Abstract
Objective
The purpose of this study was to determine MRI screening recommendations and the subsequent outcomes in women with increased risk for breast cancer evaluated by oncology subspecialists at an academic center.
Patients and Methods
Patients evaluated between 1/1/2007– 3/1/2011 under diagnosis codes for family history of breast or ovarian cancer, genetic syndromes, lobular carcinoma in situ or atypical hyperplasia were included. Patients with a history of breast cancer were excluded. Retrospective review of prospectively acquired demographics, lifetime risk of breast cancer and screening recommendations were obtained from the medical record. Retrospective review of the results of prospectively interpreted breast imaging examinations and image-guided biopsies were analyzed.
Results
282 women were included. The majority of patients were premenopausal with a median age of 43. Most (69%) were referred due to a family history of breast or ovarian cancers. MRI was recommended for 84% of patients based on a documented lifetime risk > 20%. Most women referred for MRI screening (88%) were compliant with this recommendation. A total of 299 breast MRI examinations were performed in 146 patients. Biopsy was performed for 32 (11%) exams and 10 cancers were detected for a PPV of 31% (based on biopsy performed) and an overall per exam cancer yield of 3.3%. Three cancers were detected in patients who did not undergo screening MRI. The 13 cancers were Stage 0-II; all patients were without evidence of disease with a median follow-up of 22 months.
Conclusion
In a cohort of women seen by breast subspecialty providers, screening breast MRI was recommended according to guidelines, and used primarily premenopausal women with a family history or genetic predisposition to breast cancer. Adherence to MRI screening recommendations was high and cancer yield from breast MRI was similar to that in clinical trials.
Keywords: breast cancer, screening, high risk, magnetic resonance imaging (MRI)
Introduction
Multiple genetic and non-genetic factors are associated with an increased lifetime risk of breast cancer, with the greatest risk from the highly penetrant breast cancer susceptibility genes BRCA1 and BRCA2. Other risk factors include family history and non-genetically derived risk factors including a personal history of atypical hyperplasia or lobular carcinoma in situ (LCIS), previous chest wall radiation, increased mammographic breast density and increased lifetime exposure to estrogen (i.e. early menarche, nulliparity, late menopause or use of combined modality hormone replacement therapy)(1-5).
Studies have shown that implementing more intensive surveillance for women at increased risk for breast cancer may lead to earlier cancer detection and improved outcomes (6, 7). In particular, this may be most important for premenopausal at-risk women, for whom mammography may have lower sensitivity due to higher mammographic density (8). In 2007, the American Cancer Society (ACS) released guidelines recommending the addition of annual breast magnetic resonance imaging (MRI) screening to annual mammographic screening based on breast cancer risk stratification. The addition of MRI is recommended for women with a known BRCA gene mutation and their untested first-degree relatives as well as women whose lifetime risk of breast cancer is estimated at 20-25% or higher based on models largely dependent on family history. Also included in this high-risk group are women who had radiation therapy to the chest between ages 10 and 30 and those with other familial genetic syndromes known to strongly increase risk for breast cancer (9). Although LCIS and atypical hyperplasia are associated with a significantly elevated future risk of breast cancer (10), the 2007 ACS guidelines stated that there was insufficient evidence to recommend for or against MRI screening in this patient population. Instead, MRI use should be decided on a case-by-case basis, based on factors such as age, family history, characteristics of the biopsy, breast density, and patient preference (9).
There are limitations in the available evidence for screening breast MRI in high risk patients. These studies have been substantially different in eligibility criteria, study design and MRI technique, and the initial risk stratification and recommendations for MRI screening from providers has not been routinely reported (11-16). In particular, little is known regarding how breast MRI is actually being employed in high risk patients. The purpose of this study was to evaluate MRI screening recommendations based on breast cancer risk stratification, , adherence to MRI screening and outcomes including biopsy rate, and cancer detection in women with an increased risk for breast cancer evaluated by a heterogeneous group of breast cancer providers, including breast surgeons, medical oncologists and dedicated nurse practitioners at a single large academic institution.
Materials and Methods
Study Population
After obtaining an Institutional Review Board (IRB) approval for this Health Insurance Portability and Accountability Act (HIPPA) compliant study, we identified women with an increased risk for breast cancer seen in the University of Wisconsin Cancer Center Clinics (breast clinic, medical oncology, gynecologic-oncology, or breast surgery) from January 1, 2007 through March 1, 2011. The study start date of January 1, 2007, was selected to coincide with the ACS recommendations for breast MRI screening published in February, 2007 (9). Patients were included if they were seen by a UW breast center nurse practitioner, medical or surgical oncologist with a diagnosis code of a family history of breast or ovarian cancer, personal or family history of genetic susceptibility to malignant neoplasm, LCIS, or atypical ductal or lobular hyperplasia. Patients with a diagnosis code for invasive breast cancer or ductal carcinoma in situ prior to the first encounter were excluded.
Study Design
A retrospective chart review of medical records was performed to obtain demographic information (age, ethnicity and breast cancer risk factors including genetic testing results); documented model-calculated lifetime risk of breast cancer; screening recommendations; surveillance methods and outcome; risk reduction recommendations and patients' selection of recommended chemoprevention, and risk reduction surgeries. Data was recorded through 5/31/2012, This date was at least 12 months from the latest time for patients' eligibility and allowed evaluation of patients' adherence to screening recommendations and outcomes. For patients diagnosed with cancer during the study timeframe, we obtained data on tumor type, cancer stage, receptor status, type of therapy and outcome from the medical record.
MRI Cohort
Patients with at least one screening MRI prior to 5/31/2012 were included in the MRI cohort. Results of breast imaging (including breast MRI, diagnostic and screening mammography, and image-guided biopsies) were obtained from a structured reporting system (PenRad™ Technologies Inc., Buffalo, MN). The imaging studies were interpreted and assessed according to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) lexicon (17). The following final assessment categories were assigned: BI-RADS Category 1, Negative; BI-RADS Category 2, Benign; BI-RADS Category 3, Probably Benign Finding; BI-RADS Category 4, Suspicious Abnormality; and BI-RADS Category 5, Highly Suggestive of Malignancy. Image-guided biopsies were recommended for all suspicious lesions (BI-RADS Category 4 and 5). Probably benign lesions (BI-RADS Category 3) were typically managed with a short-term follow-up MRI.
Breast MRI Technique
All study examinations were performed on a 1.5-T GE scanner (GE Healthcare, Waukesha, WI) using a dedicated breast coil (7 or 8 Channel, Invivo, Peawaukee, WI 1/2007 through 1/2011; 8 Channel Sentinelle, Hologic, Inc., Bedford, MA, 2/2011 through 5/2012). 0.1 mmol/kg of gadolinium contrast (gadodiamide (Omniscan), GE Healthcare, Inc., Princeton, NJ from 2007-2008 and gadobenate dimeglumine (MultiHance), Bracco Diagnostics, Inc., Monroe Township, NJ 2008 to 2010) was hand or power-injected at 2 cc/second followed by a 20 cc saline flush. From 1/2007 through 8/2011, a primarily sagittal imaging protocol was used. Pre-contrast sequences included a 3-plane localizer, sagittal T2-weighted 2d fast spin echo (FSE) with fat saturation (TR=5600 msec; TE=120 msec; Echo train length [ETL] =14) and diffusion weighted imaging (B=0; B=1000) of each breast. Bilateral, simultaneous sagittal T1-weighted 3d spoiled turbo gradient echo imaging was performed using Volume Imaging for Breast Assessment (VIBRANT) parallel imaging (GE Healthcare) with and without fat-saturation prior to contrast administration, and eight times following contrast injection with approximately 70 second temporal resolution. Delayed high-resolution axial and sagittal T1-weighted with fat saturation VIBRANT sequences were also obtained. In August 2011, the protocol transitioned to an axial protocol including the following pre-contrast sequences: 3-plane localizer, bilateral axial T2-weighted 2d FSE with fat saturation (TR=4000 msec; TE=85 msec; ETL=16) and bilateral axial 3d T1-weighted VIBRANT sequences with and without chemical fat saturation. Post-contrast T1-weighted VIBRANT sequences were repeated three times with approximately 180 second temporal resolution (TR=6.8 msec; TE 3.3=msec; flip angle 10°).Computer Aided Evaluation (DynaCAD, Invivo, Gainesville, FL 1/2007 through 1/2011; Aegis, Hologic, Inc., Bedford, MA 2/2011 through 5/2012) was performed for temporal kinetic evaluation and creation of reformats, including subtraction and maximum intensity projection (MIP) images. MRI-guided biopsies were performed using a 9-gauge vacuum assisted breast biopsy device (Suros ATEC, Hologic, Inc.).
Breast MRI Interpretation
Breast MRI examinations were prospectively interpreted by breast imaging specialists and interpretation criteria, assessments, and recommendations were based on the ACR BI-RADS lexicon as described above. Examinations were interpreted in conjunction with the patient's clinical history and other breast imaging studies, when available, including mammography and ultrasound. Breast MRI examinations given an initial assessment of BI-RADS Category 0 with a recommendation for additional imaging were retrospectively reviewed and assigned a final MRI BI-RADS assessment of 1–5 based on results of follow-up imaging examinations. Imaging outcomes were determined by one year follow-up. If biopsy was performed, the pathology result was recorded as described above.
Statistical Analysis
The data were entered into a computerized spreadsheet (Microsoft Excel 2010). Descriptive statistics were utilized for data summary. Statistical significance was tested using chi-square or student's T tests with a p value of less than 0.05 considered statistically significant. The positive predictive value (PPV) for screening breast MRI was calculated per ACR BI-RADS as follows: PPV2 (biopsy recommended) reflects the percentage of all examinations recommended for biopsy or surgical consultation (BI-RADS Categories 4 and 5) that resulted in a tissue diagnosis of cancer within one year and was calculated as the number of true-positive findings divided by the number of cases recommended for biopsy; PPV3 (biopsy performed) reflects the percentage of all known biopsies done as a result of positive examinations (BI-RADS Categories 4 and 5) that resulted in a tissue diagnosis of cancer within one year and was calculated as the number of true-positive findings divided by the number of biopsies. The cancer yield was calculated on a per examination basis as the number of cancers diagnosed (maximum of one per examination) divided by the number of breast MRIs performed, and on a per patient basis as the number of cancers diagnosed divided by the number of patients screened with breast MRI.
Results
A total of 282 women were included (Table 1). The median age was 43 years (range, 20-75 years) and the majority (71%) of women were premenopausal. The primary reason for referral to a subspecialist was a family history of breast or ovarian cancers (69%). A family or personal history of deleterious mutations in breast cancer susceptibility genes was the primary reason for referral in 7% of the patients. Although 68 patients (24%) were referred for other reasons, most of them (80%) also had a family history of breast or ovarian cancer. Overall, 95% of the patients (269 of 282) had a family history of breast or ovarian cancer. Mean number of relatives with breast cancer was two (range 0-9) and 77% (218 of 282) of all patients had at least one first degree relative with breast cancer. Median age of the youngest family member with breast cancer was 42 years (range 19-80). Genetics consult was recommended and performed in 221 of 282 patients (78%). Of the 282 patients, 39 (14%) had a family history of a genetic susceptibility to breast cancer. Thirty of 282 patients (11%) were confirmed to have a gene mutation. Of the 13 of 282 patients with no family history of breast or ovarian cancer, one had a family history of hereditary diffuse gastric cancer syndrome (CDH1 mutation), one had a history of Hodgkin's lymphoma and mantle radiation therapy and the remaining 11 patients were seen due to lobular carcinoma in situ (LCIS) or atypical ductal or lobular hyperplasia (ADH/ALH).
Table 1. Baseline patient characteristics.
| Characteristic | All patients (N=282) |
|---|---|
| Median age in years (range) | 43 (20-75) |
| 20-29 | 33 (12%) |
| 30-39 | 72 (25%) |
| 40-49 | 97 (34%) |
| 50-59 | 58 (21%) |
| 60+ | 22 (8%) |
| Ethnicity | |
| Caucasian | 272 (96.5%) |
| Other | 10 (3.5%) |
| Menopausal status | |
| Premenopausal | 199 (71%) |
| Postmenopausal | 79 (28%) |
| Primary reason for referral | |
| FH of breast or ovarian cancer | 194 (69%) |
| FH or personal history of a known genetic mutation | 20 (7%) |
| Breast symptom | 19 (7%) |
| Abnormal imaging | 17 (6%) |
| LCIS or atypical hyperplasia | 30 (11%) |
| Family history | |
| Average number of affected members (range) | 2 (0-9) |
| Mean age of the youngest relative with breast cancer (range) | 42 (19-80) |
| Calculated lifetime risk of breast cancer of 20% or greater | 146 (51%) |
| Personal history of a genetic mutation | 30 (11%) |
| FH of a known genetic mutation | 39 (14%) |
FH= Family History; LCIS= Lobular Carcinoma In Situ.
Lifetime risk of breast cancer was estimated based on IBIS (Tyrer-Cuzick) or Claus models (18, 19) in the majority of the patients and documented in 211 of 282 charts (75%) as part of their subspecialty care (Figure 1). Of the 211 patients with a documented lifetime risk estimate, 146 patients were estimated to have a lifetime risk of breast cancer of 20% or higher. MRI was recommended for 123 of these 146 (84%) patients. For the 23 patients with a lifetime risk > 20% who were not recommended to have breast MRI, reasons included: young age compared to family history (1 patient), history of risk reduction bilateral mastectomies (4 patients), kidney transplant (1), change in calculated risk over time (4 patients) and unknown reasons (13 patients). A total of 108 patients of 123 (88%) were compliant with the MRI recommendations and underwent at least one screening MRI. Of the 15 patients who were recommended but did not undergo an MRI, one patient was diagnosed with breast cancer and underwent surgery prior to MRI, one had the MRI outside of the study window, one MRI was declined by insurance, 4 patients declined and a further 8 patients were considering it or were lost to follow-up. Of the 211 patients with a documented risk assessment, 65 patients were estimated to have a lifetime risk of <20%. Of those, 13 patients underwent MRI screening. These 13 women had an average of 2.2 family members with breast cancer. The mean age of the youngest relative at the time of cancer diagnosis was 44 (range of 30-80). Five patients of the 13 (38%) had a calculated life time risk of 18-19% and 4 patients had a personal history of LCIS or atypical hyperplasia in addition to positive family history, which was felt to contribute enough to warrant MRI screening.
Figure 1. Total patients and MRI-screened cohort.
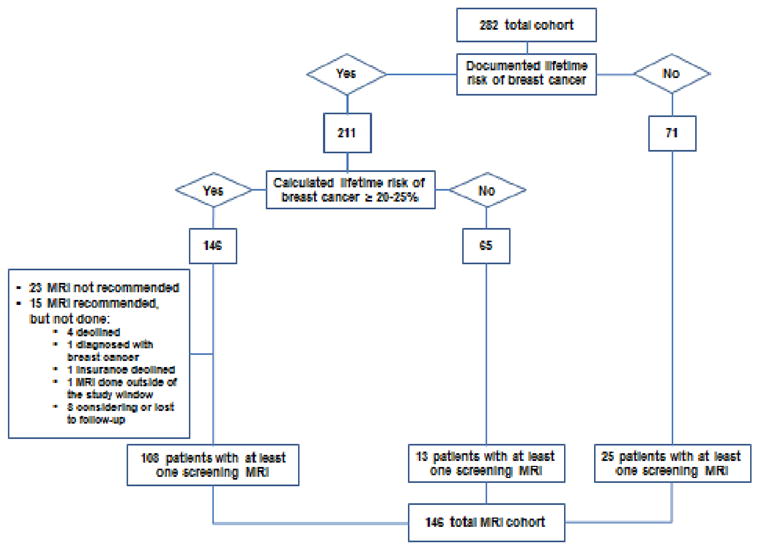
Seventy-one of 282 patients did not have a documented lifetime risk in the chart, and 25 of these patients underwent at least one screening breast MRI. Family history of BRCA mutation was the indication for MR imaging in 2 women. The rest of these patients had a family history of breast cancer, with 84% having at least one first-degree relative with breast cancer. On average, each woman had 2.9 family members with breast cancer with a mean age of 38 at the time of cancer diagnosis.
The MRI cohort includes a total of 146 patients who underwent at least one screening MRI during the study window (see Figure 1). The patients within the MRI cohort were younger than patients who did not have breast MRI (mean age 41 vs. 46, p=0.0002). More patients in the MRI cohort were premenopausal (84% in MRI cohort vs. 56% in no-MRI cohort, p <0.0001). The patients in the MRI cohort were more likely to have a documented risk calculation in the chart comparing to the no-MRI cohort (83% vs. 66%, p = 0.0012). Personal history of genetic mutations predisposing to breast cancer was significantly more common within the MRI cohort (15% vs. 6% in no-MRI cohort, p = 0.01). Family history of genetic mutations was reported in 17% and 10% patients within the MRI and no-MRI cohorts respectively, however this difference was not statistically significant (p = 0.09). In the MRI cohort family members were generally diagnosed with breast cancer at a younger age (mean age of the youngest relative was 40 years vs. 45 years in no-MRI cohort, p=0.00002).
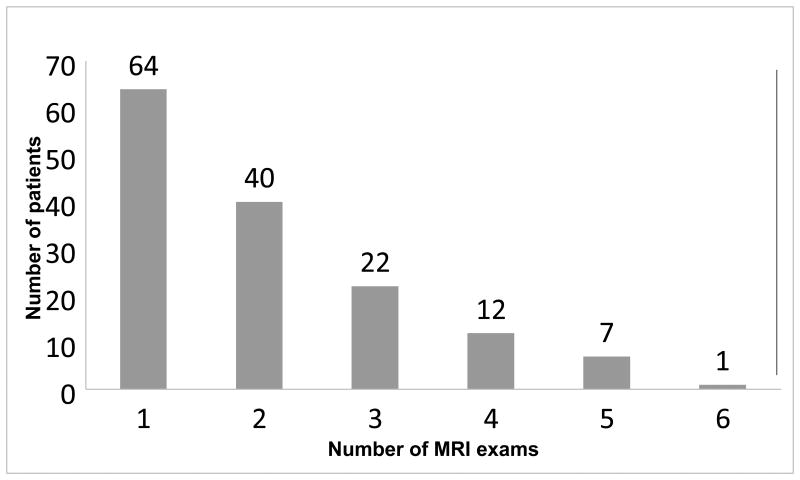
In total, 299 MRI examinations were performed in the 146 patients screened with breast MRI (Figure 2). A median of two MRI examinations per patient were performed (range of 1 to 6 examinations per patient). Final BI-RADS Assessment Categories were: BI-RADS 1 or 2, 234 exams (78%); BI-RADS 3, 31 exams (10%); BI-RADS 4 or 5, 34 exams (11%). Two of 31 patients with a probably benign, BI-RADS Category 3 assessment elected for MRI-guided biopsy, both with a benign result. Of those patients with a recommendation for biopsy (BI-RADS Category 4 or 5), 32 patients had a biopsy performed and 10 were diagnosed with cancer (9 invasive carcinomas and one DCIS). All patients with a MRI detected breast cancer had a negative mammogram within the preceding 11 months. PPV2 for a biopsy recommendation was 29% and PPV3 for biopsy performed was 31%. Cancer yield per the number of screening MRI examinations performed was 33 cancers per 1000 examinations. Cancer yield per number of patients screened (including those with multiple screening rounds) was 68 cancers per 1000 patients (Table 2).
Figure 2. Number of MRIs per patient.
Table 2. Added cancer yield of breast MRI screening.
| Total patients | Total MRI exams | Biopsy performed | Positive predictive value of biopsies performed based on MRI exams | Cancer yield per patient | Cancer yield per MRI exam |
|---|---|---|---|---|---|
| 146 | 299 | 32/299 (10.7%) | 10/32 (31%) | 10/146 (6.8%) | 10/299 (3.3%) |
A total of 13 cancers were detected in 282 patients during the study: 10 with MRI, one with mammography, one with ultrasound (performed for short interval follow-up of a previous ultrasound finding), and one was patient-detected. All cancers were Stage 0 – II. All patients were without evidence of disease with median follow-up of more than 18 months (range 10-36 months). Table 3 shows the characteristics of the patients with a detected breast cancer.
Table 3. Characteristics of the patients with detected breast cancer.
| Characteristic | MRI cohort N= 146 |
No-MRI cohort N=136 |
|---|---|---|
|
| ||
| Number of patients diagnosed with breast cancer | 10 | 3 |
|
| ||
| Median age at diagnosis in years (range) | 49 (39-62) | 61 (39-67) |
|
| ||
| Premenopausal status at diagnosis | 6 (60%) | 1 (33%) |
|
| ||
| BRCA carrier | 4 (40%) | 2 (66%) |
|
| ||
| Cancer detection method | ||
| Patient detected | 1 | |
| Ultrasound | 1 | |
| Mammogram | 1 | |
| MRI | 10 | |
|
| ||
| Stage | ||
| DCIS | 1 | 1 |
| I | 7 | 2 |
| II | 2 | |
|
| ||
| Hormone Receptor positive | 7 | 1 |
| Triple negative | 0 | 2 |
|
| ||
| Surgery | ||
| Breast-conserving surgery | 5 | 1 |
| Mastectomy | 5 | 2 |
|
| ||
| Adjuvant Therapy | ||
| Chemo | 3 | 2 |
| XRT | 6 | 2 |
| Hormonal | 8 | 2 |
|
| ||
| Median follow-up and status | 19 months, NED | 36 months, NED |
DCIS=Ductal Carcinoma in Situ; XRT= Radiation Therapy; NED=No Evidence of Disease
A total of 40 patients (14%) had a history of LCIS, ADH or ALH, with the majority of these patients (72%) also having a family history of breast cancer. Median age in this group was 49 years (range of 35 to 75) and 47% of them were premenopausal. Of these 40 women, 11 underwent MRI screening mainly due to increased risk of developing breast cancer regardless of the history of LCIS or atypical hyperplasia. Two cancers were detected by screening MRI in these patients. One was initially referred due to LCIS and family history and was found to be a BRCA2 mutation carrier and underwent screening MRI. The second patient had atypical hyperplasia and one first degree family member with a post-menopausal breast cancer. She underwent screening MRI due to estimated lifetime risk of 43% based on Gail model, rather than IBIS or Claus models.
Chemoprevention was discussed with 156 of 282 (55%) and started in 16 patients. Six of these 16 patients subsequently stopped before 5 years. Risk reduction surgeries including bilateral mastectomy or oophorectomy were primarily discussed with women with known BRCA mutations. A total of 25 patients (8.9%) underwent at least one risk reduction surgery, 19 of whom had a gene mutation.
Discussion
In this study we demonstrated that subspecialty providers evaluating women at risk for breast cancer recommend MRI screening appropriately to those women who meet the ACS guidelines. MRIs were largely recommended in premenopausal women at high risk for developing breast cancer based on current breast cancer risk assessment models. Furthermore, 88% of women referred for MRI screening were compliant with this recommendation.
Since 2007 when the ACS published the guidelines recommending annual MR imaging in addition to mammography in high-risk women, few studies have investigated providers' ability to select patients or patients' adherence to the recommendations. A recent observational cohort study of community MRI use indicated that approximately half of patients receiving screening breast MRI had a lifetime risk of breast cancer of less than 15%.(20) Another separate retrospective community-based study indicated that only 21% of patients undergoing screening breast MRI met the ACS guidelines.(21) Regarding adherence, in the American College of Radiology Imaging Network (ACRIN) 6666 trial of high-risk women with mammographically dense breasts being screened with breast ultrasound followed by MRI, 42.1% of the women declined MRI screening(22). In contrast, previous studies examining women visiting genetic counseling clinics or women from BRCA1/2 mutation families show relatively high adherence (67–90%) to clinical breast exam (CBE) and mammography screening recommendations (23, 24). Our study demonstrates that in at-risk women identified by oncology subspecialty providers, MRI is recommended primarily to patients meeting guidelines and that adherence to MRI recommendations is high.
MRI has consistently demonstrated higher sensitivity for detecting breast cancer comparing to mammography or ultrasound in women at high risk for developing breast cancer. Across studies, the mean cancer yield owing to the addition of breast MRI to screening was 22 per 1000 patients imaged (25). Additionally, 3-16% of screened patients received a recommendation for biopsy based on MRI findings. Among biopsies performed, the positive predictive value for malignancy ranged from 17% to 89% with a mean of 45% (25, 26). In our study, 52% of the cohort underwent at least one screening MRI. The majority of MRI-screened patients (84%) were premenopausal – a cohort in whom mammographic density is often a concern. Biopsy was recommended following 34 (11%) examinations; this is similar to the rates seen in other studies. The positive predictive value for biopsy performed was 31%, thus within the desirable positive biopsy rate range (25% to 40%) recommended by the American College of Radiology. The overall added cancer yield was 3.3% per exam and similar to prior published results (6, 13, 26-27). These results demonstrate that MRI screening is beneficial in a real world population at high risk for breast cancer, and that MRI screening in this patient population has a high diagnostic yield with an acceptable positive biopsy rate outside a clinical trial.
It should be noted that our patient population was different from those in previously published studies. They were a heterogeneous group of patients evaluated due to high risk of developing breast cancer. For instance, we included a total of 40 patients (14%) who had a history of LCIS, ADH or ALH. Even though most of them also had a family history of breast cancer, these patients were slightly different from the rest of cohort. They were older and less than half of them were premenopausal. Patients with LCIS have a 20-25% lifetime risk of developing breast cancer after 20 years (10); however, due to lack of published evidence, the 2007 ACS guidelines for screening breast MRI advised that MRI screening in these patients should be decided on a case-by-case basis with consideration of other risk factors and patient preference (9). However, the 2009 National Comprehensive Cancer Network guidelines indicate that annual MRI be considered for women with LCIS as an adjunct to mammography and clinical examination (28). Based on few retrospective studies in patients with LCIS, MR imaging resulted in 3.7% to 4.5% incremental cancer detection rate (29-31). In our study a total of 11 out of 40 patients with LCIS or atypical hyperplasia (27%) underwent MR screening, mainly based on the other risk factors. Breast cancer was detected in 2 patients (18%), one being a BRCA carrier. In our practice we do not recommend routine use of screening MRI in all patients with LCIS, but these data support that further prospective studies are needed to determine the impact of MR screening in this patient population.
Our study had several limitations. This was a retrospective chart review. Our patients were primarily Caucasians and the sample size was modest for a screening study. Despite routine recommendations, follow-up exams and imaging timeframes varied among the patients. Estimated risk of breast cancer was not documented in all charts and different risk assessment models were used. It is noteworthy that since 2010, high risk patients at UW Breast Center are seen in our dedicated, multidisciplinary UW Breast Cancer Prevention, Assessment and Tailored Health Screening (PATHS) Clinic. Since that time, documentation of breast cancer risk assessment using validated tools is documented in all cases.
Conclusion
In a cohort of women seen by breast subspecialty providers at a single academic center, screening breast MRI was recommended according to ACS guidelines resulting in MRI screening in primarily premenopausal women with a family history or genetic predisposition to breast cancer. Adherence to MRI screening recommendations was high. Our data demonstrates similar cancer yield from breast MRI screening compared to clinical trials of high risk women. In addition, the positive predictive value for biopsy performed of 31% is acceptable and within the range recommended by ACR-BI-RADS for mammography of 25-40% and consistent with rates in the MRI literature.
These results reinforce prior recommendations from prospective clinical trials for supplemental screening with breast MRI in conjunction with mammography in women at high risk for breast cancer.
Table 4. Characteristics of the patients with LCIS or atypical hyperplasia.
| Characteristic | Patients with LCIS or ALH/ADH N=40 |
|---|---|
|
| |
| Median age in years (range) | 49 (35-75) |
| 35-44 | 5 (12.5%) |
| 45-54 | 20 (50%) |
| 55-64 | 10 (25%) |
| 65+ | 5 (12.5%) |
|
| |
| Menopausal status | |
| Premenopausal | 19 (47%) |
| Postmenopausal | 20 (50%) |
|
| |
| Family history of breast or ovarian cancer | 29 (72%) |
| At least 1 first-degree relative with breast cancer | 16 (40%) |
|
| |
| Personal history of a known genetic mutation | 1 (2.5%) |
|
| |
| MRI performed | 11 (27%) |
|
| |
| Cancer diagnosis | 2 (5%) |
LCIS=Lobular Carcinoma in Situ; ALH=Atypical Lobular Hyperplasia; ADH=Atypical Ductal Hyperplasia.
Acknowledgments
We would like to acknowledge Linda Szalkuki, NP and Amy Stettner, Senior Genetic Counselor for their contributions to this manuscript and thank Torie Champeny for her assistance with achieving regulatory approval for this research.
Funding Source: This work was supported by the NCI Cancer Center Support Grant P30 CA014520.
Footnotes
Conflict of Interest/Disclosures: All authors report no relevant financial conflicts of interest.
References
- 1.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004 Sep;4(9):665–76. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 2.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–51. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 3.ESHRE Capri Workshop Group. Hormones and breast cancer. Hum Reprod Update. 2004;10(4):281–93. doi: 10.1093/humupd/dmh025. [DOI] [PubMed] [Google Scholar]
- 4.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin Disease. JAMA. 2003;290(4):465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 6.Passaperuma K, Warner E, Causer PA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107(1):24–30. doi: 10.1038/bjc.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FH01 collaborative teams. Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol. 2010;11(12):1127–34. doi: 10.1016/S1470-2045(10)70263-1. [DOI] [PubMed] [Google Scholar]
- 8.Saadatmand S, Rutgers EJ, Tollenaar RA, et al. Breast density as indicator for the use of mammography or MRI to screen women with familial risk for breast cancer (FaMRIsc): a multicentre randomized controlled trial. BMC Cancer. 2012;12:440. doi: 10.1186/1471-2407-12-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 10.Degnim AC, King TA. Surgical Management of High-Risk Breast Lesions. Surg Clin North Am. 2013;93(2):329–40. doi: 10.1016/j.suc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–37. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 12.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–25. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 13.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicenter cohort study (MARIBS) Lancet. 2005;365(9473):1769–78. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 14.Evans DG, Lennard F, Pointon LJ, et al. Eligibility for magnetic resonance imaging screening in the United Kingdom: effect of strict selection criteria and anonymous DNA testing on breast cancer incidence in the MARIBS Study. Cancer Epidemiol Biomarkers Prev. 2009 Jul;18(7):2123–31. doi: 10.1158/1055-9965.EPI-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmore L, Margenthaler JA. Use of breast MRI surveillance in women at high risk for breast cancer: a single-institutional experience. Ann Surg Oncol. 2010;17(Suppl 3):263–7. doi: 10.1245/s10434-010-1236-4. [DOI] [PubMed] [Google Scholar]
- 16.Wilke LG, Broadwater G, Rabiner S, et al. Breast self-examination: defining a cohort still in need. Am J Surg. 2009 Oct;198(4):575–9. doi: 10.1016/j.amjsurg.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda DM, Hylton NM, Kuhl CK, et al. BI-RADS: Magnetic Resonance Imaging, 1st edition. In: D'Orsi DJ, Mendelson EB, Ikeda DM, et al., editors. Breast Imaging Reporting and Data System: Breast Imaging Atlas. 4th. Reston, VA: American College of Radiology; 2003. BIRADS classification. [Google Scholar]
- 18.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 19.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73(3):643–51. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Wernli KJ, DeMartini WB, Ichikawa L, et al. Breast Cancer Surveillance Consortium. Patterns of Breast Magnetic Resonance Imaging in Community Practice. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.11963. Published online Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stout NK, Nekhlyudov L, Li L, et al. Rapid Increase in Breast Magnetic Resonance Imaging Use: Trends from 2000-2011. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.11958. Published online Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87. doi: 10.1148/radiol.2541090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campitelli MA, Chiarelli AM, Mirea L, et al. Adherence to breast and ovarian cancer screening recommendations for female relatives from the Ontario site of the Breast Cancer Family Registry. Eur J Cancer Prev. 2011;20(6):492–500. doi: 10.1097/CEJ.0b013e3283476217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antill YC, Reynolds J, Young MA, et al. Screening behavior in women at increased familial risk for breast cancer. Fam Cancer. 2006;5(4):359–68. doi: 10.1007/s10689-006-0006-8. [DOI] [PubMed] [Google Scholar]
- 25.DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19(3):143–50. doi: 10.1097/RMR.0b013e31818a40a5. [DOI] [PubMed] [Google Scholar]
- 26.Sickles EA. The Use of Breast Imaging to Screen Women at High Risk for Cancer. Radiol Clin North Am. 2010;48(5):859–78. doi: 10.1016/j.rcl.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671–9. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 28.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–96. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 29.Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–7. doi: 10.1245/s10434-006-9195-5. [DOI] [PubMed] [Google Scholar]
- 30.Friedlander LC, Roth SO, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology. 2011;261(2):421–7. doi: 10.1148/radiol.11103516. [DOI] [PubMed] [Google Scholar]
- 31.Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011;261(2):414–20. doi: 10.1148/radiol.11110091. [DOI] [PubMed] [Google Scholar]