Abstract
Background
In contrast to colon cancer, the implications of reduced lymph node retrieval in rectal cancer are unclear.
Methods
Using the California Cancer Registry, we performed a retrospective cohort study of 4790 patients with stage I – III rectal cancer diagnosed from 2000 to 2007 who underwent tri-modality therapy. Using multivariate Cox proportional hazards models adjusted for age, sex, race, socioeconomic status, T-stage, and lymph node numbers, we evaluated rectal cancer specific survival (RC-SS) in neoadjuvant and adjuvant cohorts in the overall population and amongst those without involved lymph nodes (pN0).
Results
Sixty one percent of evaluable patients were treated with neoadjuvant chemoradiation. Although there was no difference in RC-SS between neoadjuvant and adjuvant chemoradiation cohorts, the median number of lymph nodes examined was reduced after neoadjuvant therapy (8 vs. 11, p < 0.0001). Positive lymph nodes were associated with worse RC-SS regardless of sequence, although the effect was numerically stronger for residual lymph nodes in the neoadjuvant cohort. Compared to at least 12, eight or fewer lymph nodes retrieved was associated with worse outcome in both neoadjuvant and adjuvant cohorts. However, no association between reduced lymph nodes examined and RC-SS was seen in the neoadjuvant cohort when the analysis was restricted to pN0 patients.
Conclusions
In this large cohort of rectal cancer patients treated with tri-modality therapy, reduced lymph node retrieval in node negative patients did not provide additional prognostic information in patients treated with neoadjuvant therapy.
Keywords: Rectal neoplasms, Lymph nodes, Neoadjuvant therapy, Chemoradiotherapy, Adjuvant, Neoplasm staging
Introduction
Approximately 40,000 new cases of rectal cancer are diagnosed in the United States each year.1 Management of this disease has changed significantly since 2000, when the National Cancer Institute (NCI) revised the surgical treatment guidelines for colorectal cancer.2 In 2000, locally advanced rectal cancer was frequently managed by primary surgery followed by adjuvant chemoradiotherapy (CRT).3 However, with the publication of the German Rectal Cancer Study Group (CAO/ARO/AIO-94) trial in 2004, neoadjuvant CRT became the preferred approach for most cases of clinical stage II and III rectal adenocarcinoma.4
In the last decade, the impact of increased lymph node retrieval on outcomes of surgically-resected colorectal cancer has been identified in both population-based and clinical studies.5,6 Guidelines from the American Joint Committee on Cancer (AJCC) and College of American Pathologists (CAP) currently recommend evaluation of a minimum of 12 lymph nodes after colorectal surgery.7–9 Indeed, removal and pathologic examination of at least 12 regional lymph nodes from resected colon cancer is a National Quality Forum endorsed metric.10 Since the data supporting this recommendation are derived primarily from studies of patients undergoing surgery for colon cancer, the extent to which this measure should be extrapolated to rectal cancer is not clear.
While lymph node retrieval may be altered by surgical procedure and the intensity of pathologic examination, other predictors of lymph node retrieval in colorectal cancer include age, gender, and tumor site.11 Additionally, recent studies demonstrate that fewer total lymph nodes are recovered after CRT and that only a fraction of specimens contain adequate lymph nodes after neoadjuvant CRT for rectal cancer.11–15 Nonetheless, studies have come to conflicting conclusions as to whether increased lymph node retrieval is associated with outcome after neoadjuvant CRT.12–19
Given that lymph node examination has had important implications on patient prognosis and because lymph nodes status may influence decisions regarding adjuvant therapy and intensity of follow up, we sought to evaluate the prognostic implications of lymph node retrieval and examination in rectal cancer treated with neoadjuvant CRT in a contemporary large population-based study.
Patients and methods
Setting and subjects
We conducted a retrospective cohort study using the California Cancer Registry (CCR), a statewide, population-based cancer registry. The registry includes demographic, diagnostic, treatment and outcome information. To ensure current follow up for vital status and cause of death, the CCR database is linked annually to death certificates, hospital discharge data, Medicare files, and the National Death Index. For patients diagnosed since 2000, follow up is over 95%.
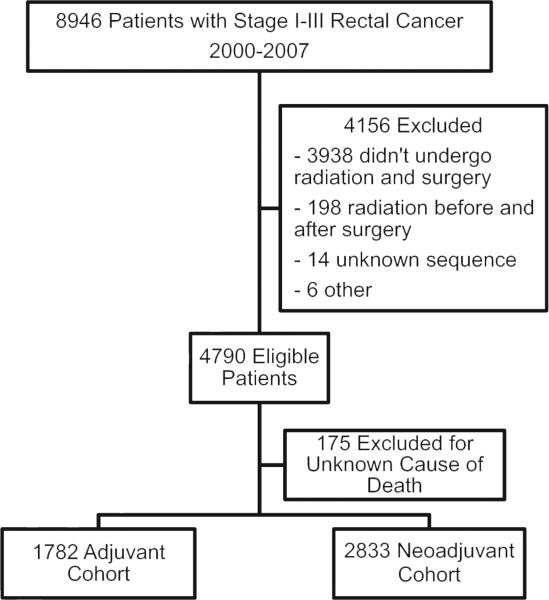
Patients were included in this study if they were 18 years of age or older; diagnosed with stage I, II, or III rectal cancer between the years 2000 and 2007; and underwent tri-modality therapy consisting of surgery, chemotherapy, and radiation (Fig. 1). Surgery is defined by the CCR as the operation performed on the primary tumor site within the first 6 months of diagnosis. Because our intention was to evaluate the outcome of patients who actually underwent the specified treatment, we did not attempt to impute missing data.
Figure 1.
Selection of rectal cancer patients included in this study.
Patients were excluded if they had a prior history of cancer, unknown cause of death, or if there was missing data regarding stage, treatment, lymph node number, or survival. Based on the radiation treatment sequence code (RADSEQ) from the CCR database, patients were divided into two cohorts: those who received chemotherapy and radiation prior to surgery (neoadjuvant CRT), and those who underwent surgery followed by adjuvant chemotherapy and radiation.
Primary outcome
The primary outcome was rectal cancer specific survival (RC-SS), which was defined as the time from diagnosis until death due to rectal cancer or December 31, 2008.
Covariates
Age, sex, race, socioeconomic status (SES), T-stage, number of positive lymph nodes, and number of examined lymph nodes were obtained from relevant registry fields. Regional lymph node data are extracted from the pathology reports by the reporting institution. After visualization of the distribution of lymph nodes retrieved, this variable was categorized as 0–2, 3–5, 6–8, 9–11, and 12 or more.
Statistical analysis
Baseline characteristics of the two cohorts were compared using the Chi-squared test. Multivariate Cox proportional hazards models adjusting for covariates were constructed for the entire population and then repeated for the subset of pathologic node negative patients. The Kaplan–Meier method was used to construct survival curves for RC-SS comparing the neoadjuvant and adjuvant cohorts. Analyses were repeated for overall survival, which may be more impacted than RC-SS by factors beyond initial treatment. However, the results for overall survival were not significantly different, and RC-SS results are reported. Two-sided P values less than 0.05 were considered statistically significant. All statistical analysis was performed using SAS software version 9.3 (SAS Inc., Cary, NC, USA). This study was approved by the University of California, Davis Institutional Review Board.
Results
We identified 8946 patients who were diagnosed with stage I – III rectal cancer between 2000 and 2007 (Fig. 1). Of these, 4790 underwent tri-modality therapy consisting of surgery, chemotherapy, and radiation. We excluded a further 175 patients for having an unknown cause of death. Within the eligible population, 2833 patients (61%) underwent CRT prior to surgery (the neoadjuvant cohort) and 1782 patients (39%) proceeded to surgery before receiving adjuvant CRT (the adjuvant cohort).
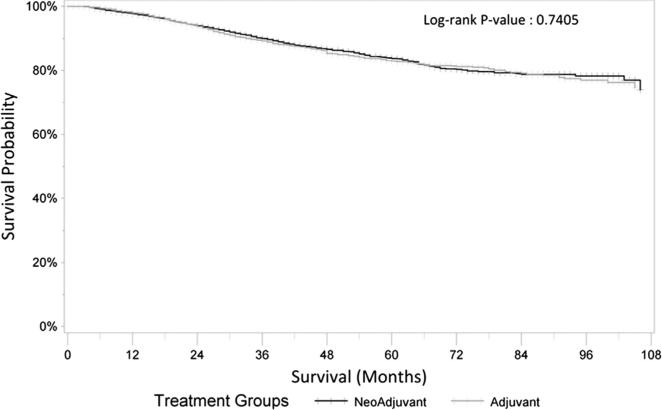
Baseline characteristics of the two cohorts are presented in Table 1. Median age at diagnosis was 59 in the neoadjuvant cohort and 62 in the adjuvant cohort. There was an approximate 3:2 male predominance in both cohorts. The most common racial background was Non-Hispanic White, with significant populations of Hispanics and Asian or Pacific Islanders. The neoadjuvant cohort was slightly more likely to have a higher SES. The majority of patients in both cohorts had primary tumor invasion through the muscularis propria (T3). There were more patients with node negative disease in the neoadjuvant cohort (68% vs. 37%) and consequently higher percentage of patients with all levels of node positivity in the adjuvant cohort. In patients who had undergone neoadjuvant CRT, the median number of lymph nodes examined was reduced (8 vs. 11, p < 0.0001). There was no overall difference in RC-SS between the neoadjuvant and adjuvant cohorts (Fig. 2).
Table 1.
Clinical and demographic characteristics of stage I – III rectal cancer patients treated with tri-modality therapy in California 2000–2007.
| Characteristic | Neoadjuvant cohort N = 2833 | Adjuvant cohort N = 1782 | p Value | |||
|---|---|---|---|---|---|---|
| Age (median) | 59 | 62 | <0.0001 | |||
| Sex: | Male | 1782 | 62.9% | 1061 | 59.5% | 0.0222 |
| Female | 1051 | 37.1% | 721 | 40.5% | ||
| Race/Ethnicity: | Non-Hispanic White | 1776 | 62.7% | 1077 | 60.4% | 0.3416 |
| Non-Hispanic Black | 140 | 4.9% | 93 | 5.2% | ||
| Hispanic | 483 | 17.0% | 343 | 19.2% | ||
| Asian/Pacific Islander | 419 | 14.8% | 257 | 14.4% | ||
| Other/Unknown | 15 | 0.5% | 12 | 0.7% | ||
| Socioeconomic status: | 1st 2nd Quintiles | 884 | 31.2% | 616 | 34.6% | 0.0343 |
| 3rd Quintile | 585 | 20.6% | 371 | 20.8% | ||
| 4th – 5th Quintiles | 1364 | 48.1% | 795 | 44.6% | ||
| T stage: | 0–1 | 268 | 9.5% | 99 | 5.6% | <0.0001 |
| 2 | 512 | 18.1% | 300 | 16.8% | ||
| 3 | 1760 | 62.1% | 1230 | 69.0% | ||
| 4 | 290 | 10.2% | 153 | 8.6% | ||
| Unknown | 3 | 0.1% | 0 | 0.0% | ||
| N stage: | N0 | 1916 | 67.6% | 658 | 36.9% | <0.0001 |
| N1a | 341 | 12.0% | 314 | 17.6% | ||
| N1b | 292 | 10.3% | 364 | 20.4% | ||
| N2a | 146 | 5.2% | 231 | 13.0% | ||
| N2b | 138 | 4.9% | 215 | 12.1% | ||
| Nodes examined: | 0–2 | 283 | 10.0% | 106 | 5.9% | <0.0001 |
| 3–5 | 569 | 20.1% | 226 | 12.7% | ||
| 6–8 | 571 | 20.2% | 305 | 17.1% | ||
| 9–11 | 469 | 16.6% | 300 | 16.8% | ||
| 12+ | 941 | 33.2% | 845 | 47.4% | ||
Figure 2.
Kaplan–Meier curves for rectal cancer specific survival amongst neoadjuvant and adjuvant cohorts.
In multivariate models for RC-SS amongst all patients, increasing age and T4 tumors were significant predictors of worse outcome regardless of treatment sequence (Table 2). There was no association between sex, race/ethnicity, or SES and RC-SS. Increasing number of positive lymph nodes was associated with worse outcome regardless of sequence, although the effect is numerically stronger and present at a lower lymph node stage in those who have been treated with neoadjuvant CRT. Those with lower numbers of lymph nodes retrieved trended towards a worse outcome, although the effect was only statistically signifi-cant for those with 8 or fewer lymph nodes retrieved (Table 2).
Table 2.
Multivariable Cox regression for rectal cancer specific survival amongst all patients with stage I – III rectal cancer treated with tri-modality therapy in California 2000–2007.
| Characteristic | Neoadjuvant cohort N = 2833 |
Adjuvant cohort N = 1782 |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age (continuous – 10 year increment): | 1.14 (1.05–1.24) | 0.0028 | 1.18 (1.07–1.32) | 0.0016 | |
| Sex: | Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.01 (0.81–1.25) | 0.9598 | 0.91 (0.71–1.16) | 0.4353 | |
| Race/Ethnicity: | Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | ||
| Non-Hispanic Black | 1.34 (0.86–2.10) | 0.1984 | 0.96 (0.54–1.69) | 0.8764 | |
| Hispanic | 1.02 (0.76–1.37) | 0.8733 | 1.04 (0.74–1.46) | 0.8137 | |
| Asian/Pacific Islander | 0.82 (0.59–1.14) | 0.2431 | 0.93 (0.64–1.36) | 0.7189 | |
| Socioeconomic status: | 1st – 2nd Quintile | 1.00 (reference) | 1.00 (reference) | ||
| 3rd Quintile | 0.97 (0.73–1.30) | 0.8400 | 1.32 (0.95–1.85) | 0.0986 | |
| 4th – 5th Quintile | 0.86 (0.67–1.12) | 0.2603 | 1.07 (0.79–1.44) | 0.6684 | |
| T stage: | 0–1 | 1.00 (reference) | 1.00 (reference) | ||
| 2 | 0.86 (0.50–1.48) | 0.5789 | 1.89 (0.66–5.42) | 0.2350 | |
| 3 | 1.53 (0.96–2.44) | 0.0745 | 3.74 (1.38–10.17) | 0.0097 | |
| 4 | 3.10 (1.87–5.14) | <0.0001 | 5.46 (1.92–15.59) | 0.0015 | |
| N stage: | N0 | 1.00 (reference) | 1.00 (reference) | ||
| N1a | 1.89 (1.36–2.61) | 0.0001 | 1.14 (0.75–1.73) | 0.5266 | |
| N1b | 2.18 (1.57–3.03) | <0.0001 | 1.54 (1.07–2.22) | 0.0210 | |
| N2a | 3.75 (2.61–5.40) | <0.0001 | 2.60 (1.76–3.83) | <0.0001 | |
| N2b | 6.37 (4.40–9.23) | <0.0001 | 5.43 (3.71–7.93) | <0.0001 | |
| Nodes examined: | 0–2 | 1.67 (1.10–2.54) | 0.0168 | 1.74 (1.10–2.54) | 0.0680 |
| 3–5 | 1.77 (1.28–2.46) | 0.0006 | 2.19 (1.48–3.23) | <0.0001 | |
| 6–8 | 1.68 (1.23–2.29) | 0.0111 | 1.79 (1.26–2.53) | 0.0010 | |
| 9–11 | 1.35 (0.97–1.88) | 0.0747 | 1.30 (0.92–1.85) | 0.1401 | |
| 12+ | 1.00 (reference) | 1.00 (reference) | |||
Similar trends for worse outcomes in both cohorts were observed for increasing age and T stage amongst those patients without positive lymph nodes identified in the surgical specimen (Table 3). Improved outcomes were observed in the highest SES group receiving neoadjuvant CRT and Asian/Pacific Islanders receiving adjuvant CRT. There was no statistically significant difference in RC-SS with lower numbers of lymph nodes retrieved in node negative patients in the neoadjuvant cohort. Compared to 12 or more lymph nodes examined, those with 3–5 or 6–8 removed before adjuvant therapy had significant inferior outcomes.
Table 3.
Multivariable Cox regression for rectal cancer specific survival amongst pathologic node-negative patients treated with tri-modality therapy in California 2000–2007.
| Characteristic | Neoadjuvant cohort N = 1916 |
Adjuvant cohort N = 658 |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p Value | ||
| Age (continuous – 10 year increment): | 1.14 (1.00–1.29) | 0.0468 | 1.22 (0.92–1.51) | 0.0748 | |
| Sex: | Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.08 (0.79–1.49) | 0.6288 | 1.02 (0.62–1.68) | 0.4353 | |
| Race/Ethnicity: | Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | ||
| Non-Hispanic Black | 1.39 (0.72–2.69) | 0.3274 | 0.43 (0.10–1.80) | 0.2467 | |
| Hispanic | 1.03 (0.66–1.59) | 0.9074 | 0.78 (0.39–1.57) | 0.4873 | |
| Asian/Pacific Islander | 0.96 (0.61–1.52) | 0.8695 | 0.33 (0.12–0.93) | 0.0364 | |
| Socioeconomic status: | 1st – 2nd Quintile | 1.00 (reference) | 1.00 (reference) | ||
| 3rd Quintile | 0.83 (0.55–1.26) | 0.3776 | 2.04 (1.09–3.82) | 0.0253 | |
| 4th – 5th Quintile | 0.69 (0.47–1.00) | 0.0498 | 1.14 (00.61–2.12) | 0.6909 | |
| T stage: | 0–1 | 1.00 (reference) | 1.00 (reference) | ||
| 2 | 1.27 (0.65–2.45) | 0.4835 | 2.03 (0.44–9.45) | 0.3681 | |
| 3 | 1.76 (0.98–3.16) | 0.0594 | 2.20 (0.53–9.19) | 0.2806 | |
| 4 | 4.64 (2.47–8.74) | <0.0001 | 4.18 (0.90–19.34) | 0.0672 | |
| Nodes examined: | 0–2 | 1.38 (0.84–2.27) | 0.2074 | 1.67 (0.67–4.20) | 0.2723 |
| 3–5 | 1.38 (0.88–2.17) | 0.1638 | 3.02 (1.49–6.13) | 0.0021 | |
| 6–8 | 1.20 (0.74–1.93) | 0.4552 | 2.18 (1.08–4.40) | 0.0301 | |
| 9–11 | 1.10 (0.64–1.87) | 0.7390 | 1.84 (0.84–4.02) | 0.1274 | |
| 12+ | 1.00 (reference) | 1.00 (reference) | |||
Discussion
In this retrospective study of over 4700 rectal cancer patients treated with tri-modality therapy, we investigated the prognostic implications of reduced lymph node retrieval after neoadjuvant therapy. This study corroborates prior studies that show a reduced number of lymph nodes retrieved in patients who have received neoadjuvant CRT. More importantly, we found no association between the number of lymph nodes retrieved and RC-SS in node negative patients after neoadjuvant CRT. This finding highlights a key difference between rectal and colon cancer and underscores careful interpretation of the pathologic findings when combined modality therapy is undertaken.
Although colon and rectal cancer have many biologic similarities, their treatment paradigms have diverged due to anatomic considerations. During the period of observation in this study, the proportion of patients with nonmetastatic rectal cancer receiving CRT before surgery increased. This corresponds temporally with the 2004 publication of the German Rectal Cancer Study Group trial, which showed improved local control and reduced toxicity with neoadjuvant treatment.4 As observed in the clinical trial, there was no difference in survival outcomes based on the treatment sequence in this population-based study.
The AJCC, CAP and other groups currently recommend that a minimum of twelve lymph nodes be examined at the time of colorectal cancer resection.7–10 The data driving this cutoff is based primarily on colon cancer studies, where the available data strongly support this recommendation.5,6 In this study, we did observe this expected association between reduced lymph node retrieval and survival in the neoadjuvant CRT cohort. However, a statistically significant effect could only be observed with 8 or fewer lymph nodes retrieved. Moreover, similar findings were observed in the adjuvant cohort. However, the association could not be confirmed amongst patients treated with neoadjuvant therapy who did not have pathologically involved lymph nodes in their surgical specimens despite relatively robust cohort sizes to detect such an effect. This suggests that the prognostic information gained by additional lymph node assessment in those patients treated with neoadjuvant therapy is concentrated in those with node positive disease and a higher risk of recurrence.
Several studies – including this one – have demonstrated that the total number of lymph nodes retrieved at the time of primary resection is reduced after neoadjuvant CRT.12–15 The major finding of this study is the failure to demonstrate that reduced lymph node retrieval is associated with worse outcome in node negative rectal cancer treated with neoadjuvant therapy. We did not find a threshold lymph node number below which there was an association with survival. This corroborates the findings of 2 much smaller institutional series and suggests that failure to retrieve 12 lymph nodes in this patient population is not associated with inferior outcome.17,20 We suggest that limited lymphadenectomy should not be used to assign a separate prognosis in node negative patients when neoadjuvant trimodality treatment is planned. This study provides some support to the argument that nodal assessment should not be used as a quality care endpoint for rectal cancer patients who have undergone CRT.21–23
Another important observation from this study relates to the implications of residual node positivity in patients who underwent neoadjuvant CRT. In this study, more pathologic node-negative cancers were observed in the neoadjuvant cohort compared to the adjuvant cohort. This is the expected result of the eradication of positive lymph nodes by neoadjuvant CRT. However, patients with node positive disease in the neoadjuvant cohort had a numerically worse prognosis compared to those in the adjuvant cohort. This result is consistent with multiple other studies and most likely relates to more resistant tumor biology, where the persistence of cancer within a lymph node after CRT portends a greater likelihood of recurrence.24,25 Future studies should investigate intensification of therapy or novel strategies in this subset of patients with a particularly poor prognosis.
There are several limitations to this study. As in all retrospective cohort studies, the results may have been affected by unmeasured confounding variables. Importantly, in this study we were not able to account for variability in skill among surgeons or pathologists, a factor that may impact lymph node retrieval. Pathologic assessment was performed locally and reported to the registry; therefore, we were unable to standardize the assessment. Additionally, the details of radiation and chemotherapy administration are not supplied to the registry, limiting our ability to account for variation in how treatment was administered amongst the groups. Recurrence information is not captured by the registry and we are unable to calculate recurrence free survival. Furthermore, the California Cancer Registry suffers from the same difficulties associated with other large cancer registries, including incomplete or inaccurate cancer reporting. Race and ethnicity data are particularly susceptible to misclassification bias. Nonetheless, this study assesses a large patient registry with excellent reporting standards.
In conclusion, we observed similar survival outcomes for patients treated with CRT preoperatively compared to those treated with CRT postoperatively in this large population-based study. The use of neoadjuvant CRT is increasing in frequency in California, and the implications of this transition include a lower rate of lymph node retrieval after surgery and a poor prognosis for those with residual positive lymph nodes after neoadjuvant therapy. Finally, we did not find an association between reduced lymph node number and survival in node negative patients treated with neoadjuvant CRT, suggesting that this measure does not provide useful information to inform therapeutic decision-making in this group of patients with rectal cancer.
Acknowledgments
Role of the funding source
Dr. Semrad is supported by the National Cancer Institute of the National Institutes of Health under award number K12CA138464.
Footnotes
Prior presentation: Presented at the 2012 ASCO Annual Meeting (Abstract #3566).
Conflict of interest statement
All authors have no conflicts of interest to disclose.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–96. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 3.Gastrointestinal Tumor Study Group Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–72. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–41. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 6.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–9. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 7.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 9.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–51. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Quality Forum [09.09 14];At least 12 regional lymph nodes are removed and pathologically examined for resected colon cancer. Available at: http://www.qualityforum.org/QPS/0225.
- 11.Chou JF, Row D, Gonen M, et al. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. 2010;116:2560–70. doi: 10.1002/cncr.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter NN, Morris AM, Rothenberger DA, et al. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426–31. doi: 10.1016/j.ijrobp.2004.06.259. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente SG, Manson RJ, Ludwig KA, et al. Neoadjuvant chemo-radiation for rectal cancer reduces lymph node harvest in proctectomy specimens. J Gastrointest Surg. 2009;13:269–74. doi: 10.1007/s11605-008-0717-2. [DOI] [PubMed] [Google Scholar]
- 14.Rullier A, Laurent C, Capdepont M, et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Safar B, Wexner S, et al. Lymph node harvest after proctectomy for invasive rectal adenocarcinoma following neoadjuvant therapy: does the same standard apply? Dis Colon Rectum. 2009;52:549–57. doi: 10.1007/DCR.0b013e31819eb872. [DOI] [PubMed] [Google Scholar]
- 16.de Campos-Lobato LF, Stocchi L, de Sousa JB, et al. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think!. Ann Surg Oncol. 2013;20:3398–406. doi: 10.1245/s10434-013-3010-x. [DOI] [PubMed] [Google Scholar]
- 17.Govindarajan A, Gonen M, Weiser MR, et al. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568–73. doi: 10.1200/JCO.2011.37.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidner TB, Ozao-Choy JJ, Yoon J, et al. Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am J Surg. 2012;204:843–7. doi: 10.1016/j.amjsurg.2012.05.003. discussion 7e8. [DOI] [PubMed] [Google Scholar]
- 19.Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272–9. doi: 10.1016/j.ejca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Persiani R, Biondi A, Gambacorta MA, et al. Prognostic implications of the lymph node count after neoadjuvant treatment for rectal cancer. Br J Surg. 2014;101:133–42. doi: 10.1002/bjs.9341. [DOI] [PubMed] [Google Scholar]
- 21.Mathoulin-Pelissier S, Becouarn Y, Belleannee G, et al. Quality indicators for colorectal cancer surgery and care according to patient-, tumor-, and hospital-related factors. BMC Cancer. 2012;12:297. doi: 10.1186/1471-2407-12-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald JR, Renehan AG, O'Dwyer ST, et al. Lymph node harvest in colon and rectal cancer: current considerations. World J Gastrointest Surg. 2012;4:9–19. doi: 10.4240/wjgs.v4.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel EM, Jacobsen PB, Lee JH, et al. Florida initiative for quality cancer care: improvements on colorectal cancer quality of care indicators during a 3-year interval. J Am Coll Surg. 2013;218:16–25. e4. doi: 10.1016/j.jamcollsurg.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang GJ, Rodriguez-Bigas MA, Eng C, et al. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–40. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 25.Seery TE, Ziogas A, Lin BS, et al. Mortality risk after preoperative versus postoperative chemotherapy and radiotherapy in lymph node-positive rectal cancer. J Gastrointest Surg. 2013;17:374–81. doi: 10.1007/s11605-012-2116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]