Abstract
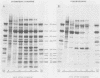
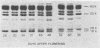
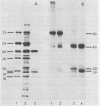
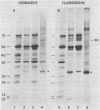
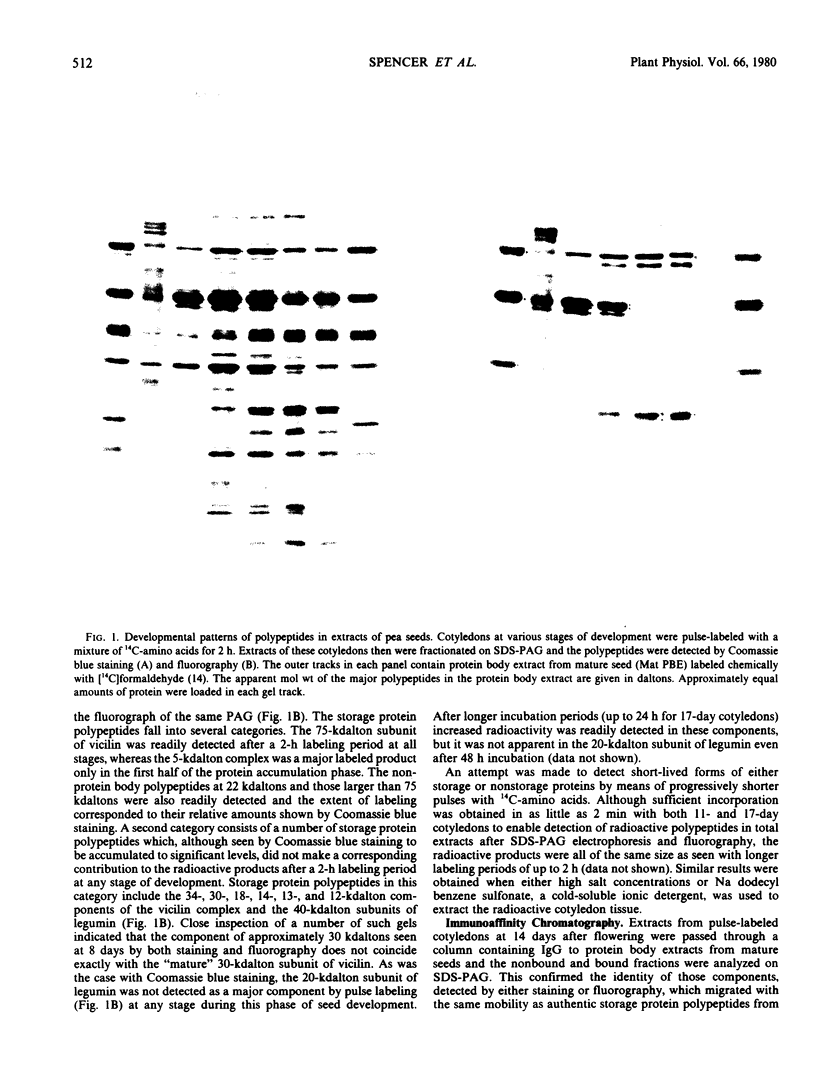
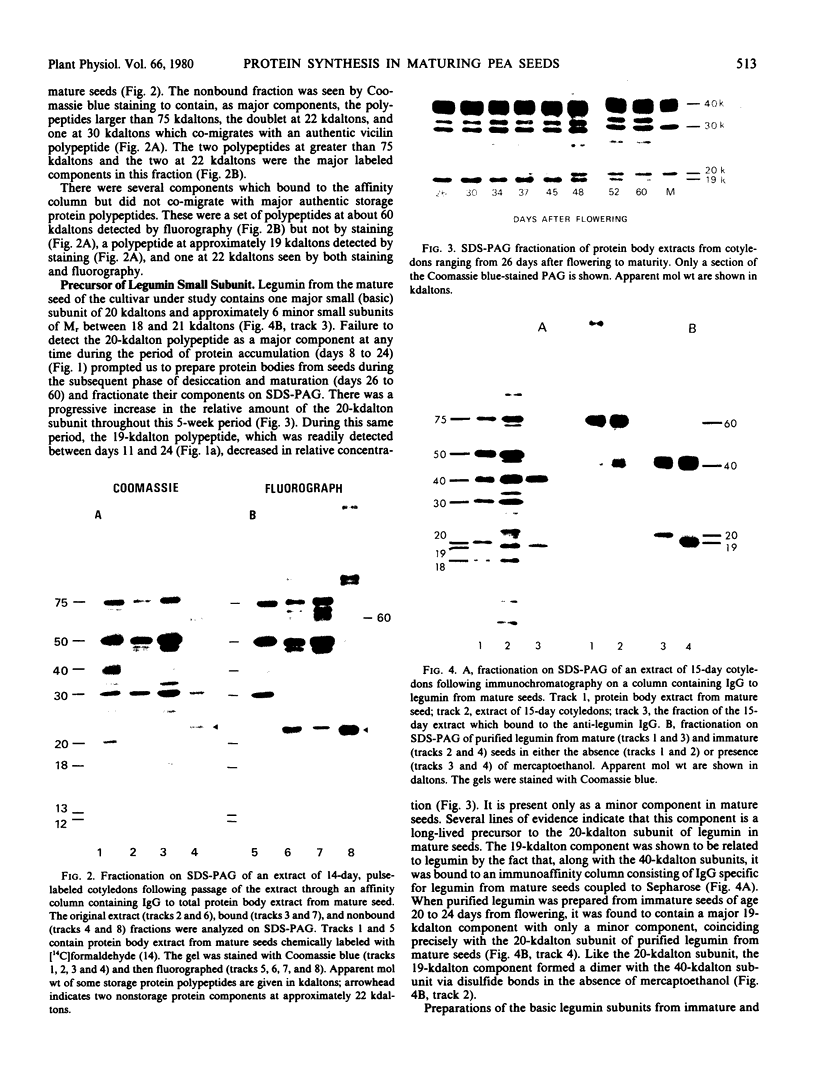
Intact cotyledons were taken from pea seeds at various stages during seed development and pulse-labeled with 14C-amino acids. Salt-soluble proteins then were extracted and fractionated on Na dodecyl sulfate-polyacrylamide gels. Storage proteins in these extracts were identified by their binding to immunoaffinity columns. The labeling studies showed that the synthesis of storage protein polypeptides accounts for a major part of total protein synthesis of developing cotyledons between 10 and 22 days after flowering. The distribution of the incorporated radioactivity between individual storage protein polypeptides varied with stage of development. For example, the synthesis of the 50 kilodalton complex of vicilin subunits dominated the early stages of protein accumulation but was a negligible proportion of the total incorporation in the later stages. On the other hand, the 75 kilodalton vicilin subunit was synthesized throughout this entire period. The major small subunit of legumin (20 kilodaltons) was not detected by either Coomassie blue staining or by 2-hour labeling during this period. It was found to arise during the desiccation phase of seed maturation from a long-lived precursor with a relative electrophoretic mobility equivalent to 19 kilodaltons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Casey R. Immunoaffinity chromatography as a means of purifying legumin from Pisum (pea) seeds. Biochem J. 1979 Feb 1;177(2):509–520. doi: 10.1042/bj1770509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Higgins T. J., Spencer D. Cell-free Synthesis of Pea Seed Proteins. Plant Physiol. 1977 Nov;60(5):655–661. doi: 10.1104/pp.60.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Sun S. M., Mutschler M. A., Bliss F. A., Hall T. C. Protein Synthesis and Accumulation in Bean Cotyledons during Growth. Plant Physiol. 1978 Jun;61(6):918–923. doi: 10.1104/pp.61.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. Isolation and chemical characterization of a mitogenic lectin from Pisum sativum. J Biol Chem. 1974 Sep 25;249(18):6004–6012. [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]