Abstract
Lysyl oxidase-like 2 (LOXL2) is involved in a wide range of physiological and pathological processes, including fibrosis and tumor progression, implicating intracellular and extracellular functions. To explore the specific in vivo role of LOXL2 in physiological and tumor contexts, we generated conditional gain- and loss-of-function mouse models. Germ-line deletion of Loxl2 promotes lethality in half of newborn mice mainly associated to congenital heart defects, while Loxl2 overexpression triggers male sterility due to epididymal dysfunction caused by epithelial disorganization, fibrosis and acute inflammation. Remarkably, when challenged to chemical skin carcinogenesis, Loxl2-overexpressing mice increased tumor burden and malignant progression, while Loxl2-deficient mice exhibit the opposite phenotypes. Loxl2 levels in premalignant tumors negatively correlate with expression of epidermal differentiation markers and components of the Notch1 pathway. We show that LOXL2 is a direct repressor of NOTCH1. Additionally, we identify an exclusive expression pattern between LOXL2 and members of the canonical NOTCH1 pathway in human HNSCC. Our data identify for the first time novel LOXL2 roles in tissue homeostasis and support it as a target for SCC therapy.
Keywords: epidermal differentiation, Loxl2 mouse models, male sterility, Notch1, squamous cell carcinoma
Introduction
Lysyl oxidase-like 2 (LOXL2) belongs to the lysyl oxidase (LOX) family that includes five closely related proteins (LOX and LOXL1–LOXL4). LOX enzymes are secreted to the extacracellular matrix (ECM) where they participate in the proper modification of ECM components essential to the biogenesis of the connective tissues (Csiszar, 2001; Kagan & Li, 2003; Lucero & Kagan, 2006; Kim et al, 2011). All members of the family present a highly conserved C-terminus region containing the catalytic domain and a more divergent N-terminal part potentially responsible for their specific functions (Maki & Kivirikko, 2001; Maki et al, 2001). Apart from the extracellular role of some LOX enzymes in the maturation of the ECM, recent studies have suggested novel functions for these proteins in a wide spectrum of biological processes. In particular, LOXL2 has been involved in gene transcription, cell motility/migration and adhesion, angiogenesis and differentiation, demonstrating their ability to affect both extra and intracellular cell functions (Peinado et al, 2005; Payne et al, 2007; Fujimoto & Tajima, 2009; Hollosi et al, 2009; Peng et al, 2009; Barker et al, 2011; Bignon et al, 2011; Moreno-Bueno et al, 2011; Cano et al, 2012; Herranz et al, 2012; Millanes-Romero et al, 2013). Regarding LOXL2 intracellular actions, we previously described its participation in epithelial–mesenchymal transition (EMT), a key event for tumor invasion and metastasis, by repressing E-cadherin in a Snail1-dependent manner (Peinado et al, 2005), and recently described that LOXL2 catalytic activity is dispensable for EMT induction (Cuevas et al, 2014). The role of LOXL2 in several human cancers has been increasingly described (Cano et al, 2012). In this pathological context, our previous studies in large series of human tumor samples demonstrated that intracellular localization of LOXL2 and its overexpression are associated to poor prognosis in laryngeal SCC (squamous cell carcinoma) (Peinado et al, 2008) and to distant metastasis in basal-like breast carcinomas (Moreno-Bueno et al, 2011). Moreover, mechanistic studies in human cancer cell lines revealed the implication of intracellular LOXL2 in EMT, invasion and metastasis through regulation of cell polarity and differentiation programs, dependent and independent of Snail1 (Peinado et al, 2008; Moreno-Bueno et al, 2011). Other groups have also suggested the involvement of extracellular LOXL2 in metastasis of breast and other tumor types (Barry-Hamilton et al, 2010; Barker et al, 2011; Wong et al, 2014). Additionally, different members of the LOX family, highlighting LOX and LOXL2, have been involved in pre-metastatic niche formation (Erler et al, 2009; Wong et al, 2011, 2014; Canesin et al, 2015), an event presently considered essential for tumor cell homing and micrometastasis (Psaila & Lyden, 2009). Despite the increasing evidence supporting multiple LOXL2 roles in tumorigenesis and metastasis, the mechanistic bases of these pathological functions and their relevance for future therapeutic interventions have not been fully established. Moreover, in vivo data concerning LOXL2 involvement in tissue homeostasis, the downstream effectors and its potential redundant action with other LOX members remain elusive.
In the current study, we have generated for the first time conditional genetic mouse models lacking and overexpressing Loxl2 to deepen into the in vivo biological implication of LOXL2 in both physiological and tumor prone contexts. Constitutive abrogation of Loxl2 results in perinatal lethality with incomplete penetrance associated, in one-third of the cases, to congenital heart defects and/or distension of the hepatic blood vessels. On the other hand, males overexpressing Loxl2 are sterile due to testicular degeneration and epididymal dysfunction caused by altered epithelial organization, increased fibrosis and acute inflammation. Moreover, phenotypic analysis of both mouse models subjected to the two-step skin carcinogenesis protocol, as a model system of human head and neck squamous cell carcinoma (HNSCC) (Yuspa, 1994), uncovers a key role for Loxl2 during tumor initiation and progression. Thus, Loxl2 deficiency significantly decreases the size of skin lesions and their malignant progression, while Loxl2 overexpression markedly diminishes latency and increases tumor burden and malignancy. Mechanistic studies indicate that Loxl2 negatively regulates epidermal differentiation and the Notch1 signaling pathway in premalignant lesions. Remarkably, LOXL2 binds to, at least, two different regions of NOTCH1 promoter, reducing the methylation status of H3K4me3 and RNA polymerase II recruitment, therefore repressing NOTCH1 transcription. Finally, the relevance of the negative regulation of NOTCH1 by LOXL2 in human tumors is shown by the exclusive expression pattern between LOXL2 and components of the NOTCH1 pathway in large cohorts of human HNSCC and cervical SCC. The present data highlight the critical role of LOXL2 in homeostasis of specific tissues and strengthen the potential value of LOXL2 as a druggable target for novel therapeutic interventions in SCC, where the in vivo Loxl2 models presented here can provide valuable pre-clinical models.
Results
Generation of transgenic mice
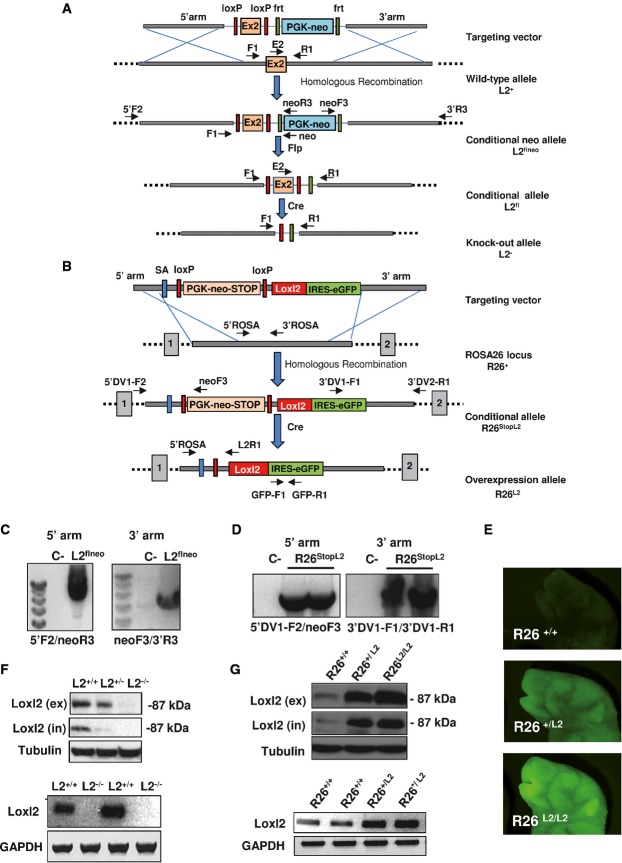
Constitutive KO (L2−/−) and overexpressing (R26L2/L2) mice for Loxl2 were generated by gene-targeting techniques as described in Materials and Methods and Fig1. Briefly, we placed the endogenous loci of Loxl2 or ROSA26 genes in specific plasmid constructions based on the Cre/loxP system which, ultimately, after CMV-Cre-mediated recombination gave rise to generalized deletion or overexpression of Loxl2, respectively (Fig1A and B). Homologous recombination was confirmed by diagnostic PCR in embryonic stem cell positive clones and chimeric founders (Fig1C and D). Germ-line Loxl2 KO and overexpressing animals were generated by intercrossing heterozygous mice. Fluorescence imaging as well as RNA and Western blot analysis confirmed targeted Loxl2 expression (Fig1E–G). Considering that the two genetically engineered Loxl2 mouse strains have different genetic backgrounds, all the experiments presented hereafter were performed with littermate controls for each model.
Figure 1.
- A Schematic representation of different Loxl2-targeted alleles. Exon 2 (orange box) and PGK-neomycin selection marker (blue box) flanked by LoxP (red rectangles) and frt (green rectangles) sites, respectively, are indicated in the targeting vector. Homologous recombination resulted in the generation of L2flneo allele and subsequent recombination events mediated by Flp (flippase) and Cre enzymes yielded L2fl and L2− alleles, respectively. Position of primers used in PCRs is indicated.
- B Schematic representation of different ROSA26 allele variants used in the study. Targeting vector containing PGK-neomycin-stop cassette (orange box) flanked by LoxP sites (red rectangles), splicing acceptor signal (SA) (blue rectangle) and Loxl2 cDNA sequence (red box) followed by the IRES-GFP reporter gene (green box), are represented. R26StopL2 allele is initially generated by homologous recombination. After Cre-mediated excision, the PGK-neomycin-stop element is removed and the Loxl2/GFP tandem is expressed under the control of ROSA26 promoter (R26L2 allele). Primer pairs for PCR analysis are also detailed.
- C,D Diagnostic PCR analysis of positive ES cell clones showing the detection of the recombinant L2flne (C) and R26StopL2 (D) alleles. Negative controls were also used in parallel (C-).
- E GFP images from wild-type, heterozygous and homozygous Loxl2-overexpressing newborn mice.
- F,G Western blot analysis (upper panels) of whole-cell extracts (in) and conditioned medium (ex), and semi-quantitative PCR (lower panels) performed in MEFs from the indicated genotypes. α-tubulin and GAPDH were used as loading controls in Western blot analysis and semi-quantitative PCR, respectively.
Deletion of Loxl2 provokes perinatal lethality
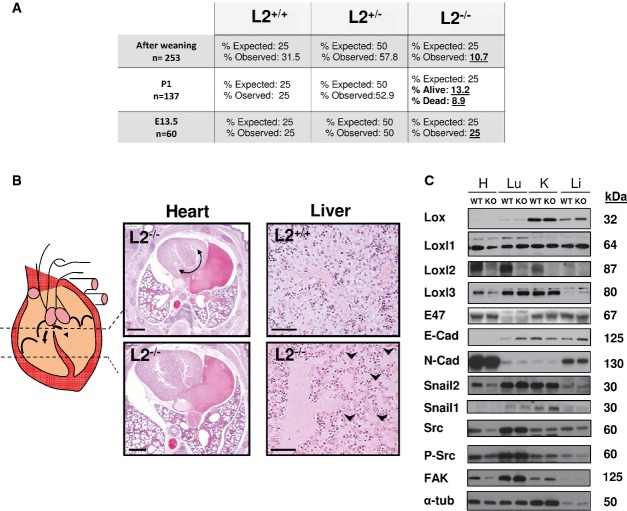
Deleted Loxl2 mutants were viable and fertile, but analysis of the offspring (n = 253) at weaning revealed that L2−/− mice were present at twofold lower frequency than expected from a Mendelian ratio in each of the two conditional KO established lines (Fig2A, and data not shown). Since normal numbers of L2−/− embryos were observed at mid-gestation (E13.5) (Fig2A), we decided to proceed with perinatal analysis. At P1, some L2−/− neonates were grossly affected with a cyanotic appearance dying in a few hours. Histopathological examination of serial sections from healthy and dead L2−/− neonates showed that two out of eight dead L2−/− mice displayed disrupted ventricular septa formation (Fig2B, left). This alteration was accompanied by a distension of the hepatic blood vessels in one of the cases (Fig2B, right), a defect also found in one additional dead L2−/− mouse. Thus, almost 40% (three out eight) of dead L2−/− neonates presented dramatic alterations in the heart and/or liver tissue homeostasis, likely responsible for their death. In agreement with the heart phenotype, high expression levels of Loxl2 were found in heart and lung, and at lesser extent in kidney and liver (Fig2C). No major differences in the expression of other LOX members (Lox, Loxl1 and Loxl3) were detected in different tissues between L2−/− and control mice except for Loxl3 downregulation in heart (Fig2C), discarding main compensatory mechanisms. Analysis of EMT factors and Lox-related pathways revealed increased E-cadherin levels in lung and liver and significantly decreased Snail2, FAK and Src (and P-Src) levels, mainly in heart extracts from L2−/− mice (Fig2C). Considering that some of these molecules have been critically implicated in cardiac morphogenesis, particularly the FAK/Src signaling pathway (Peng et al, 2008) and the reported LOXL2 modulation of FAK in other contexts (Peng et al, 2009; Moreno-Bueno et al, 2011; Barker et al, 2013), these findings support the heart defects detected in the absence of Loxl2.
Figure 2.
- Percentage of observed surviving animals compared to the expected Mendelian ratio in wild-type, heterozygous and knock-out animals at mid-gestation (E13.5), P1 and after weaning. Mendelian values reflecting perinatal lethality are in bold.
- Diagram and H&E of transversal sections from Loxl2 KO heart at different levels showing aberrant ventricular communication (left). Curved arrow points to disrupted ventricular septa. H&E sections of livers (right) from Loxl2 WT (top) and KO (bottom) neonates. Arrowheads indicate regions of capillary distension. Scale bars: 1,500 μm (heart), 100 μm (liver).
- Western blot analysis of Lox members, EMT markers and Src/FAK pathway in the indicated tissues derived from a pool of two P1 neonates of each Loxl2 genotype. H, heart; Lu, lung; K, kidney and L, liver. Molecular mass of each marker is shown on the right. Notice that the 4 right lanes corresponding to Lox and Loxl3 markers were rotated 180°C from the original blots because of a mistake in the loading order of kidney and liver samples. Original blots for Lox, Loxl1, Loxl2 and Loxl3 detection are shown in the source data for this figure.
Loxl2 overexpression results in male sterility
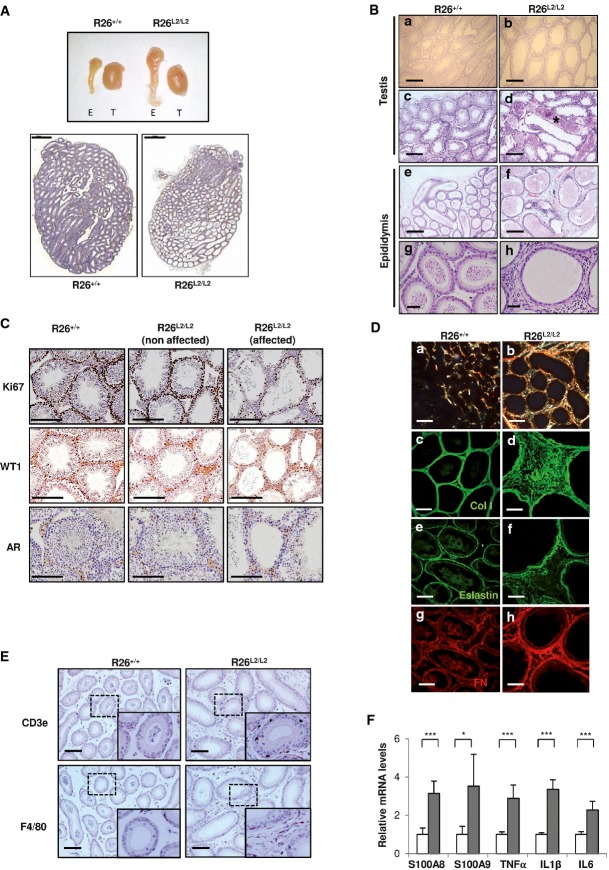
Regarding constitutive Loxl2 overexpression mutants, male and female R26L2/L2 mice were born according to the expected Mendelian ratio and survived without showing any gross histological abnormality (data not shown). However, 90% of the R26L2/L2 males were sterile. Histological examination of reproductive organs of R26L2/L2 males showed epididymis hypertrophy and testicular degeneration, affecting more than 60% of the tissue, as early as 3 months after birth (Fig3A and B, upper); analysis at latter points revealed a dramatic reduction of spermatozoa content in the epididymis lumen (Fig3B, bottom). Additionally, a clear disorganization of seminiferous tubules with reduced proliferation index and prominent spermatoceles was observed in degenerated areas of mutant testis (Fig3B and C). Characteristic meiosis I and II figures were identified in R26L2/L2 testis as well as normal ratios of supporting and interstitial cell populations, Sertoli and Leydig cells, respectively (Fig3C, and data not shown), discarding a problem in the spermatogenesis process as a cause of R26L2/L2 male sterility.
Figure 3.
- Macroscopic view of testis and epididymis from R26+/+ and R26L2/L2 mice (top) and H&E image of whole testis from both groups of animals showing testicular degeneration in the overexpressing mice (bottom). T: testis, E: epididymis. Scale bar in lower panels: 1,000 μm.
- H&E from paraffin-embedded tissues of testis (upper panels) and epididymis (lower panels) from R26+/+ and R26L2/L2 mice showing testicular degeneration (a, b), spermatocele formation (asterisk) (c, d), abnormal epididymal architecture (e, f) and complete absence of spermatozoa in testis (g, h) from R26L2/L2 mice of 4 months of age in comparison with their respective controls. Scale bars: 250 μm (a–f) and 50 μm (g, h).
- Immunohistochemical analysis of testis derived from 3-month-old mutant and control animals for Ki67, WT1 and androgen receptor (AR) markers to evaluate proliferation, Sertoli and Leydig cell populations, respectively. Affected and non-affected testis region from R26L2/L2 mice (middle and right panels) compared to control R26+/+ testis (left panels) are shown. Scale bar: 250 μm.
- Picrosirius red staining images of epididymis from control and R26L2/L2 mice of 3 months of age under polarized light (a, b). Confocal immunofluorescence images for collagen I (Col I) (c, d), elastin (e, f) and fibronectin (FN) (g, h) in OCT sections of epididymis from 3-month-old control R26+/+ and R26L2/L2 mice. Scale bars: 250 μm (a, b) and 100 μm (c–h).
- Immunohistochemical analysis for inflammatory cell markers CD3e (T-lymphocytes) (top) and F4/80 (macrophages) (bottom) in epididymis from R26+/+ and R26L2/L2 mice of 3 months of age. Scale bars: 100 μm. Inserts show a 2.5-fold amplified region.
- Quantitative RT–PCR of S100A8, S100A9, TNFα, IL1β and IL6 cytokine expression in epididymis from R26+/+ (white) and R26L2/L2 (gray) mice. *P < 0.05; ***P < 0.001 (Student's t-test; unpaired, 2-tailed). Error bars represent standard error.
A detailed examination of epididymis from R26L2/L2 mice indicated distended tubular architecture, accompanied by acute tissue inflammation and fibrosis (Fig3D–F). Concerning the fibrotic phenotype, large fibronectin, elastin and collagen deposits were found in the stromal epididymal component of R26L2/L2 mice that also showed a denser ECM than controls, as revealed by immunofluorescence and picrosirius red staining (Fig3D). Moreover, the acute inflammatory response detected in the surrounding connective tissue and epithelial component of the R26L2/L2 epididymal tubules (Fig3E) was confirmed in terms of both recruitment of specific immune cell populations and increased inflammatory cytokine expression compared to controls (Fig3E and F).
Regarding the epithelial component of epididymis, atrophic flattened cells as well as regions with squamous cell metaplasia were observed in R26L2/L2 mice (Fig4A a and b). Further, increased proliferating epithelial and stromal cells were present in Loxl2-overexpressing epididymis (Fig4A c and d), which, interestingly, correlate with the augmented levels of Loxl2 found in both tissue compartments compared to paired controls (Fig4A e–h).
Figure 4.
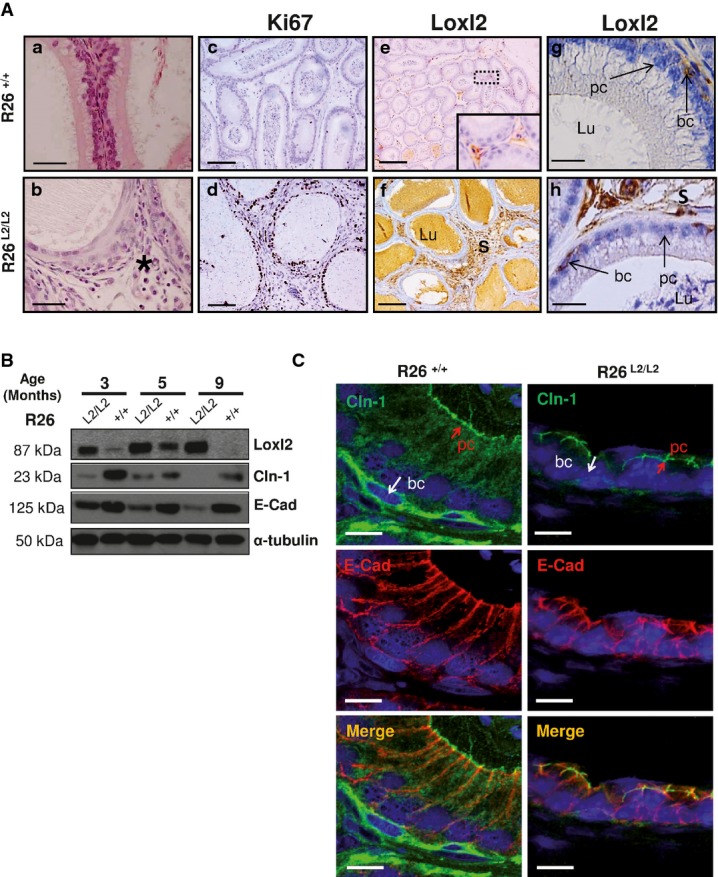
- Detailed region of squamous cell metaplasia in epididymis from Loxl2-overexpressing mice (b, black asterisk) compared to the normal epididymal architecture found in control counterparts (a). Immunohistochemical analysis shows increased Ki67 staining in stroma and epithelium components of R26L2/L2 epididymal tissue (d) compared to controls (c). Immunohistochemical staining of Loxl2 in epididymis from control (e, g) and R26L2/L2 mice (f, h) showing Loxl2 overexpression in both surrounding stroma (f) and basal cells (h) of R26L2/L2 in comparison with basal levels detected in R26+/+ mice (e, g). Insert in (e) shows a fourfold amplified region. Arrows in (g, h) indicate Loxl2 expression in basal cells. Scale bars: 50 μm (a, b), 100 μm (c, d), 250 μm (e, f) and 25 μm (g, h).
- Western blot analysis of Loxl2, E-cadherin (E-cad) and claudin-1 (Cdn-1) protein levels in epididymal extracts from mice of the indicated Loxl2 genotypes and age. Two independent experiments with 2 different animals per genotype and age were performed.
- Immunofluorescence analysis of E-cadherin and claudin-1 in OCT sections from epididymis of control and 3-month-old R26L2/L2 mice. Merged images are shown in the bottom panels. Scale bar: 10 μm.
Epididymis is a pseudo-stratified epithelium composed of principal and basal cells lying on a basement membrane. This structure is mainly preserved by apical tight junctions, including ZO and claudin proteins, and adherent junctions (Gregory et al, 2001; Cyr et al, 2007; Dube et al, 2007). Since LOXL2 is involved in the downregulation of different components of the intercellular junction machinery such as E-cadherin and claudin-1 (Peinado et al, 2005; Moreno-Bueno et al, 2011), we decided to explore the status of both players in epididymis. Analysis of claudin-1 and E-cadherin levels in epididymal tissue from R26L2/L2 males revealed a conspicuous fall of their expression compared to control mice, which increased dramatically with age (Fig4B). Strikingly, claudin-1 and E-cadherin decrease was concentrated to basal cells of R26L2/L2 epididymis (Fig4C), in tight agreement with Loxl2 cellular localization in this specific cell compartment (Fig4A g and h).
Collectively, these results suggest that epithelial disorganization and altered epithelial–stromal cross talk in the epididymis promoted by Loxl2 overexpression are responsible for aberrant seminiferous tubules, spermatocele formation and, ultimately, compromised fertility.
Loxl2 is required for skin tumor progression
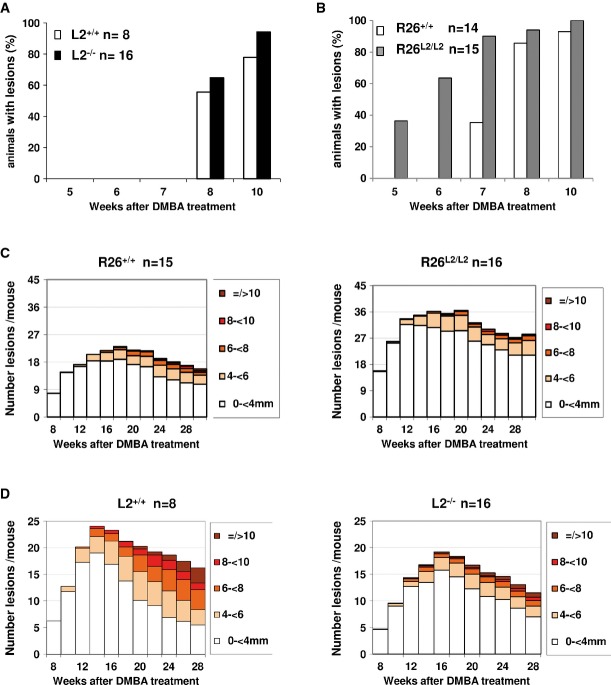
LOXL2 has been proposed as a prognostic marker in larynx SCC (Peinado et al, 2008), one of the most frequently diagnosed tumors worldwide. To study the effect of Loxl2 abrogation and overexpression on SCC tumor formation and progression, we took advantage of the well-established two-stage mouse skin carcinogenesis as a model system of the evolution of HNSCC, sharing similar molecular alterations and response to TGFβ1 during malignant progression (Yuspa, 1994; Glick, 2012). Whereas control (L2+/+) and KO (L2−/−) mice started to develop lesions after 8 weeks of initiating DMBA application (Fig5A), the onset of lesion formation was markedly accelerated in Loxl2-overexpressing mice (R26L2/L2) in which the first lesions appeared just 5 weeks post-DMBA application (Fig5B). Another striking difference concerned the increased number of lesions observed in R26L2/L2 animals compared to their control mice (R26+/+). In particular, at week 20, the number of lesions in R26L2/L2 mice almost doubled those of control mice with an average of 36 lesions/mouse compared to controls with an average of 22 lesions/mouse (Fig5C, Supplementary Figs S1 and S2). Noticeably, the opposite phenotype was observed in Loxl2 KO mice that showed a reduction of tumor burden during most of the carcinogenesis protocol in comparison with wild-type controls (Fig5D, Supplementary Figs S1 and S2). Moreover, an important fraction of the neoplastic lesions developed in the absence of Loxl2 were significantly smaller than those expressing normal Loxl2 levels. Thus, at the end of the experiment (week 28), 50% of neoplasias in L2+/+ mice reached more than 6 mm in size while this percentage fell to 25% in L2−/− counterparts where in fact most of the lesions remained smaller than 4 mm (Fig5D, Supplementary Figs S1 and S2).
Figure 5.
- A,B The onset of lesion formation after DMBA-TPA treatment is indicated as the percentage of (A) L2+/+ and L2−/− and (B) R26+/+ and R26L2/L2 mice with tumors from 5 to 10 weeks post-initiation.
- C,D Number and size of lesions per mouse are shown from (C) 8 to 30 weeks post-DMBA application in R26+/+ (left) and R26L2/L2 (right) mice, and from (D) 8 to 28 weeks post-DMBA application in L2+/+ (left) and L2−/− (right) mice. n: number of mice used in each experimental group. Statistical analysis is provided in Supplementary Fig S2.
Histopathological analyses revealed that the vast majority of the lesions from all genotypes were papillomas. However, 7.7% (seven out of 90) of lesions in L2+/+ mice progressed to SCC, and, importantly, a threefold decrease in SCC incidence (2.8%; two out of 72) was observed in L2−/− mice (Fig6A). Noticeably, the opposite phenotype was detected in R26L2/L2 mice showing a 4% incidence (four out of 100) of SCC contrasting with a complete absence of SCC in their control mice (Fig6A). The different behavior in terms of tumor progression between the two cohorts of control mice used in the carcinogenesis experiments reflects the strong sensitivity dependence on the genetic background, as consistently reported by other groups (Naito & DiGiovanni, 1989; Woodworth et al, 2004).
Figure 6.
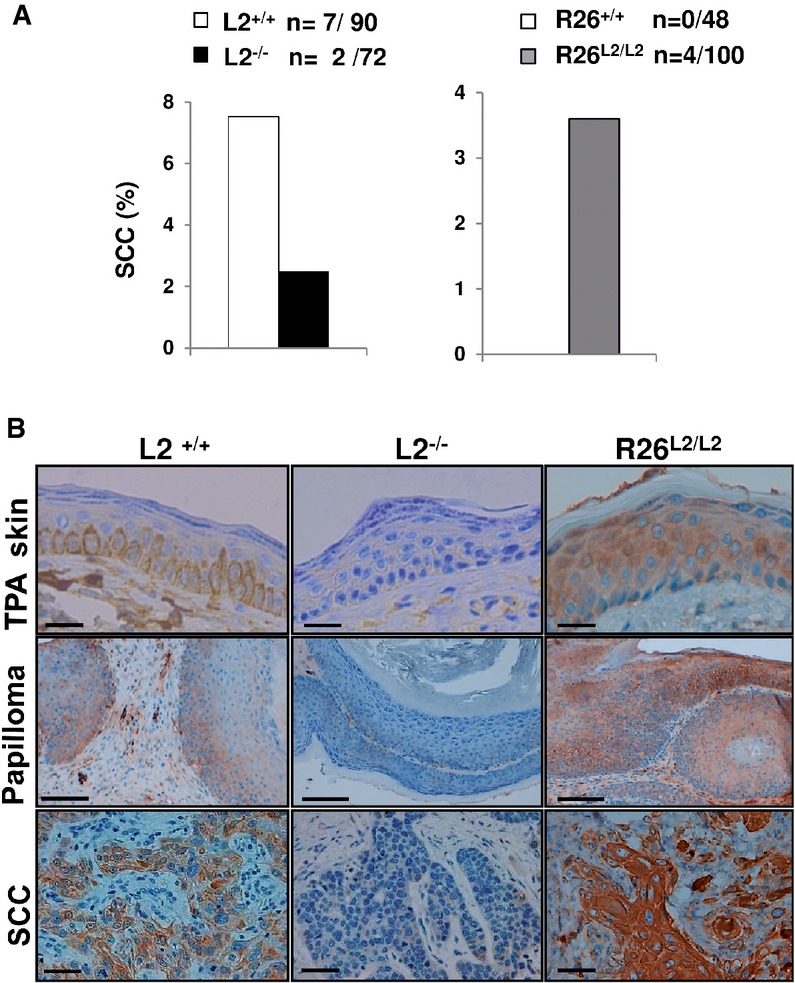
- Percentage of squamous cell carcinomas (SSC) per mouse observed in L2−/− (left, black bars) and R26L2/L2 (right, gray bars) mutant mice compared to their respective controls (white bars); n: absolute number of SCC regarding total number of analyzed lesions per each Loxl2 genotype.
- Immunohistochemical staining for Loxl2 in TPA-treated skins, papillomas and SCC from control L2+/+, L2−/− and overexpressing R26L2/L2 mice. Scale bars: 100 μm (top and bottom panels) and 250 μm (central panels). Immunohistochemical images are representative of three analyzed animals per genotype.
Loxl2 expression was restricted to the basal skin layer in TPA-treated and normal wild-type mice (Fig6B, upper left panel; and data not shown), in agreement with previous observations (Fujimoto & Tajima, 2009). As expected, no Loxl2 expression was detected in TPA-treated skins from L2−/− mice, while similar hyperplastic epidermis from R26L2/L2 mice exhibited a clear Loxl2 staining in all of the epidermal layers (Fig6B, upper central and right panels). In the context of tumor lesions, Loxl2 expression was expanded to hyperplastic areas of papillomas from wild-type and R26L2/L2 mice, with increased levels detected to most cell layers in the latter case (Fig6B, middle left and right panels). In the same way, higher amounts of Loxl2 were observed in SCC derived from R26L2/L2 mice compared to those appearing in control mice (Fig6B, bottom left and right panels). Noteworthy, Loxl2 expression was mainly confined to the intracellular compartment in papillomas and particularly SCC, thus corroborating our previous observations that linked augmented intracellular LOXL2 to human HNSCC malignancy (Peinado et al, 2008). The complete absence of Loxl2 protein in papillomas and SCC developed by L2−/− mice was also confirmed (Fig6B, central middle and bottom panels).
In sum, all these results support a relevant role of Loxl2 in both the initiation and malignant progression of skin tumors.
Loxl2 inhibits epidermal differentiation
LOXL2 has been previously proposed as a negative regulator of keratinocyte differentiation (Peinado et al, 2008; Lugassy et al, 2012). To deepen into this Loxl2 function in in vivo contexts and its potential contribution to the carcinogenic behavior, the expression of a panel of epidermal differentiation genes was evaluated in papillomas from the two genetically modified Loxl2 mouse models compared to their littermate controls. Reduced malignant progression of papillomas derived from L2−/− mice correlated with increased differentiation, as assessed by upregulated expression of early and late differentiation markers such as K1, loricrin and involucrin (Fig7A). Importantly, Lox expression levels prominently increased in the absence of Loxl2 (Fig7A), supporting a reciprocal regulation mechanism in this tumor context, similar to that proposed in normal epidermis (Fujimoto & Tajima, 2009). Regarding other Lox members, Loxl3 was also upregulated while Loxl1 and Loxl4 remained unaltered in the absence of Loxl2 (Supplementary Fig S3A). As expected, the opposite behavior was found in R26L2/L2 papillomas showing significant downregulation of involucrin and Lox and a more modest reduction in loricrin and K1 (Fig7B). Additionally, Loxl3 and Loxl4 mRNA levels were markedly decreased in papillomas from Loxl2-overexpressing mice, strengthening the transcriptional connection between these four Lox members (Fig7B and Supplementary Fig S3B). Together, these analyses support that Loxl2 promotes a dedifferentiation status in premalignant skin lesions that likely favors their malignant progression. Importantly, a similar but less marked behavior in terms of differentiation has been observed in normal skin. In fact, IF staining indicated altered terminal differentiation layers, although no major changes were observed in the overall skin architecture of L2−/− and R26L2/L2 mice compared to controls (Supplementary Fig S4A). Parallel in vitro studies with mutant and control primary keratinocytes from both Loxl2 mouse models revealed that protein levels of loricrin, suprabasal cytokeratins and prototypic Lox inversely correlated with Loxl2 levels (Supplementary Fig S4B), despite of which no major alterations in Ca2+-induced differentiation and cell adhesion to a Collagen IV matrix (Supplementary Fig S4C) were detected. Collectively, these data argue in favor of a relevant role of Loxl2 in the negative regulation of terminal differentiation in normal epidermis and much more prominently in premalignant skin lesions.
Figure 7.
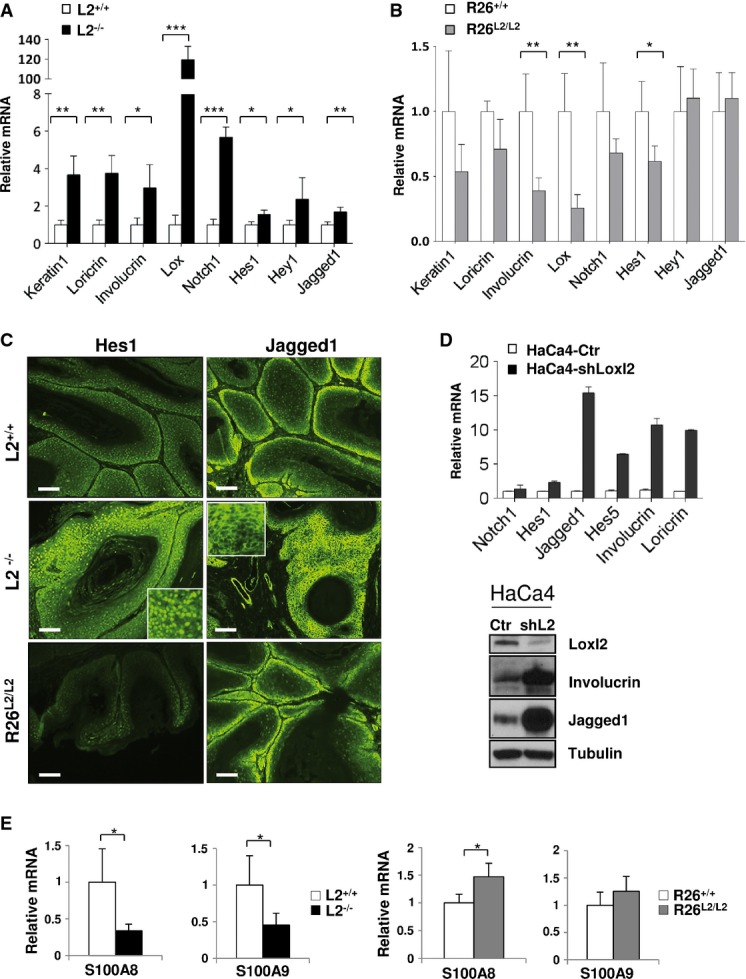
- Quantitative RT–PCR analysis of epidermal differentiation markers (keratin-1, loricrin, involucrin and Lox) and Notch1 pathway-related genes (Notch1, Hes1, Hey1 and Jagged1) in papillomas from control and L2−/− mice.
- The same quantitative RT–PCR analysis as in (A) was carried out in papillomas from control and R26L2/L2 mice.
- Representative immunofluorescence staining for Hes1 (left panels) and Jagged1 (right panels) in papillomas of the indicated Loxl2 genotypes. Sixteen lesions from four different mice of each genotype were examined. Scale bars: 250 μm. Inserts in central panels show representative threefold amplified regions.
- Quantitative RT–PCR (top) and Western blot analysis (bottom) of differentiation markers and Notch1-related genes in control (HaCa4-Ctr) and Loxl2-interfered (HaCa4-shLoxl2) HaCa4 cells. Detection of Loxl2 levels is shown as a control of the knockdown efficiency. Two experimental replicates were carried out.
- Quantitative RT–PCR analyses of S100A8 and S100A9 expression in papillomas from L2−/− (left) and R26L2/L2 mice (right) compared to their respective controls.
Loxl2 negatively regulates the Notch1 pathway in skin tumors
The critical implication of the Notch1 signaling pathway in epidermal homeostasis promoting differentiation and tumor suppression in non-melanoma skin cancer has been previously established (Lefort & Dotto, 2004; Watt et al, 2008; Panelos & Massi, 2009; Guinea-Viniegra et al, 2012; Brooks et al, 2014). Therefore, we were prompted to investigate whether Loxl2 might be influencing the Notch1 pathway during mouse skin tumor progression. To this end, we analyzed the differentiation-inducing Notch1 signaling pathway in papillomas derived from both L2−/− and R26L2/L2 mice compared to their corresponding controls. When Loxl2 was deleted, papillomas exhibited increased Notch1 mRNA levels compared to controls, together with a concomitant induction of the canonical Notch1 target genes Hey1 and Hes1, as well as a consistent increase in the expression of Jagged1 (JAG1), one of the Notch ligands (Fig7A). Confirming these data, immunofluorescence analysis revealed a strong upregulation of Hes1 and Jagged1 proteins in L2−/− papillomas compared to controls (Fig7C, upper and middle panels). Importantly, analysis of the Notch1 pathway in papillomas derived from overexpressing R26L2/L2 mice revealed a different scenario: Notch1 and Hes1 expression levels were altered, being downregulated compared to controls (Fig7B), although to a lesser degree than in L2−/− tumors. These later data suggest that although normal levels of Loxl2 are sufficient to maintain a basal Notch1 signaling activity, the pathway can be negatively modulated by increased Loxl2 levels. In fact, immunofluorescence analyses (Fig7C and Supplementary Fig S5) indicated that expression of Hes1 protein was dramatically reduced in papillomas from overexpressing Loxl2 mice (Fig7C, bottom left panel) validating the mRNA data. Despite the observed alterations in the Notch1 signaling pathways, no substantial changes occurred in p53 at both mRNA and protein levels (Supplementary Fig S6), critically involved in the induction of Notch1 transcription in skin (Lefort et al, 2007; Yugawa et al, 2007; Kolev et al, 2008; Guinea-Viniegra et al, 2012). However, when p21 expression was analyzed as a read out of p53 activity, we found that increased amounts of Loxl2 diminished p21 at both mRNA and protein levels (Supplementary Fig S6).
The functional consequences of altering Loxl2 expression levels on the Notch1 signaling pathway were further assessed in mouse squamous cell carcinoma HaCa4 cells where we previously reported the induction of a differentiated phenotype and suppression of invasion and tumorigenicity by Loxl2 silencing (Peinado et al, 2005, 2008). HaCa4 cells were lentivirally infected with a shRNA for Loxl2 that interferes with its protein expression by more than 80% (Fig7D, lower panel). Real-time RT–PCR and immunoblot analysis revealed that Loxl2 silencing in HaCa4 cells induces a dramatic upregulation of different Notch1-related players as well as differentiation genes (Fig7D).
In addition to the above-described alterations, Loxl2 levels affect the inflammatory component of papillomas. Thus, inflammation was increased in R26L2/L2 while decreased in L2−/− lesions compared to their respective controls (Supplementary Fig S7). In agreement with this, striking alterations in expression of inflammatory cytokines S100A9 and/or S100A8 were detected in papilloma lesions. Papillomas from L2−/− mice displayed strong downregulation of S100A8 and S100A9 transcripts, while R26L2/L2 lesions exhibited increased S100A8 mRNA levels compared to their wild-type controls (Fig7E). These data are in agreement with the downregulation of inflammation by Notch1 in skin (Dumortier et al, 2010) and further link Loxl2 to downregulation of the Notch1 pathway in skin carcinogenesis.
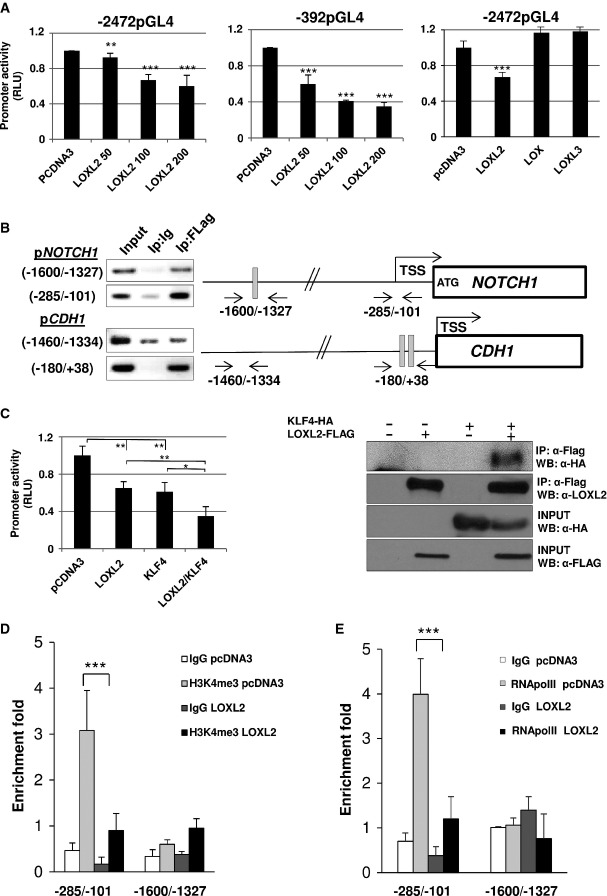
Loxl2 binds to NOTCH1 promoter increasing H3K4me3 deamination and impairing RNA polymerase II recruitment
To get mechanistic insights into the Loxl2-mediated regulation of the Notch1 pathway, the potential of Loxl2 to mediate transcriptional regulation of Notch1 was investigated. To this end, the response to LOXL2 of two different fragments of the human NOTCH1 promoter differing in their 5′ end length and p53 binding elements (Lambertini et al, 2010) was analyzed. The activity of both promoter constructs, even in the case of the short one (-392pGL4) devoid of p53 binding elements (Lambertini et al, 2010), was repressed by LOXL2 in a dose-dependent manner (Fig8A, left and central panels). This repressor activity seems to be LOXL2 specific since other members of the family, such as LOX and LOXL3, whose in vivo expression levels inversely correlated to Loxl2 status, did not cause any effects on the full-length promoter activity (Fig8A right panel). Furthermore, chromatin immunoprecipitation (ChIP) assays showed the binding of LOXL2 to the endogenous NOTCH1 promoter. LOXL2 interacts with the NOTCH1 promoter in a distal region that contains an E-box element, as well as in a proximal region lacking E-boxes and p53 binding regions but containing binding sites for the repressors KLF4 and Sp3 (Lambertini et al, 2010) (Fig8B). These data indicate that LOXL2 also recognizes and binds to the NOTCH1 promoter independently of E-boxes, in contrast to its action on the E-cadherin (CDH1) promoter (Fig8B) (Cuevas et al, 2014). In this regard, it has been shown that the transcriptional repressors KLF4 and Sp3 can modulate NOTCH1 expression through their binding to DNA sequences preferentially located in the minimal functional region of the NOTCH1 promoter (Lambertini et al, 2010). Additionally, our group has previously reported a repression mechanism of E-cadherin where LOXL2 collaborates with different transcription factors, such as Snail1 and E47, that could play a role as intermediate proteins for LOXL2 binding to the E-boxes of CDH1 promoter (Peinado et al, 2005; Canesin et al, 2015; Cuevas et al, 2014). Thus, we next focused on the identification of accessory proteins capable to cooperate with LOXL2 in the regulation of NOTCH1 transcription and therefore potentially mediate LOXL2 binding to different regions of the NOTCH1 promoter. To this end, we analyzed whether increased levels of LOXL2 can synergize with ectopically overexpressed Snail1/E47 or KLF4/Sp3 in the repression of −2472pGL4 and −392pGL4 NOTCH1 promoter activity, respectively. No synergistic effect on reporter silencing was observed by the concomitant expression of LOXL2 and Snail1 or E47, which prevented us to postulate them as intermediate factors responsible for LOXL2 binding to the E-box of the NOTCH1 promoter (Supplementary Fig S8A and B). No synergistic effect between LOXL2 and Sp3 (Supplementary Fig S8C) on the activity of the shortest variant of NOTCH1 promoter was also observed. However, combined expression of LOXL2 and KLF4 resulted in a significant increase, up to 63%, in the repression activity (Fig8C, left panel), suggesting that KLF4 might facilitate the binding of LOXL2 to the proximal NOTCH1 promoter to repress gene transcription. This notion was further reinforced by co-immunoprecipitation experiments that suggested an in vivo interaction between LOXL2 and KLF4 (Fig8C, right panel).
Figure 8.
- A NOTCH1 promoter activity assays performed in HEK293T cells using full-length (−2472pGL4; left panel) and minimal functional (−392pGL4; middle panel) promoter regions in the presence of pcDNA3 control (200 ng) and increasing amounts of human LOXL2. NOTCH1 promoter activity was also measured using −2472pGL4 promoter region (right panel) in the presence of human LOX and LOXL3 (100 ng). Results are the average of at least three different experiments performed on triplicate samples.
- B Chromatin immunoprecipitation (ChIP) assays in HEK293T cells transiently expressing LOXL2-Flag for binding to endogenous NOTCH1 (upper panels) and E-cadherin (CDH1) (lower panels) promoters in distal and proximal regions. Negative control with rabbit IgG (Ip: Ig) and positive input non-immunoprecipitated fractions are included. Positions of primers for amplification and E-boxes (gray) in both promoters are indicated. Transcription start site (TSS) for both genes and ATG initiating codon in NOTCH1 are also represented (Lambertini et al, 2010). Results show one representative experiment out of three performed for each condition and gene.
- C Left: NOTCH1 promoter activity assays performed in HEK293T cells using −392pGL4 promoter region in the presence of pcDNA3 control (200 ng), human LOXL2 or KLF4 (100 ng pcDNA3 + 100 ng plasmid of interest), and combined expression of LOXL2 and KLF4 (100 ng of each). Results show the average of three different experiments performed on triplicate samples. Right: coimmunoprecipitation assays in HEK293T cells transiently transfected with KLF4-HA and LOXL2-FLAG. Cell extracts were immunoprecipitated with anti-FLAG and analyzed with anti-HA for detecting interaction between LOXL2 and KLF4 (upper panel). As a positive control of immunoprecipitation, membranes were also incubated with specific LOXL2 antibody (middle upper panel). Lower panels show input levels of the indicated proteins.
- D,E ChIP assay for the detection of endogenous H3K4me3 levels (D) and binding of RNA polymerase II (E) in the indicated regions of the NOTCH1 promoter in HEK293T cells transiently expressing LOXL2 or pcDNA3 as a control. All ChIP samples were examined in parallel by PCR amplification of a negative control region (see Supplementary Table S4). Results are expressed as fold enrichment for each indicated binding site relative to the negative control region. The average of two experimental triplicates is shown.
We next asked whether LOXL2 downregulation of NOTCH1 gene transcription could also be caused through counteraction of the p53 upregulatory effect on NOTCH1 expression. To this end, we analyzed in mouse immortalized MCAD3 and human HaCaT keratinocyte cell lines the activity of the NOTCH1 reporter (−2472pGL4) in the presence of p53 and LOXL2. Thus, while increased p53 levels caused augmented NOTCH1 reporter activity, co-expression of LOXL2 interfered with this induction in both cell lines (Supplementary Fig S9), suggesting that LOXL2 could also interfere with p53-mediated transcriptional activation of NOTCH1.
Finally, since LOXL2 has been recently attributed a novel role as a histone-modifying enzyme catalyzing deamination of tri-methylated lysine 4 in histone H3 (H3K4me3) promoting gene silencing (Herranz et al, 2012), we decided to investigate the relevance of this particular mechanism in the context of NOTCH1 transcriptional regulation. ChIP analysis showed a significant decrease in H3K4me3 level at the NOTCH1 proximal promoter region which strongly correlates with a clear reduction of RNA polymerase II binding when LOXL2 is overexpressed (Fig8D and E). In contrast, H3K4me3 level at the −1,600/−1,327 region was unaltered by LOXL2 expression and RNA polymerase II recruitment could not be detected (Fig8D and E), suggesting that LOXL2 binding to this region regulates NOTCH1 expression by a distinct mechanism that remains to be elucidated.
Considering all the above evidence, we conclude that the tumor-prone role played by Loxl2 in skin carcinogenesis depends on its ability to negatively modulate epidermal differentiation by repressing the NOTCH1 promoter activity and thus decreasing the steady state level of the Notch1 signaling pathway.
Negative correlation between LOXL2 and Notch1 pathway in human SCC
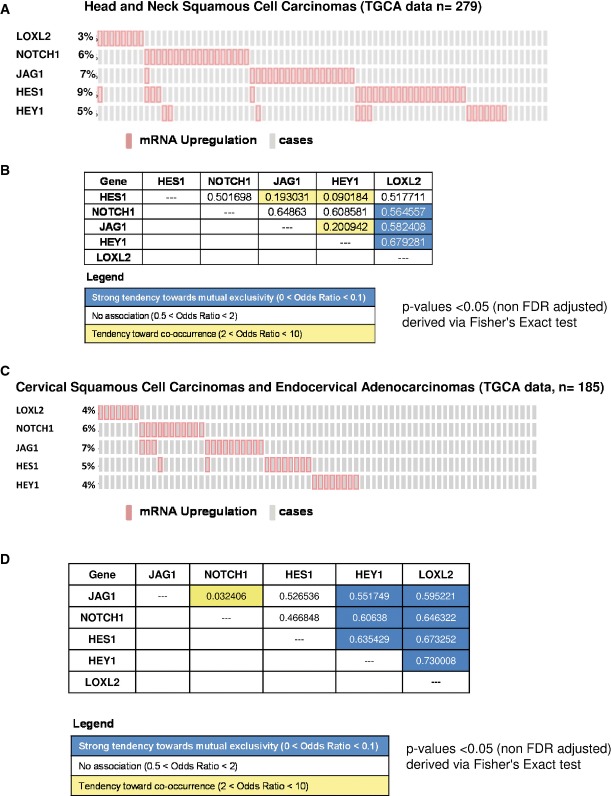
We next analyzed whether the Loxl2 negative regulation of the Notch1 pathway characterized in the mouse skin tumors is relevant for human tumors. To this end, we first analyzed HNSCC and cervical SCC because (i) of our previous identification of LOXL2 as a prognostic marker in laryngeal SCC (Peinado et al, 2008) and (ii) the mouse skin carcinogenesis model faithfully reproduces the pathology of SCC from stratified epithelia (Yuspa, 1994; Raimondi et al, 2009; Glick, 2012). For the correlation analysis of LOXL2 and canonical Notch1 pathway, we have used the available TCGA data (http://www.cbioportal.org/public-portal/index.do) from HNSCC (n = 279) and endocervical adenocarcinomas (n = 185) (Fig9). We studied the correlation between expression data of selected genes (HES1, NOTCH1, JAG1, HEY1, LOXL2) according to the values obtained from the RNAseq study using a 2.0 of z-score threshold. In the case of HNSCC, a total of 25.1% of samples (70 carcinomas) showed overexpression in some of the selected genes (LOXL2: 3%, JAG1: 6%, NOTCH1: 7%, HES1: 9%, HEY1: 5%) (Fig9A). A similar ratio of overexpression in those selected genes was found in cervical SCC and endocervical adenocarcinomas (24%, 44 cases) (LOXL2: 4%, JAG1: 7%, NOTCH1: 6%, HES1: 5%, HEY1: 4%) (Fig9C). In order to analyze the co-occurrence tendency among these genes, a mutual exclusivity study using the cBioPortal tool was performed (Fig9B and D). When gene expression was evaluated in a pair-way fashion, a P-value was obtained which refers to the association tendency. Non-relation between LOXL2 mRNA levels and those of any member of the canonical Notch1 pathway analyzed was observed in both series of tumors. In fact, the expression level of LOXL2 in comparison with NOTCH1, JAG1 and HEY1 shows mutual exclusivity, reinforcing the negative impact of LOXL2 on the Notch1 signaling pathway in human tumors.
Figure 9.
- A–D Meta-study of 279 head and neck carcinomas (A) and 185 cervical squamous cell carcinomas and endocervical adenocarcinomas (C) from TCGA (http://www.cbioportal.org/public-portal/index.do) to analyze the percentage of samples with altered expression of LOXL2, NOTCH1, JAG1, HEY1 and HES1 (ref: pink, cases with overexpression of the indicated genes; gray, individual cases). The representation only includes the cases with alteration in at least one of the indicated genes. (B, D) Mutual exclusivity study among genes indicated in (A, B). The P-values shown in the tables were calculated using a Fisher test in a BioCarta tool (http://www.cbioportal.org/public-portal/index.do). For this analysis, we selected a z-score threshold of 2.0. The legend (lower) represents the level of tendency to occur.
Discussion
During the last few years, accumulated evidence has supported a complex and pleiotropic role for proteins of the LOX family. Apart from their classical and widely reported function as ECM remodeling factors (Csiszar, 2001; Lucero & Kagan, 2006), emerging data have demonstrated the involvement of lysyl oxidases in a plethora of intracellular and extracellular processes (Barker et al, 2012; Cano et al, 2012). Generation of loss-of-function mouse models for Lox and Loxl1 has shed some light on tissue-specific functions of these two LOX family members. The prototypic Lox plays a key role in the cardiovascular system (Maki et al, 2002; Hornstra et al, 2003), and Loxl1 was shown to be critical during the elastogenesis process (Liu et al, 2004). However, in vivo data concerning other members of the LOX family remain unexplored although similar multifunctional actions have been described, in particular for LOXL2 (Cano et al, 2012). Therefore, we generated deletion and overexpression mice for Loxl2 by conventional gene-targeting techniques to investigate the in vivo consequences of Loxl2 deregulation in normal tissue homeostasis and in response to a carcinogenic insult.
According to our results, most of the tissues properly tolerate defective or excessive amounts of Loxl2. Just heart and male reproductive compartments were more prominently sensitized to Loxl2 perturbations, but not in a complementary fashion. While Loxl2 abrogation produces severe cardiac defects in 25% of the affected KO animals, hearts of overexpression mice are unaffected by its overproduction. In this sense, we show that complete depletion of Loxl2 in heart associates with a significant reduction of FAK and Src expression levels whose downregulation has been shown to severely affect ventricular septal formation at late embryonic developmental stages but with incomplete penetrance (Peng et al, 2008). Moreover, the recent characterization of the helicase-like transcription factor (Hltf) KO mice revealed partial perinatal lethality of Hltf-deleted animals due to a defective collagen biogenesis in the cardiac tissue that strongly correlated with a significant reduction of Loxl2 levels (Helmer et al, 2013). All these data allow us to attribute to Loxl2 a relevant role during cardiac morphogenesis that we are currently investigating in more detail.
In a similar way, male sterility detected in overexpressing R26L2/L2 mice does not correlate with any functional abnormality observed in the gonads of KO males. Regarding this phenotype, it is worth remarking that downregulation of different components of the junctional machinery found in Loxl2-overexpressing epididymis, such as claudin-1 and E-cadherin, constitutes the first in vivo evidence that validates Loxl2 involvement in the control of the intercellular adhesion and differentiation processes in defined tissue contexts.
On the other hand, aberrant expression of different LOX family members, including LOXL2, has been reported in several fibrotic and cancer diseases (Barker et al, 2012). In this sense, the epididymal alterations observed in the R26L2/L2 mice associated to a fibrotic phenotype provide the first in vivo genetic proof for Loxl2 contribution to fibrosis, probably due to the increased expression levels of extracellular Loxl2. The finding that other tissues are apparently refractory to the Loxl2-mediated fibrotic induction might suggest that further increased levels of Loxl2 can be required and/or that additional environmental cues need to simultaneously occur to promote a fibrotic response in less sensitive tissues. The genetic Loxl2 models here described can provide excellent tools to further investigate pathological fibrosis in different experimental settings.
Regarding cancer, several LOX members are highly expressed in invasive tumors and closely associated with metastasis and poor patient outcome (Barker et al, 2012; Cano et al, 2012). Nevertheless, in some particular neoplasias, like HNSCC, both upregulation and reduced expression of LOX and LOXL2 have been described (Rost et al, 2003; Peinado et al, 2008). To obtain insights into the in vivo role played by LOXL2 in SCC, we subjected our Loxl2 gain- and loss-of-function mouse models to the classical DMBA/TPA protocol. The results presented herein clearly demonstrate a tumor prone role for Loxl2 during epidermal carcinogenesis being important for both tumor initiation and malignant progression. Importantly, intracellular Loxl2 was mainly detected in papillomas and SCC of Loxl2-expressing mice, additionally validating the prognostic value of intracellular LOXL2 we previously reported in human larynx SCC (Peinado et al, 2008). Noteworthy, Loxl2 abrogation in mice induced increased expression of epidermal differentiation markers within papilloma lesions, extending the proposed inhibitory role of Loxl2 during the keratinization process in normal epidermis (Peinado et al, 2008; Lugassy et al, 2012) to a pathological context. Moreover, the upregulation of the Notch1 signaling pathway detected in papillomas derived from L2−/− mice, and the reverse scenario found in R26L2/L2 lesions, strongly supports a LOXL2-mediated action on this key-signaling pathway for epidermal differentiation (Lefort et al, 2007) during premalignant keratinocyte differentiation, thus impeding the maintenance of the differentiation status. Concordant with these results, silencing of Loxl2 in HaCa4 SCC cell line (Peinado et al, 2005, 2008) confirmed the ability of Loxl2 to negatively modulate epidermal differentiation and the Notch1 signaling pathway in a more aggressive tumor context. Interestingly, no alterations in the expression of p53, an established transcriptional inducer of Notch1 (Lefort et al, 2007; Yugawa et al, 2007; Guinea-Viniegra et al, 2012), have been found in papillomas developed by Loxl2 KO or overexpressing mice. However, the reduced expression levels of p21 detected in Loxl2-overexpressing lesions indicate an alteration, at some extent, of p53 transcriptional activity. In this line of evidence, promoter activity assays indicate that Loxl2 interferes with p53-dependent upregulation of Notch1. Therefore, our findings demonstrate that Loxl2 is a novel negative transcriptional modulator of the Notch1 pathway in skin tumor context, which could be acting independently of other previously identified regulators that impinge on the p53–Notch1 regulatory axis, by downregulating p53 at transcriptional or post-transcriptional levels (Kolev et al, 2008; Dotto, 2009; Guinea-Viniegra et al, 2012).
Remarkably, Loxl2-mediated downregulation of the Notch1 pathway turned out to be specific for this particular member of the LOX family, since other related proteins, such as LOX and LOXL3, failed to induce repression of the full-length NOTCH1 promoter. Confirming these data, abrogation of Loxl2 in mouse papillomas is able to increase Notch1 mRNA levels despite the concomitant induction of Lox and Loxl3.
Importantly, LOXL2 binds to the NOTCH1 promoter, at least, in two different regions that, in the case of the most proximal sequence to the TSS (transcription start site), might be mediated by KLF4 according to its capacity to interact with LOXL2 in co-immunoprecipitation experiments and the synergistic effect observed in the repression of the NOTCH1 promoter activity assays. Interestingly, overexpression of KLF4 has been reported during progression of skin SCC (Chen et al, 2008) reinforcing the present connection with LOXL2. Although further experimental approaches are required, we propose that KLF4/LOXL2-mediated repression of NOTCH1 gene expression might be caused, at least in part, by the LOXL2-dependent deamination of the H3K4me3 which finally results in a reduced binding of RNA polymerase II. This novel role of LOXL2 can open new avenues to deepen into its potential regulation of Notch1 during normal skin differentiation and development.
On the other hand, the altered inflammatory response and expression of S100A9/S100A8 cytokines occurring in papilloma lesions depending on the Loxl2 levels provide additional support to a LOXL2/Notch1 regulatory axis to control SCC tumor progression, also in agreement with previous data on the negative regulation of inflammation by Notch1 in skin diseases (Dumortier et al, 2010). These data, together with our recent observations of Loxl2 upregulation of S100A9/S100A8 and other cytokines in syngeneic models of mouse breast carcinomas (Canesin et al, 2015), provide strong support for a major role of LOXL2 in the regulation of a pro-tumoral inflammatory response. In agreement with this, given the ubiquitous deletion and overexpression of Loxl2, we cannot discard that this protein could control Notch1 signaling also in dermal cells, where this pathway plays an important role in the control of skin tumor formation (Hu et al, 2012). Importantly, the expression of LOXL2 and members of the canonical Notch1 signaling pathway in human HNSCC and cervical SCC shows a mutual exclusive pattern further supporting the negative regulation of LOXL2 of this key pathway in human tumor contexts of SCC. Our present data, together with the significant finding that Loxl2 is not essentially required for normal epidermal homeostasis, open new therapeutics opportunities for the treatment of LOXL2-overexpressing SCC tumors.
In sum, this is the first comprehensive in vivo approach performed to systematically study the role of LOXl2 in tissue homeostasis and tumorigenesis, demonstrating the active role of Loxl2 in cardiac morphogenesis, epididymis dysfunction and mouse skin carcinogenesis. The great effort dedicated to the design of lysyl oxidase inhibitors during the last years (Barker et al, 2012) makes our Loxl2 mouse models highly valuable tools to test and challenge the anticipated results of a future LOXL2-based therapy and provide suitable pre-clinical model systems to evaluate the efficiency and specificity of these drugs. We suggest that targeting LOXL2, although not sufficient to achieve a complete tumor regression, could be successfully combined with epidermal differentiation inducers such as those impinging on the NOTCH1 pathway, such as EGFR or TACE inhibitors (Kolev et al, 2008; Guinea-Viniegra et al, 2012), to collectively improve therapeutic responses of SCC.
Materials and Methods
Mice
All mouse studies were conducted in accordance with protocols approved by the Use Committee for Animal Care from the Universidad Autónoma de Madrid (UAM) (Re# CEI-25-587). We generated by gene-targeting technique, mouse models of both gain- and loss-of-function where Loxl2 expression was elevated (overexpressing mice) or endogenously abrogated (knock-out mice) (see general schemes in Fig1). For Loxl2 depletion, a gene-targeting vector comprising exon two (containing the translation start site) and a downstream neomycin cassette, flanked by loxP and frt sites, respectively, was generated. In the case of the overexpressing mutant, a single copy of the Loxl2 cDNA followed by the GFP reporter gene was inserted downstream to a loxP-flanked neo-STOP cassette by homologous recombination into the ROSA26 (R26) locus (Nyabi et al, 2009). By this way, transcription of the exogenous Loxl2 cDNA is under the control of R26 promoter allowing a reproducible and stable overexpression of the transgene. Both gene-targeting constructs were electroporated into ES cells, and positive clones were screened by diagnostic PCR. Microinjection of the recombinant clones led to the generation of chimeric animals, and those able to transmit the desired recombination event were used to establish different conditional transgenic strains. To analyze the in vivo consequences of ubiquitous Loxl2 deletion, conditional KO animals were crossed with the CMV-Cre strain in order to obtain a constitutive Loxl2− allele. Germline Loxl2 KO (L2−/−) animals were generated by intercrossing heterozygous mice. Regarding Loxl2 overexpression mutants, homozygous R26L2/L2 was obtained from conditional progenitors as described for KO mice.
Targeting vectors
For the generation of KO mice, targeting construction was ordered to Gene Bridges Company. In the case of Loxl2-overexpressing mice, homologous recombination vector was generated following the Gateway® cloning DNA technology. Briefly, for the Gateway LR reaction, 100 ng of the pEntry plasmid harboring Loxl2 cDNA and 150 ng pROSA26-DV (Nyabi et al, 2009) in the LR Clonase II mix were left overnight at RT. After in vitro recombination, LR mixture was electroporated into DH5α strain. Bacteria containing the pROSA26-based expression clones were propagated at 28°C in 300 μl of LB medium without antibiotics for 90 min shaking and plated out on LB-AMP agar plates also grown at 28°C. Subsequent bacterial expansion was also performed at 28°C to avoid recombination. Twenty to 50 colonies were typically obtained, and analysis of 10 colonies by enzymatic restriction yielded 7–10 correctly recombined clones. Correct clones were sequence-verified using the S1for (ATCATGTCTGGATCCCCATC) and S2rev (GGGGCGGAATTCGATATCAAG) primers. To confirm correct sequence of the Loxl2 insert of pROSA26-derived expression clones, sequence verification was carried out.
Diagnostic PCR and genotyping
PCR-based screening of targeted conditional R26-Loxl2 and Loxl2 KO ES cell clones were performed using external and internal primers in both 5′ and 3′ recombinant regions. Diagnostic PCR fragments were obtained using Takara LA Taq hot Start Version enzyme. Primers for conventional PCR genotyping are described in Fig1 and Supplementary Table S1.
qRT–PCR
Total RNA from papillomas was extracted by homogenization with TRIzol reagent (Invitrogen) followed by phenol–chloroform extraction protocol. cDNA synthesis (Superscript II RNase H reverse transcriptase; Invitrogen) was prepared starting from equal amounts of RNA. Quantitative real-time PCR was performed with Iq5 Bio-Rad Multicolor Real-Time PCR Detection System and the associated software (iQ5 Optical System Software), using the manufacturer's recommended conditions. Each reaction was performed in biological triplicates with 20 ng of cDNA by using SYBR Green reagent (Quanta Biosciences). Values were normalized to actin levels (primer pairs are listed in Supplementary Table S2).
Western blots
Tissue samples and cell pellets were processed as previously described (Moreno-Bueno et al, 2011). Primary antibodies are listed in Supplementary Table S3.
Immunofluorescence
Tissues were embedded in optimal cutting temperature compound (OCT; Tissue-Tek) and frozen on dry ice. For epidermal differentiation marker analyses, 5-μm frozen tissue sections were fixed in methanol and acetone (−20°C, 4 and 2 min, respectively) and blocked with EnVision FLEX ANTIBODY DILUENT (Dako) for 45 min at 37°C. Subsequent incubations with primary and secondary antibodies were performed during 1 h at 37°C. In the case of Jagged1 and Hes1 staining, immunofluorescence was performed in paraffin-embedded sections and antigen retrieval was carried out in 10 mM Na-citrate pH 6.0, 20 min in autoclave. Primary antibodies were incubated in 3% BSA, 20 mM MgCl2, 0.3% Tween-20 and 5% FBS in PBS overnight. Slides were incubated with HRP-conjugated secondary antibodies (Dako) at 1:100 for 90 min RT and developed using the tyramide amplification system (TSA-Plus Cyanine3/Fluorescein System; PerkinElmer). Sections were mounted in Vectashield medium with DAPI (Vector). Stainings were visualized using a LSM710 confocal microscope (Zeiss) and images acquired with the associate image software. For antibody details, see Supplementary Table S3.
Histology and immunohistochemistry
Tissue samples were collected, fixed in 10% formaldehyde and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin solutions. Immunohistochemical analysis was performed and analyzed as described (Peinado et al, 2008; Moreno-Bueno et al, 2011). Antibodies used are listed in Supplementary Table S3.
Picrosirius red staining
Picrosirius red analysis was performed on 5-μm frozen sections of tissue stained with 0.1% picrosirius red (Sigma) and counterstained with Weigert's hematoxylin to reveal fibrillar collagen. Sections were imaged using an Olympus IX81 fluorescence microscope fitted with an analyzer (U-ANT) and polarizer (U-POT; Olympus) oriented parallel and orthogonal to each other.
Acute TPA treatment
Mouse dorsal skins were shaved and treated three times with 12.5 μg of TPA every 2 days during 1 week. Then, mice were euthanized, and the dorsal skins were harvested for histological analysis and immunohistochemical staining.
DMBA-TPA mouse skin carcinogenesis
The two-stage mouse skin carcinogenesis (single 7,12-dimethylbenz(a)anthracene application, followed by twice weekly 12-O-tetradecanoylphorbol-13-acetate applications for 16 weeks) was performed following standard protocols (Quintanilla et al, 1986). Tumors were collected 28–30 weeks after beginning of treatment and processed for qPCR, histological, immunohistological or immunofluorescence analysis.
Promoter assays
Co-transfections were carried out in the presence of different amounts of human LOXL2, LOX, LOXL3, KLF4 (Addgene), p53 (a gift from Mathias Drosten, Centro Nacional de Investigaciones Oncológicas, Madrid, Spain), SP3 (a gift from Paolo Dotto, Massachusetts General Hospital, MA, USA), SNAIL1, E47 expression vectors, 50–200 ng of the indicated human NOTCH1 promoters (a gift of Paolo Dotto) and 10 ng of pCMV-β-gal as control of transfection efficiency. Total DNA amount was normalized with empty pcDNA3 vector. Luciferase and β-galactosidase activities were measured using the luciferase and β-Glo assay (Promega) and normalized to promoter activity detected in cells transfected with pcDNA3 empty vector. All experiments were performed on triplicate samples.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed in HEK293T cells transiently transfected with human LOXL2-Flag and empty vector, using formaldehyde before sonication, as described (Cuevas et al, 2014). For detection of interaction between LOXL2 with the endogenous NOTCH1 promoters, anti-Flag M2 affinity gel (Sigma), or unspecific mouse IgG (Jackson ImmunoResearch Laboratories), and Protein G agarose beads (Sigma) were used. ChIP assays for detecting the specific histone methylation marker, H3K4me3, and RNA polymerase II binding to NOTCH1 promoter distal and proximal regions were performed with the corresponding antibodies (see Supplementary Table S3) and unspecific rabbit IgG (ImmunoResearch Laboratories). 273- and 184-bp fragments of the human NOTCH1 promoter (from −1,620 to −1,327 and −285 to −101 from the ATG; Lambertini et al, 2010) and 126- and 218-bp fragments of the human CDH1 promoter (−1,460 to −1,334 and −180/ + 38 from the transcription start site; Cuevas et al, 2014) were amplified by PCR or qRT–PCR. Primer pairs are listed in Supplementary Table S4.
Coimmunoprecipitation
HEK293T cells were lysed 36 h after transfection in 500 μl of IPH buffer (50 mM Tris–HCl [pH 8.0], 200 mM NaCl, 5 mM EDTA, 0.5% NP-40) at 4°C for 30 min and then centrifuged at 16,200 g for 15 min to remove cell debris. 250 μg of lysate was pre-cleared with mouse IgG-agarose beads (Sigma) for 3 h and then subjected to immunoprecipitation with ANTI-FLAG M2 Affinity Gel covalently attached to agarose (Sigma) for 5 h at 4°C. Precipitates were washed four times with IPH buffer (1 ml) and then resuspended in Laemmli buffer for Western blot analysis. Antibodies used are listed in Supplementary Table S3.
Lentiviral vector infection in HaCa4 cells
Target cells were cultured in 10-cm plates along with recombinant lentivirus encoding for shRNA against Loxl2 (V3LMM_455747; Open Biosystem) (Canesin et al, 2015) and its corresponding control pGIPZ (Open Biosystem) in Ham's F12/DMEM complete medium [10% fetal bovine serum (FBS), 20 mM glutamine and 10 U/ml penicillin and 10 μg/ml streptomycin] containing 8 μg/ml polybrene at 37°C and 5% CO2. Stable pool cultures were selected with 3 μg/ml puromycin for 4–5 days after infection.
Statistics
P-values were generated using Student's t-test (unpaired, 2-tailed); a P-value < 0.05 was considered significant. To test association between categorical variables in the Supplementary Fig S2, we used the χ2 test. Error bars were calculated as standard error in all the statistical analysis shown along the work. Sample size and number of experiments are indicated in each of the Figure legends.
Acknowledgments
We would like to thank Valerie Weaver for reading the manuscript and valuable suggestions, Mathias Drosten and Paolo Dotto for providing reagents, Génesis Martín for help in maintenance of mice colonies and members of Amparo Cano's lab for helpful discussions. This work has been supported by grants from the Spanish Ministry of Economy and Innovation SAF2010-21143 (AC), SAF2010-20175 (GMB), SAF2013-44739-R (AC and FP) and CONSOLIDER-INGENIO 2010 CSD2007-00017 (AC); the AICR (12-1057) (AC and AM), the Spanish Instituto de Salud Carlos III [(RETIC-RD12/0036/0007 (AC), RETICC-RD12/0036/0054 (AB), PI13/00132 (GMB)], Comunidad de Madrid (S2010/BMD-2303) (AC and GMB) and AECC-2011 (GMB). AM and EPC are founded by postdoctoral contracts from S2010/BMD-2303 and AICR grants, respectively; FS was founded by a Jae-pre contract from the CSIC; and SM and VS are founded by technician contracts from the RETIC-RD12/0036/0007 and AICR grants, respectively.
Author contributions
AC, FP, AM and FS conceived and designed experiments; AM and FS performed most of the experiments; AF carried out epidermal marker characterization and promoter assays; VS performed tissue preparation; EPC performed promoter and ChIP assays; SM controlled the mice colonies, performed genotyping and helped in the mouse skin carcinogenesis studies; CR-H performed IF analyses of Notch1 pathway; GM-B performed Loxl2 IHC and dataset analyses; PD and GM-B carried out pathological analyses; JJH, KC and AB provided reagents; AC, FP, AM and FS wrote the manuscript; and all authors read and discussed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Figures S1–S9, Supplementary Text, Supplementary Tables S1–S4
Review Process File
Source Data for Figure 2
References
- Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- Barker HE, Bird D, Lang G, Erler JT. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res. 2013;11:1425–1436. doi: 10.1158/1541-7786.MCR-13-0033-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- Bignon M, Pichol-Thievend C, Hardouin J, Malbouyres M, Brechot N, Nasciutti L, Barret A, Teillon J, Guillon E, Etienne E, Caron M, Joubert-Caron R, Monnot C, Ruggiero F, Muller L, Germain S. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118:3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- Brooks YS, Ostano P, Jo SH, Dai J, Getsios S, Dziunycz P, Hofbauer GF, Cerveny K, Chiorino G, Lefort K, Dotto GP. Multifactorial ERbeta and NOTCH1 control of squamous differentiation and cancer. J Clin Invest. 2014;124:2260–2276. doi: 10.1172/JCI72718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesin G, Cuevas EP, Santos V, Lopez-Menendez C, Moreno-Bueno G, Huang Y, Csiszar K, Portillo F, Peinado H, Lyden D, Cano A. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene. 2015;34:951–964. doi: 10.1038/onc.2014.23. [DOI] [PubMed] [Google Scholar]
- Cano A, Santamaria PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8:1095–1108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Wu CY, Chang CC, Ma CJ, Li MC, Chen CM. Nuclear Kruppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7:777–782. doi: 10.4161/cbt.7.5.5768. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Cuevas EP, Moreno-Bueno G, Canesin G, Santos V, Portillo F, Cano A. LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition. Biol Open. 2014;3:129–137. doi: 10.1242/bio.20146841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl. 2007;9:463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9:587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol Reprod. 2007;76:1034–1044. doi: 10.1095/biolreprod.106.059246. [DOI] [PubMed] [Google Scholar]
- Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, Ferrero I, Demehri S, Song LL, Farr AG, Leonard WJ, Kopan R, Miele L, Hohl D, Finke D, Radtke F. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto E, Tajima S. Reciprocal regulation of LOX and LOXL2 expression during cell adhesion and terminal differentiation in epidermal keratinocytes. J Dermatol Sci. 2009;55:91–98. doi: 10.1016/j.jdermsci.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Glick AB. The role of TGFbeta signaling in squamous cell cancer: lessons from mouse models. J Skin Cancer. 2012;2012:249063. doi: 10.1155/2012/249063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–863. doi: 10.1210/endo.142.2.7975. [DOI] [PubMed] [Google Scholar]
- Guinea-Viniegra J, Zenz R, Scheuch H, Jimenez M, Bakiri L, Petzelbauer P, Wagner EF. Differentiation-induced skin cancer suppression by FOS, p53, and TACE/ADAM17. J Clin Invest. 2012;122:2898–2910. doi: 10.1172/JCI63103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer RA, Martinez-Zaguilan R, Dertien JS, Fulford C, Foreman O, Peiris V, Chilton BS. Helicase-like transcription factor (hltf) regulates g2/m transition, wt1/gata4/hif-1a cardiac transcription networks, and collagen biogenesis. PLoS One. 2013;8:e80461. doi: 10.1371/journal.pone.0080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz N, Dave N, Millanes-Romero A, Morey L, Diaz VM, Lorenz-Fonfria V, Gutierrez-Gallego R, Jeronimo C, Di Croce L, Garcia de Herreros A, Peiro S. Lysyl oxidase-like 2 deaminates lysine 4 in histone H3. Mol Cell. 2012;46:369–376. doi: 10.1016/j.molcel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF. Lysyl oxidase-like 2 promotes migration in noninvasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125:318–327. doi: 10.1002/ijc.24308. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R, Raffoul W, Hoetzenecker W, Hofbauer GF, Dotto GP. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–1220. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim EC, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38:145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, Neel V, Dummer R, Wagner EF, Dotto GP. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–911. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini C, Pantano S, Dotto GP. Differential control of Notch1 gene transcription by Klf4 and Sp3 transcription factors in normal versus cancer-derived keratinocytes. PLoS One. 2010;5:e10369. doi: 10.1371/journal.pone.0010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort K, Dotto GP. Notch signaling in the integrated control of keratinocyte growth/differentiation and tumor suppression. Semin Cancer Biol. 2004;14:374–386. doi: 10.1016/j.semcancer.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, Devgan V, Lieb J, Raffoul W, Hohl D, Neel V, Garlick J, Chiorino G, Dotto GP. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21:562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugassy J, Zaffryar-Eilot S, Soueid S, Mordoviz A, Smith V, Kessler O, Neufeld G. The enzymatic activity of lysyl oxidase-like-2 (LOXL2) is not required for LOXL2-induced inhibition of keratinocyte differentiation. J Biol Chem. 2012;287:3541–3549. doi: 10.1074/jbc.M111.261016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Kivirikko KI. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem J. 2001;355:381–387. doi: 10.1042/0264-6021:3550381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–496. doi: 10.1016/s0945-053x(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Millanes-Romero A, Herranz N, Perrera V, Iturbide A, Loubat-Casanovas J, Gil J, Jenuwein T, Garcia de Herreros A, Peiro S. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol Cell. 2013;52:746–757. doi: 10.1016/j.molcel.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, Portillo F, Peinado H, Palacios J, Cano A. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, DiGiovanni J. Genetic background and development of skin tumors. Carcinog Compr Surv. 1989;11:187–212. [PubMed] [Google Scholar]
- Nyabi O, Naessens M, Haigh K, Gembarska A, Goossens S, Maetens M, De Clercq S, Drogat B, Haenebalcke L, Bartunkova S, De Vos I, De Craene B, Karimi M, Berx G, Nagy A, Hilson P, Marine JC, Haigh JJ. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37:e55. doi: 10.1093/nar/gkp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther. 2009;8:1986–1993. doi: 10.4161/cbt.8.21.9921. [DOI] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer–a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Peinado H, Iglesias-de DC, la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, Zhao P, Yang ZH. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- Peng X, Wu X, Druso JE, Wei H, Park AY, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, Guan JL. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci USA. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Raimondi AR, Molinolo A, Gutkind JS. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009;69:4159–4166. doi: 10.1158/0008-5472.CAN-08-4645. [DOI] [PubMed] [Google Scholar]
- Rost T, Pyritz V, Rathcke IO, Gorogh T, Dunne AA, Werner JA. Reduction of LOX- and LOXL2-mRNA expression in head and neck squamous cell carcinomas. Anticancer Res. 2003;23:1565–1573. [PubMed] [Google Scholar]
- Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Tse AP, Huang YP, Zhu YT, Chiu DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL, Tsang FH, Lo RC, Shi J, Zheng YP, Wong CM, Ng IO. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004;25:1771–1778. doi: 10.1093/carcin/bgh170. [DOI] [PubMed] [Google Scholar]
- Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol. 2007;27:3732–3742. doi: 10.1128/MCB.02119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis–thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S9, Supplementary Text, Supplementary Tables S1–S4
Review Process File
Source Data for Figure 2