Abstract
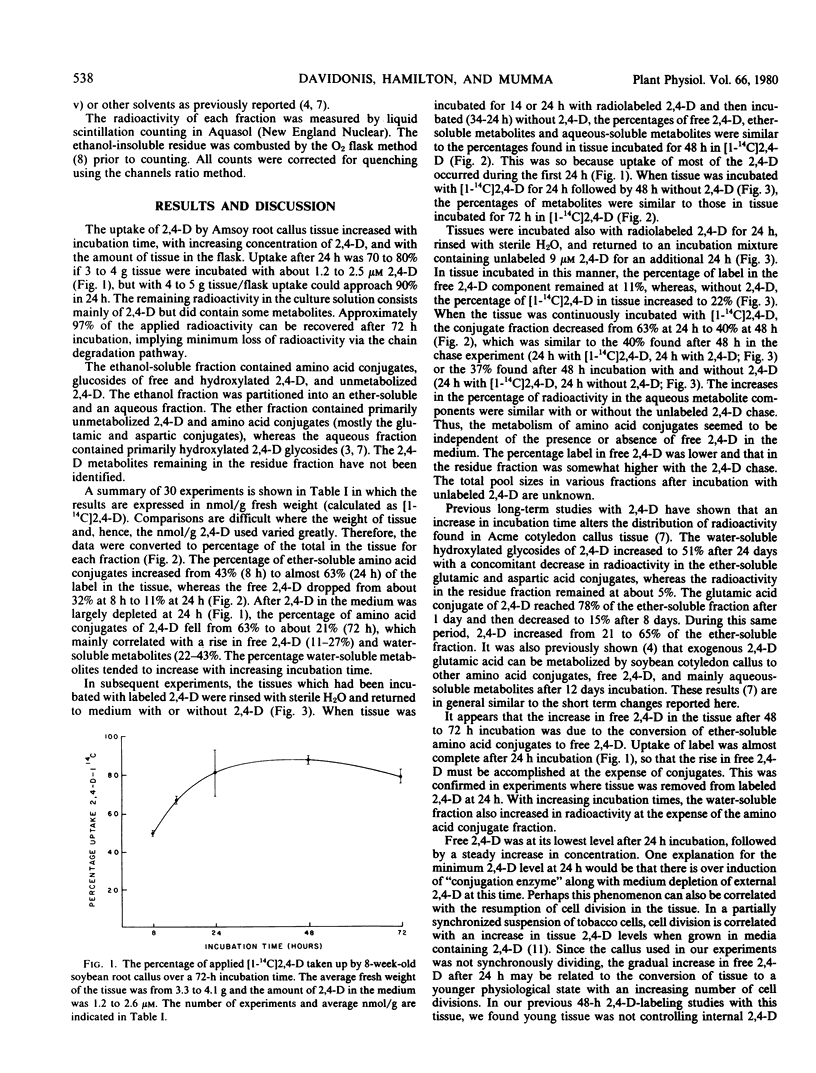
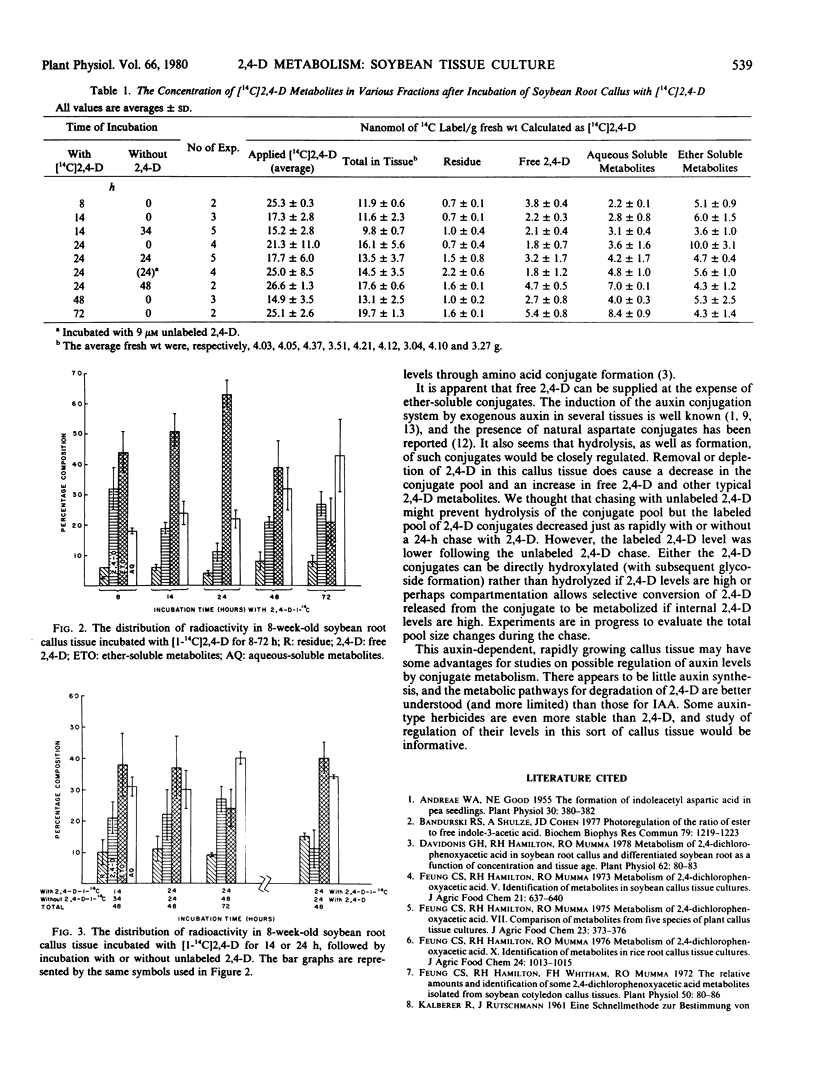
An auxin-requiring soybean root callus metabolized [1-14C]-2,4-dichlorophenoxyacetic acid (2,4-D) to diethyl ether-soluble amino acid conjugates and water-soluble metabolites. The uptake in tissue varied with incubation time, concentration, and amount of tissue. Uptake was essentially complete (80%) after a 24-hour incubation and the percentage of free 2,4-D in the tissue fell to its lowest point at this time. At later times, the percentage of free 2,4-D increased and the percentage of amino acid conjugates decreased, whereas the percentage of water-soluble metabolites increased only slightly. Similar trends were seen if the tissue was incubated for 24 hours in radioactive 2,4-D, followed by incubation in media without 2,4-D for 24 hours. Inclusion of nonlabeled 2,4-D during the 24-hour chase period did not reduce amino acid conjugate disappearance but did reduce the percentage of free [1-14C]2,4-D. Thus, an external supply of 2,4-D does not directly prevent amino acid conjugate metabolism in this tissue. It is concluded that 2,4-D amino acid conjugates were actively metabolized by this tissue to free 2,4-D and water-soluble metabolites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Davidonis G. H., Hamilton R. H., Mumma R. O. Metabolism of 2,4-dichlorophenoxyacetic Acid in soybean root callus and differentiated soybean root cultures as a function of concentration and tissue age. Plant Physiol. 1978 Jul;62(1):80–83. doi: 10.1104/pp.62.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feung C. S., Hamilton R. H., Witham F. H., Mumma R. O. The relative amounts and identification of some 2,4-dichlorophenoxyacetic Acid metabolites isolated from soybean cotyledon callus cultures. Plant Physiol. 1972 Jul;50(1):80–86. doi: 10.1104/pp.50.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feung C., Hamilton R. H., Mumma R. O. Metabolism of 2,4-dichlorophenoxyacetic acid. 10. Identication of metabolites in rice root callus tissue cultures. J Agric Food Chem. 1976 Sep-Oct;24(5):1013–1015. doi: 10.1021/jf60207a011. [DOI] [PubMed] [Google Scholar]
- Feung C., Hamilton R. H., Mumma R. O. Metabolism of 2,4-dichlorophenoxyacetic acid. V. Identification of metabolites in soybean callus tissue cultures. J Agric Food Chem. 1973 Jul-Aug;21(4):637–640. doi: 10.1021/jf60188a058. [DOI] [PubMed] [Google Scholar]
- Feung C., Hamilton R. H., Mumma R. O. Metabolism of 2,4-dichlorophenoxyacetic acid. VII. Comparison of metabolities from five species of plant tissue cultures. J Agric Food Chem. 1975 May-Jun;23(3):373–376. doi: 10.1021/jf60199a065. [DOI] [PubMed] [Google Scholar]
- Kopcewicz J., Ehmann A., Bandurski R. S. Enzymatic Esterification of Indole-3-acetic Acid to myo-Inositol and Glucose. Plant Physiol. 1974 Dec;54(6):846–851. doi: 10.1104/pp.54.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis M. A. Auxin-induced Conjugation Systems in Peas. Plant Physiol. 1972 Jan;49(1):24–27. doi: 10.1104/pp.49.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


