Abstract
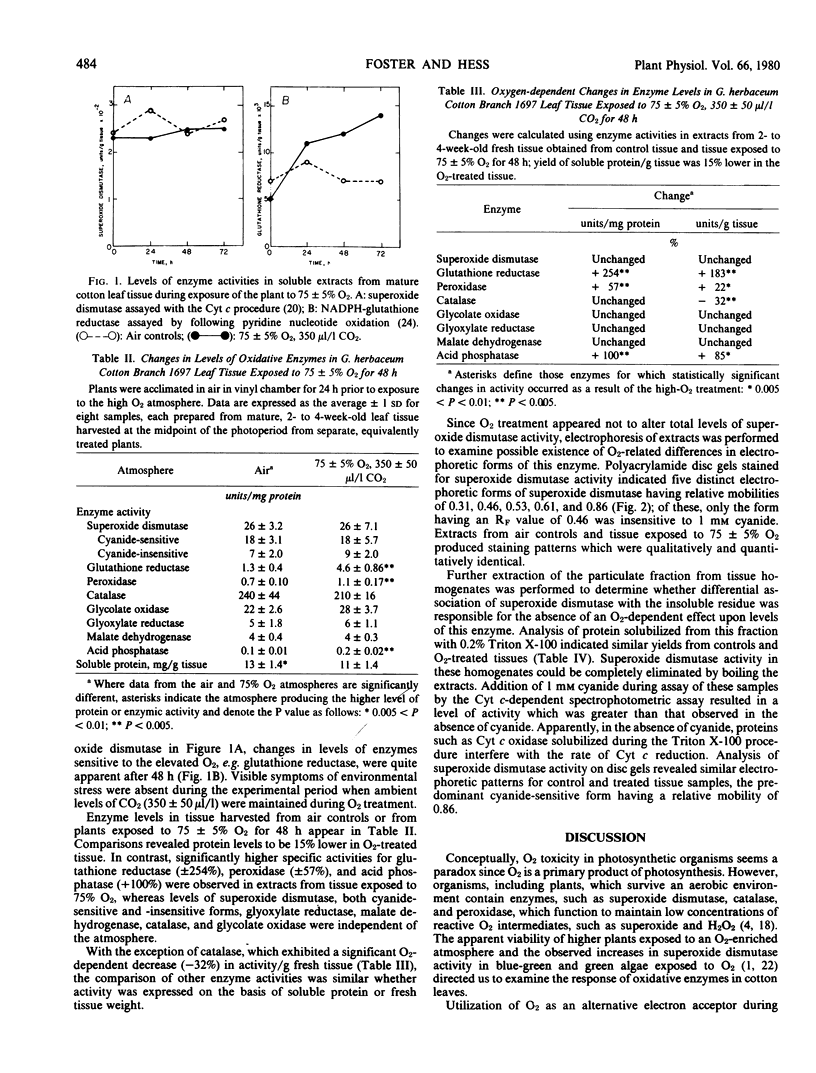
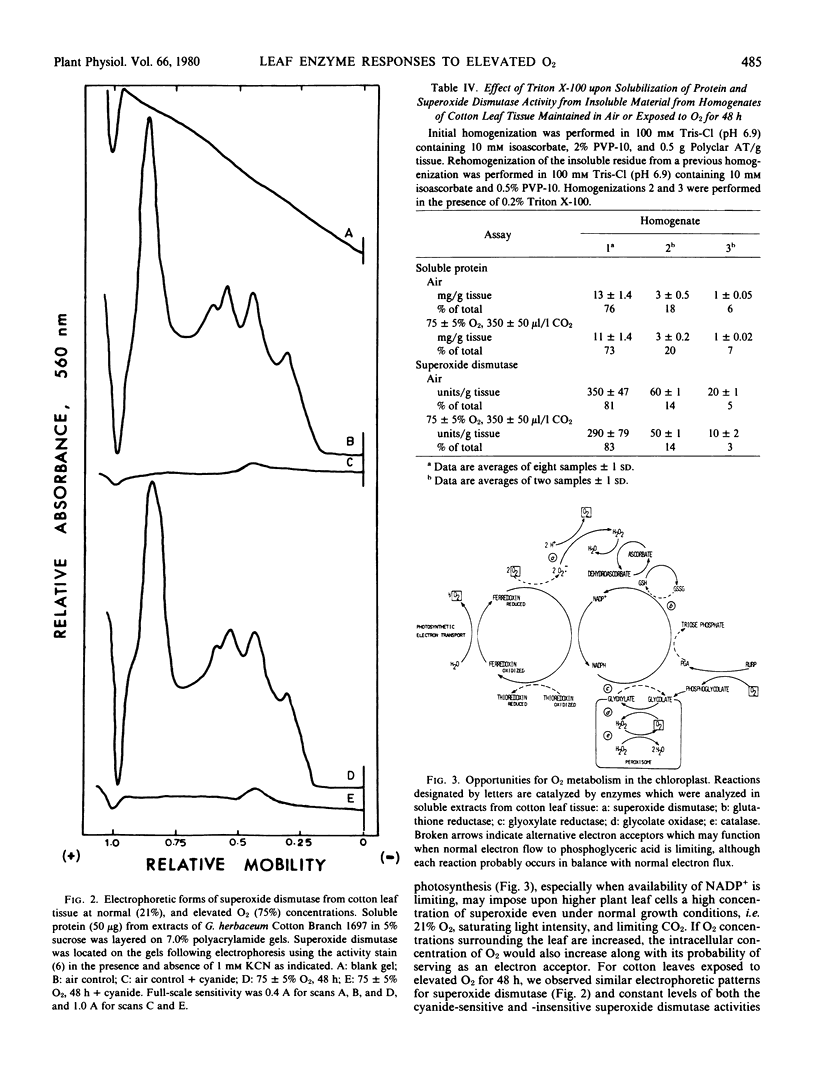
Responses of superoxide dismutase (EC 1.15.1.1) and glutathione reductase (EC 1.6.4.2) activities were evaluated in leaf tissue from intact cotton plants (Cotton Branch 1697) which were exposed to 75% O2, 350 microliters per liter CO2 for 48 hours. Soluble protein was extracted from O2-treated and control tissue, and enzyme levels were determined. Superoxide dismutase activity in cotton leaf tissue was high (26 units per milligram protein) under normal conditions of 21% O2, saturating light, and limiting CO2, and neither qualitative nor quantitative differences in the cyanide-sensitive or -insensitive forms of the enzyme occurred in response to hyperoxic conditions. Glutathione reductase activity, however, was 2- to 3-fold higher in extracts from tissue exposed to 75% O2. No increase in activity was observed for the peroxisomal enzymes, glycolate oxidase (EC 1.1.3.1) and catalase (EC 1.11.1.6). Results are consistent with an integrated pathway involving superoxide dismutase and glutathione reductase for protection of sensitive leaf components against detrimental effects of intermediate reduction products of O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Kellenberg D., Shilo M. Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol. 1974 May;19(5):379–382. doi: 10.1111/j.1751-1097.1974.tb06526.x. [DOI] [PubMed] [Google Scholar]
- Anderson D. G., Stafford H. A., Conn E. E., Vennesland B. THE DISTRIBUTION IN HIGHER PLANTS OF TRIPHOSPHOPYRIDINE NUCLEOTIDE-LINKED ENZYME SYSTEMS CAPABLE OF REDUCING GLUTATHIONE. Plant Physiol. 1952 Oct;27(4):675–684. doi: 10.1104/pp.27.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kanematsu S., Uchida K. Superoxide dismutases in photosynthetic organisms: absence of the cuprozinc enzyme in eukaryotic algae. Arch Biochem Biophys. 1977 Feb;179(1):243–256. doi: 10.1016/0003-9861(77)90109-6. [DOI] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen H. J. The use of diaminobenzidine for spectrophotometric and acrylamide gel detection of sulfite oxidase and its applicability to hydrogen peroxide-generating enzymes. Anal Biochem. 1973 May;53(1):208–222. doi: 10.1016/0003-2697(73)90423-5. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- De Leo P., Sacher J. A. Control of ribonuclease and acid phosphatase by auxin and abscisic acid during senescence of Rhoeo leaf sections. Plant Physiol. 1970 Dec;46(6):806–811. doi: 10.1104/pp.46.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Grill D. Seasonal Variation of Glutathione and Glutathione Reductase in Needles of Picea abies. Plant Physiol. 1978 Jan;61(1):119–121. doi: 10.1104/pp.61.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Marsho T. V., Behrens P. W. Photosynthetic oxygen reduction in isolated intact chloroplasts and cells in spinach. Plant Physiol. 1979 Oct;64(4):656–659. doi: 10.1104/pp.64.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Pulich W. M., Jr Resistance to high oxygen tension, streptonigrin, and ultraviolet irradiation in the green alga Chlorella sorokiniana strain ors. J Cell Biol. 1974 Sep;62(3):904–907. doi: 10.1083/jcb.62.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. E., Hess J. L. Partial purification and characterization of aspartate aminotransferases from seedling oat leaves. J Biol Chem. 1975 Jun 25;250(12):4456–4461. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]



