A. K., Eisenberg J. B. Chimeric peptides as a vehicle for peptide pharmaceutical delivery through the blood-brain barrier. Biochem Biophys Res Commun. 1987 Jul 15;146(1):307–313. doi: 10.1016/0006-291x(87)90726-1. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Triguero D., Buciak J. Transport of histone through the blood-brain barrier. J Pharmacol Exp Ther. 1989 Dec;251(3):821–826. [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G., Fenstermacher J. D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983 Mar;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Perosio P. M., Brooks J. J. Expression of nerve growth factor receptor in paraffin-embedded soft tissue tumors. Am J Pathol. 1988 Jul;132(1):152–160. [PMC free article] [PubMed] [Google Scholar]
- Pioro E. P., Cuello A. C. Distribution of nerve growth factor receptor-like immunoreactivity in the adult rat central nervous system. Effect of colchicine and correlation with the cholinergic system--I. Forebrain. Neuroscience. 1990;34(1):57–87. doi: 10.1016/0306-4522(90)90304-m. [DOI] [PubMed] [Google Scholar]
- Pioro E. P., Cuello A. C. Distribution of nerve growth factor receptor-like immunoreactivity in the adult rat central nervous system. Effect of colchicine and correlation with the cholinergic system--II. Brainstem, cerebellum and spinal cord. Neuroscience. 1990;34(1):89–110. doi: 10.1016/0306-4522(90)90305-n. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Curran G. L., Dyck P. J. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4879–4883. doi: 10.1073/pnas.85.13.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F., Curran G. L. Increased permeability across the blood-nerve barrier of albumin glycated in vitro and in vivo from patients with diabetic polyneuropathy. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2218–2222. doi: 10.1073/pnas.89.6.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F. Glycoprotein molecular-weight estimation using sodium dodecyl sulfate-pore gradient electrophoresis: comparison of tris-glycine and tris-borate-EDTA buffer systems. Anal Biochem. 1981 Jun;114(1):131–139. doi: 10.1016/0003-2697(81)90463-2. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Low P. A., Nickander K. K., Dyck P. J. Mammalian endoneurial fluid: collection and protein analysis from normal and crushed nerves. Brain Res. 1985 Apr 15;332(1):91–102. doi: 10.1016/0006-8993(85)90392-0. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Low P. A., Windebank A. J., Dyck P. J., Berg C. T., Schmelzer J. D. Altered blood-nerve barrier in experimental lead neuropathy assessed by changes in endoneurial albumin concentration. J Neurosci. 1982 Oct;2(10):1507–1514. doi: 10.1523/JNEUROSCI.02-10-01507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. I., Ohno K., Pettigrew K. D. Drug entry into the brain. Brain Res. 1979 Aug 24;172(2):354–359. doi: 10.1016/0006-8993(79)90546-8. [DOI] [PubMed] [Google Scholar]
- Raub T. J., Newton C. R. Recycling kinetics and transcytosis of transferrin in primary cultures of bovine brain microvessel endothelial cells. J Cell Physiol. 1991 Oct;149(1):141–151. doi: 10.1002/jcp.1041490118. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Fine R. E., Sandra A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J Cell Sci. 1993 Feb;104(Pt 2):521–532. doi: 10.1242/jcs.104.2.521. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Pham H., Keller B. T., Borchardt R. T., Pardridge W. M. Demonstration and structural comparison of receptors for insulin-like growth factor-I and -II (IGF-I and -II) in brain and blood-brain barrier. Biochem Biophys Res Commun. 1987 Nov 30;149(1):159–166. doi: 10.1016/0006-291x(87)91618-4. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Figlewicz D. P., Baskin D. G., Woods S. C., Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992 Aug;13(3):387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- Seitz R. J., Heininger K., Schwendemann G., Toyka K. V., Wechsler W. The mouse blood-brain barrier and blood-nerve barrier for IgG: a tracer study by use of the avidin-biotin system. Acta Neuropathol. 1985;68(1):15–21. doi: 10.1007/BF00688950. [DOI] [PubMed] [Google Scholar]
- Shimon-Hophy M., Wadhwani K. C., Chandrasekaran K., Larson D., Smith Q. R., Rapoport S. I. Regional blood-brain barrier transport of cationized bovine serum albumin in awake rats. Am J Physiol. 1991 Aug;261(2 Pt 2):R478–R483. doi: 10.1152/ajpregu.1991.261.2.R478. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol. 1981 Sep;90(3):605–613. doi: 10.1083/jcb.90.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. R., Borchardt R. T. Permeability and mechanism of albumin, cationized albumin, and glycosylated albumin transcellular transport across monolayers of cultured bovine brain capillary endothelial cells. Pharm Res. 1989 Jun;6(6):466–473. doi: 10.1023/a:1015960205409. [DOI] [PubMed] [Google Scholar]
- Smith K. R., Kato A., Borchardt R. T. Characterization of specific receptors for atrial natriuretic factor on cultured bovine brain capillary endothelial cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):308–314. doi: 10.1016/s0006-291x(88)80048-2. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Springer J. E. Nerve growth factor receptors in the central nervous system. Exp Neurol. 1988 Dec;102(3):354–365. doi: 10.1016/0014-4886(88)90231-2. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Potter B. J., Berk P. D. Studies of albumin binding to rat liver plasma membranes. Implications for the albumin receptor hypothesis. Biochim Biophys Acta. 1983 Mar 15;756(1):20–27. doi: 10.1016/0304-4165(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Schweitzer J. B., Johnson E. M., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988 Feb;8(2):664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D., Buciak J. B., Yang J., Pardridge W. M. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4761–4765. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T., Hano T., Hamada M., Nishio I., Masuyama Y. New role of nerve growth factor--an inhibitory neuromodulator of adrenergic transmission. Brain Res. 1991 Sep 20;559(2):293–296. doi: 10.1016/0006-8993(91)90014-m. [DOI] [PubMed] [Google Scholar]
- Unger J. W., Livingston J. N., Moss A. M. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36(5):343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- Van Bree J. B., De Boer A. G., Danhof M., Breimer D. D. Drug transport across the blood-brain barrier. II. Experimental techniques to study drug transport. Pharm Weekbl Sci. 1992 Dec 11;14(6):338–348. doi: 10.1007/BF01970169. [DOI] [PubMed] [Google Scholar]
- Van Bree J. B., De Boer A. G., Danhof M., Breimer D. D. Drug transport across the blood-brain barrier. III. Mechanisms and methods to improve drug delivery to the central nervous system. Pharm World Sci. 1993 Feb 19;15(1):2–9. doi: 10.1007/BF02116163. [DOI] [PubMed] [Google Scholar]
- Villegas J. C., Broadwell R. D. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. II. Adsorptive transcytosis of WGA-HRP and the blood-brain and brain-blood barriers. J Neurocytol. 1993 Feb;22(2):67–80. doi: 10.1007/BF01181571. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W., Trowbridge R. S. Ultrastructural study of transcellular transport of native and cationized albumin in cultured goat brain microvascular endothelium [corrected]. J Neurocytol. 1991 Dec;20(12):998–1006. doi: 10.1007/BF01187917. [DOI] [PubMed] [Google Scholar]
- Wadhwani K. C., Murphy V. A., Smith Q. R., Rapoport S. I. Saturable transport of manganese(II) across blood-nerve barrier of rat peripheral nerve. Am J Physiol. 1992 Feb;262(2 Pt 2):R284–R288. doi: 10.1152/ajpregu.1992.262.2.R284. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A., Curran G. L., Poduslo J. F. Blood-nerve transfer of albumin and its implications for the endoneurial microenvironment. Brain Res. 1989 Aug 7;494(1):114–121. doi: 10.1016/0006-8993(89)90149-2. [DOI] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]
- Woolf N. J., Gould E., Butcher L. L. Nerve growth factor receptor is associated with cholinergic neurons of the basal forebrain but not the pontomesencephalon. Neuroscience. 1989;30(1):143–152. doi: 10.1016/0306-4522(89)90360-6. [DOI] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988 Sep;8(9):3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr Immunohistochemical localization and biochemical characterization of nerve growth factor receptor in adult rat brain. J Comp Neurol. 1989 Dec 22;290(4):585–598. doi: 10.1002/cne.902900411. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Sobue G., Mitsuma T., Takahashi A., Hashizume Y. Nerve growth factor receptor immunoreactivity in human benign peripheral nerve sheath tumor. Acta Neuropathol. 1989;77(6):591–598. doi: 10.1007/BF00687886. [DOI] [PubMed] [Google Scholar]
- Zettler C., Head R. J., Rush R. A. Chronic nerve growth factor treatment of normotensive rats. Brain Res. 1991 Jan 11;538(2):251–262. doi: 10.1016/0006-8993(91)90437-z. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V., Skundric D. S., Segal M. B., Lipovac M. N., Mackic J. B., Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp Neurol. 1990 Mar;107(3):263–270. doi: 10.1016/0014-4886(90)90144-h. [DOI] [PubMed] [Google Scholar]
- del Valle M. E., Alvarez-Méndez J. C., Calzada B., Vega J. A. Nerve growth factor receptor immunoreactivity in non-nervous structures of the adult rat brain. Eur J Histochem. 1992;36(4):435–444. [PubMed] [Google Scholar]



