Abstract
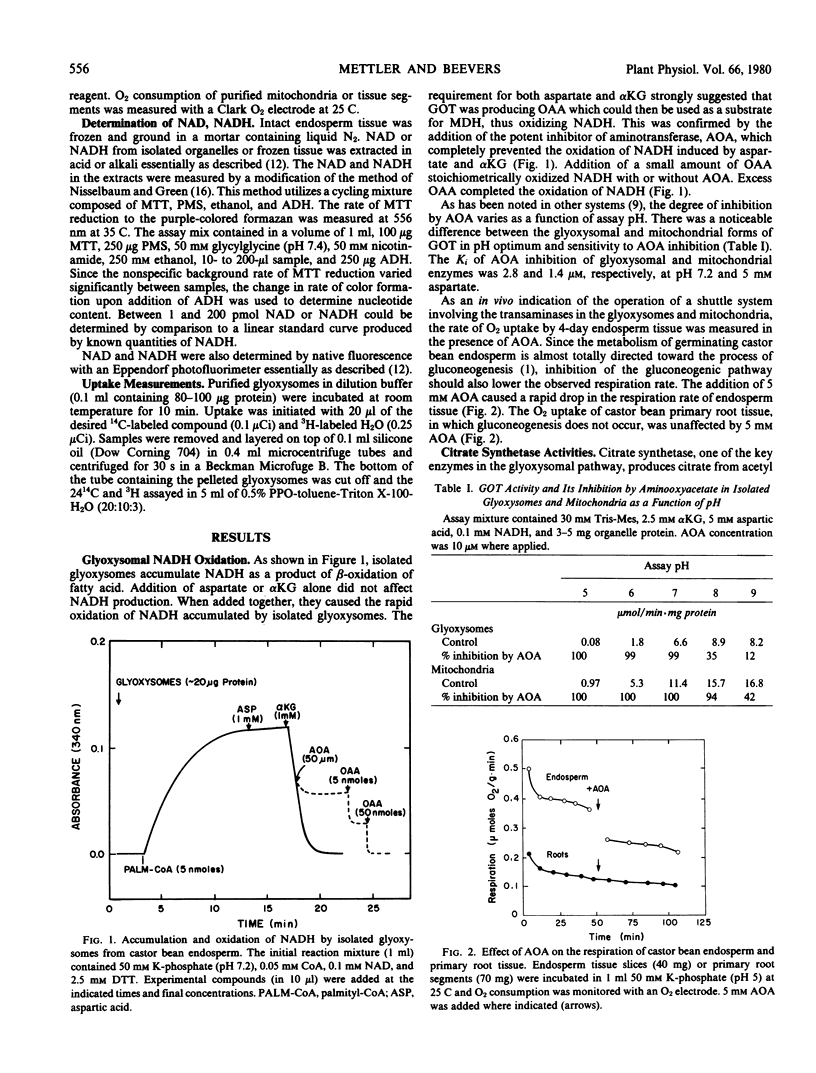
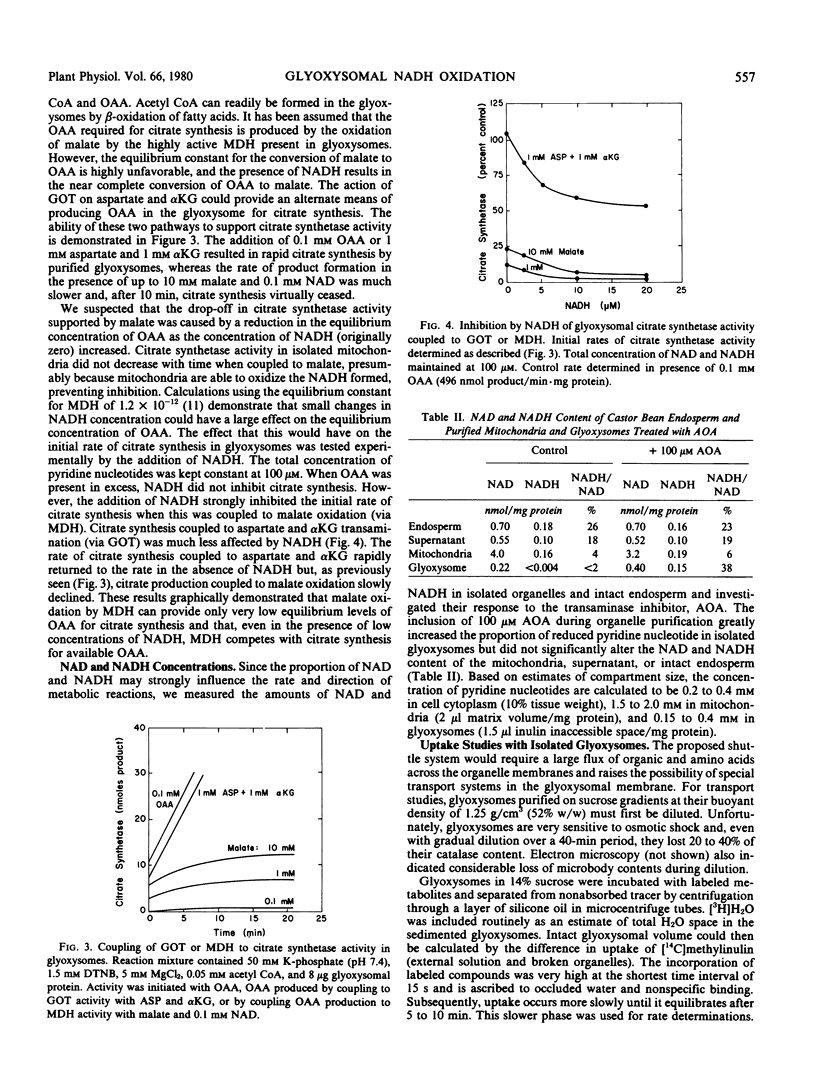
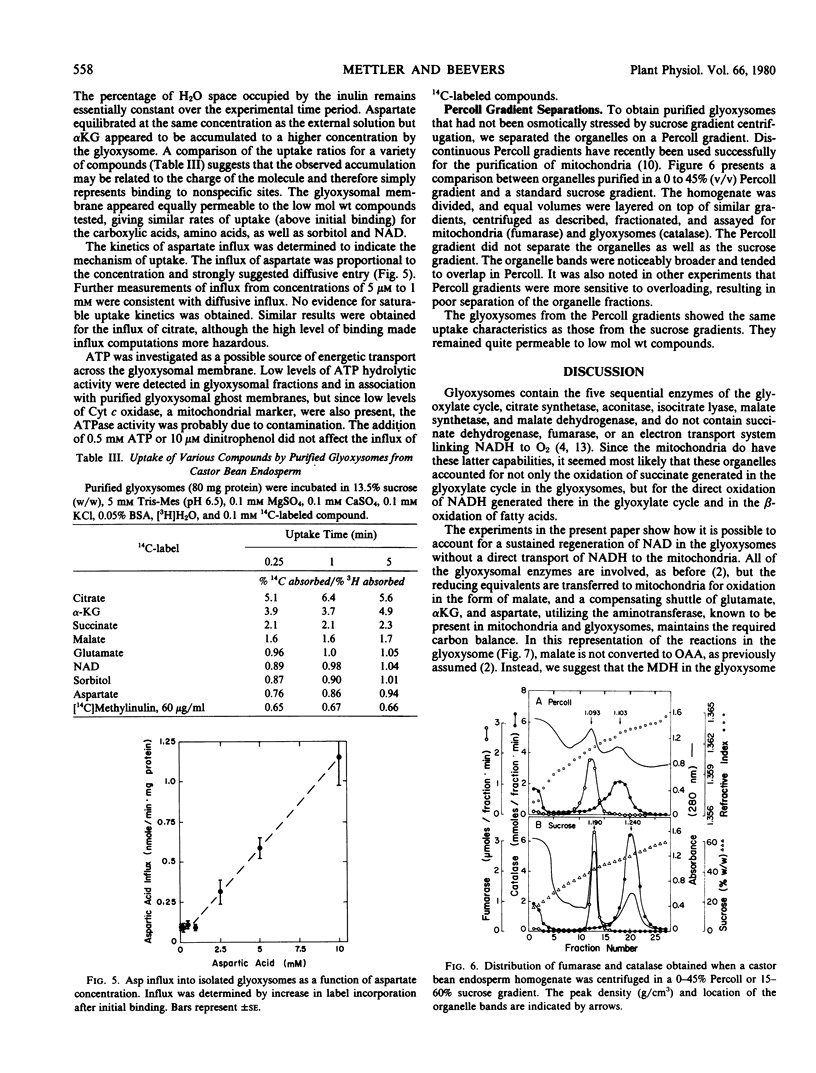
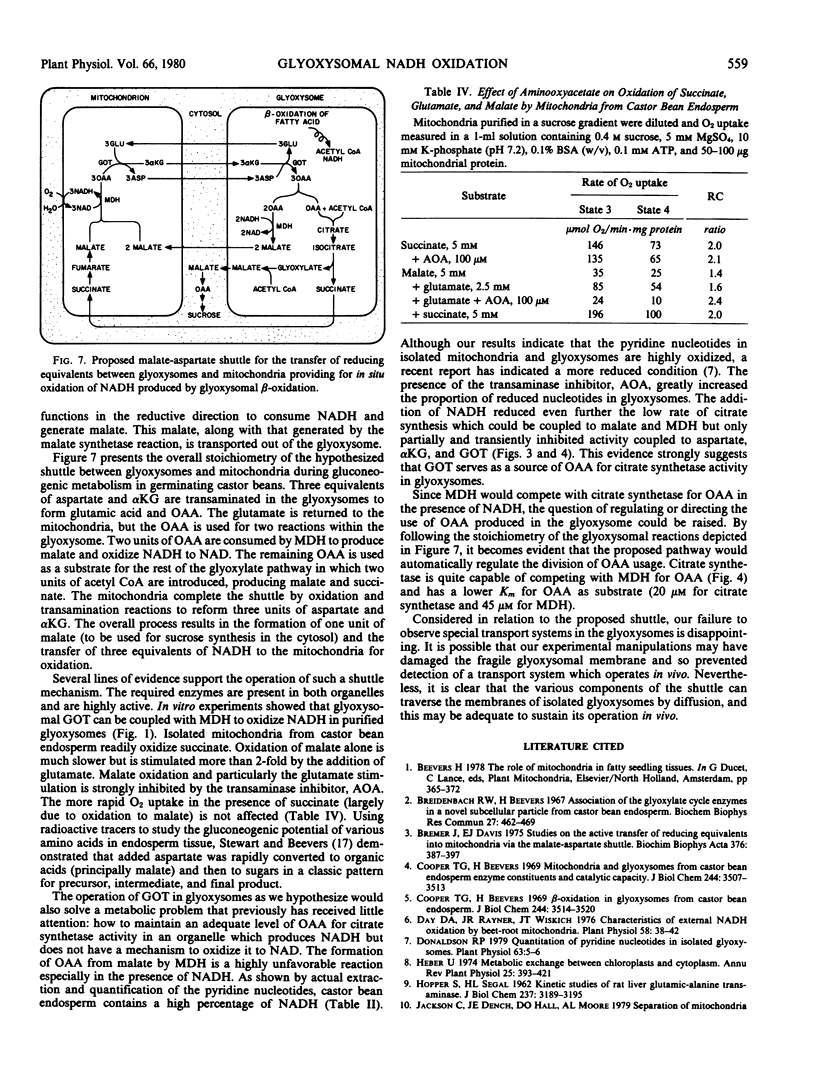
Glyoxysomes isolated from germinating castor bean endosperm accumulate NADH by β-oxidation of fatty acids. By utilizing the glutamate: oxaloacetate aminotransferase and malate dehydrogenase present in glyoxysomes and mitochondria, reducing equivalents could be transferred between the organelles by a malate-aspartate shuttle. The addition of aspartate plus α-ketoglutarate to purified glyoxysomes brought about a rapid oxidation of accumulated NADH, and the oxidation was prevented by aminooxyacetate, an inhibitor of aminotransferase activity. Citrate synthetase activity in purified glyoxysomes could be coupled readily to glutamate: oxaloacetate aminotransferase activity as a source of oxaloacetate, but coupling to malate dehydrogenase and malate resulted in low rates of citrate formation. Glyoxysomes purified in sucrose or Percoll gradients were permeable to low molecular weight compounds. No evidence was obtained for specific transport mechanisms for the proposed shuttle intermediates. The results support a revised model of gluconeogenic metabolism incorporating a malate-aspartate shuttle in the glyoxysomal pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Bremer J., Davis E. J. Studies on the active transfer of reducing equivalents into mitochondria via the malate-aspartate shuttle. Biochim Biophys Acta. 1975 Mar 20;376(3):387–397. doi: 10.1016/0005-2728(75)90161-9. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Day D. A., Rayner J. R., Wiskich J. T. Characteristics of External NADH Oxidation by Beetroot Mitochondria. Plant Physiol. 1976 Jul;58(1):38–42. doi: 10.1104/pp.58.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPER S., SEGAL H. L. Kinetic studies of rat liver glutamicalanine transaminase. J Biol Chem. 1962 Oct;237:3189–3195. [PubMed] [Google Scholar]
- Lord J. M., Beevers H. The problem of reduced nicotinamide adenine dinucleotide oxidation in glyoxysomes. Plant Physiol. 1972 Feb;49(2):249–251. doi: 10.1104/pp.49.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Isolation and characterization of the protein body membrane of castor beans. Plant Physiol. 1979 Sep;64(3):506–511. doi: 10.1104/pp.64.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Beevers H. Gluconeogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967 Nov;42(11):1587–1595. doi: 10.1104/pp.42.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



