Ezrin was first detected in epidermal growth factor-treated skin epithelial cells as a protein-tyrosine kinase substrate prior to its purification and characterization as a structural component of microvilli of chicken intestinal epithelial cell brush borders (1, 2). Subsequently, other closely related proteins including radixin and moesin were identified, that together with ezrin constitute the ERM proteins family. ERM family members have the ability to cross-link proteins of the plasma membrane with the sub-cortical cytoskeleton and were shown to be important for the stabilization of the cell cortex structure and regulation of several signal transduction pathways (3).
ERM proteins are localized at the interface between the plasma membrane and the cortical actin cytoskeleton, and are organized into three functional domains: an N-terminal FERM (Four point one ERM) domain, an extended coiled-coil region and a short C-terminal domain. Through its C-terminal region, ERM proteins bind directly to F-actin whereas binding to transmembrane proteins that occurs directly or indirectly (i.e. NHE3, CFTR) is achieved via the FERM domain. Indirect binding of ERM proteins with transmembrane proteins occurs via adaptor proteins such as EBP50 (ERM-Binding Phosphoprotein 50, also termed NHERF1 for Na+/H+ exchanger regulatory factor 1) or NHERF2 (i.e. E3KARP). Interestingly, the expression and localization of ezrin and EBP50 are inter-dependent. Indeed, in intestinal epithelial cells of Ez−/− mice, EBP50 is no longer apically concentrated and instead is diffusely localized in the cytoplasm (4). Conversely, in EBP50−/− mice, expression of ezrin is reduced in brush border membrane of both kidney proximal tubules and small intestine cells (5).
In the liver, ERM proteins are differentially expressed between the epithelial cell populations, i.e. hepatocytes and biliary cells. Moesin is expressed in hepatocytes and not in biliary epithelial cells (BEC) (6). However, both populations express radixin whereas ezrin is exclusively expressed in the apical domain of BEC (6, 7). The specific expression pattern of ezrin already exists in the developing liver where ezrin is only detected in cells of the ductal plate and early bile ducts, indicating that ezrin is specific of the biliary lineage. However, thus far, the biliary functions of ezrin have remained unexplored. In this issue of Hepatology, Hatano et al.(8), shed some light on this matter by analyzing the consequences of ezrin deficiency on liver physiology. Ezrin-deficient mice (Vil2kd/kd) exhibit a marked liver phenotype characterized by a cholestatic liver injury. Cholestasis observed in ezrin-deficient mice is primarily caused by a deregulation of biliary secretory function since no morphological abnormalities were observed in biliary epithelial cells suggesting that ezrin may not be essential for the integrity of the biliary epithelium. This observation should be more carefully addressed since it is slightly in contrast with what has been previously described in the intestine (4) where deletion of the mouse ezrin gene yields severe morphological consequences both in the developing intestinal epithelium and in the intestinal homeostasis in the adult (i.e. incomplete villus morphogenesis, junctional remodeling, cell geometry). A few points could be raised to explain this incongruence. First, a very low residual level of expression of ezrin is detectable in Vil2kd/kd mice that could be responsible for a milder phenotype in the liver. Second, ezrin has different roles in different tissues and other ERM members (i.e. radixin) may partially substitute for its function in biliary cells. Moreover, ezrin deletion in the intestine affects not only the apical villi structure but also the cell-junction organization. In fact, functional ERM proteins are also important for the organization of the F-actin cytoskeleton that is tightly associated with proteins of the apical junction complex (AJC). One can speculate that an evaluation of the morphology of AJC in the liver of ezrin-defective mice might have revealed a similar defect. Indeed, it is well known that the functional integrity of cell junctions is frequently impaired in cholestasis (9).
The work from Hatano et al. clearly shows that the absence of ezrin interferes with the physiological function of BECs or cholangiocytes. The biliary epithelium is mainly involved in the regulation of the fluidity and alkalinity of the primary canalicular bile secreted by the hepatocytes. This function depends on a number of specific transport systems and ion channels, where the most important are the anion exchanger-2 (AE2) and the PKA-regulated chloride channel CFTR, both expressed in the apical membrane of cholangiocytes and those activities are tightly coupled (10). Hatano et al. show that the apical expression of CFTR and EBP50 proteins is reduced in BECs of ezrin-deficient mice, suggesting that in absence of ezrin, CFTR·EBP50·ezrin complex is disrupted and PKA-dependent signaling is impaired leading to mislocalization and deregulation of CFTR. Thus, ezrin appears to be an essential functional organizer of the sub apical membrane. This is in line with evidence demonstrating that ezrin controls PKA-mediated phosphorylation of CFTR by completing the function of A-kinase anchoring protein (AKAP), a protein binding the regulatory subunit of PKA, therefore localizing the kinase in proximity to CFTR (11, 12).
But what happens if EBP50 or CFTR is removed from the complex? Does the liver phenotype reproduce the one described in ezrin-deficient mice? Although, phenotype and secretory function of BECs have not been investigated in the liver of EBP50-deficient mice, in vitro studies in a human BEC cell line, demonstrates that EBP50 regulates PKA-dependent chloride secretion (13). Moreover, since EBP50 is also expressed in hepatocytes, loss of EBP50 also leads to a reduced bile-acid independent bile flow linked to a lower expression of the MRP2 protein in hepatocytes (14), which would imply a more severe cholestatic phenotype. However, in contrast to ezrin-deficient mice, no significant elevation of plasma concentrations of liver enzymes is observed in EBP50-deficient mice (L Fouassier, unpublished data), indicating an absence of cholestasis. One explanation that may be raised is the existence of compensatory mechanisms such as the upregulation of EBP50-related proteins (NHERF2) (5). Interestingly, EBP50 expression has shown to be aberrantly distributed in the liver from patients with CF, PBC and PSC and a similar redistribution was also confirmed in the proliferating biliary cells of BDL rats, a model of intrahepatic cholestasis (15).
Similarly to ezrin, CFTR is selectively expressed by BECs in the liver and although CFTR has a major role in bile secretion, CFTR-deficient mice do not spontaneously develop liver disease (16). Accordingly, only a small percentage of CF patients progress to severe liver disease, in spite of a defective biliary secretory function. In this regard, it has been recently reported (17) that absence of CFTR at the membrane affects the innate immune properties of the biliary epithelium in response to bacterial products. In fact, lack of CFTR increases the TLR4-mediated response to endotoxins of the biliary epithelium causing biliary damage and inflammation. Induction of chemical colitis in Cftr-KO mice by treatment with DSS causes a biliary injury with proliferation of bile ducts similar to the ezrin-defective mouse suggesting that the secretory defect is a predisposing factor to further damage and a second hit is necessary to develop the disease. However, differently from Hatano et al., a strong peri-portal infiltration of neutrophils and macrophages was described in Cftr-KO mice treated with DSS. There is a possibility that ezrin-defective mice exposed for example to endotoxins would potentially develop an inflammatory phenotype as well. How is CFTR linked with TLR4 and innate immunity in biliary cells? Unpublished data suggest that CFTR through its association with EBP50 might participate in the regulation of TLR4 signaling transduction (18).
In ezrin-deficient mice, additional transporters other than CFTR are deregulated that may explain the development of cholestasis. About a decade ago, it was suggested that CFTR, AE2 and AQP1 were co-regulated since they co-localized in intracellular vesicles in the sub-apical domain of BEC. Furthermore, the cAMP/PKA pathway regulated insertion of these vesicles to the apical plasma membrane of the cells (19). Interestingly, ezrin-deficient mice display an accumulation of sub apical vesicles in BECs, which is correlated with decreased apical localization of CFTR, AE-2, AQP1 and EBP50. In vitro, loss of ezrin function in isolated BECs causes an impairment of translocation in the apical membrane of the transporters in basal and stimulated condition after PKA activation. Ezrin may therefore be an underlying mechanism for trafficking and/or stabilization of the transporters to the apical membrane, and for their regulation through discrete compartmentalization of PKA. Nonetheless, the molecular mechanisms by which ezrin anchors AQP1 and AE2 to the cortical F-actin cytoskeleton deserve further investigations.
In conclusion, these studies by Hatano et al. add another piece to the puzzle of the pathogenesis of ductal cholestasis by defining the importance of ezrin in the regulation of bile secretory mechanisms. Indeed, multiple studies suggest that transporters involved in bile secretion are organized in macromolecular complexes and their interaction with the cytoskeleton mediated by accessories proteins (i.e. EBP50, ezrin) plays a critical role in coordinating their function (Figure 1). Moreover, these macromolecular complexes also contain signaling molecules and kinases and therefore their regulation may provide a link with other key functions of biliary cells (i.e. cell polarity, innate immunity, proliferation, inflammation). In future studies, it will be important to dissect these interactions and how they account for the broad spectrum of cholangiopathies.
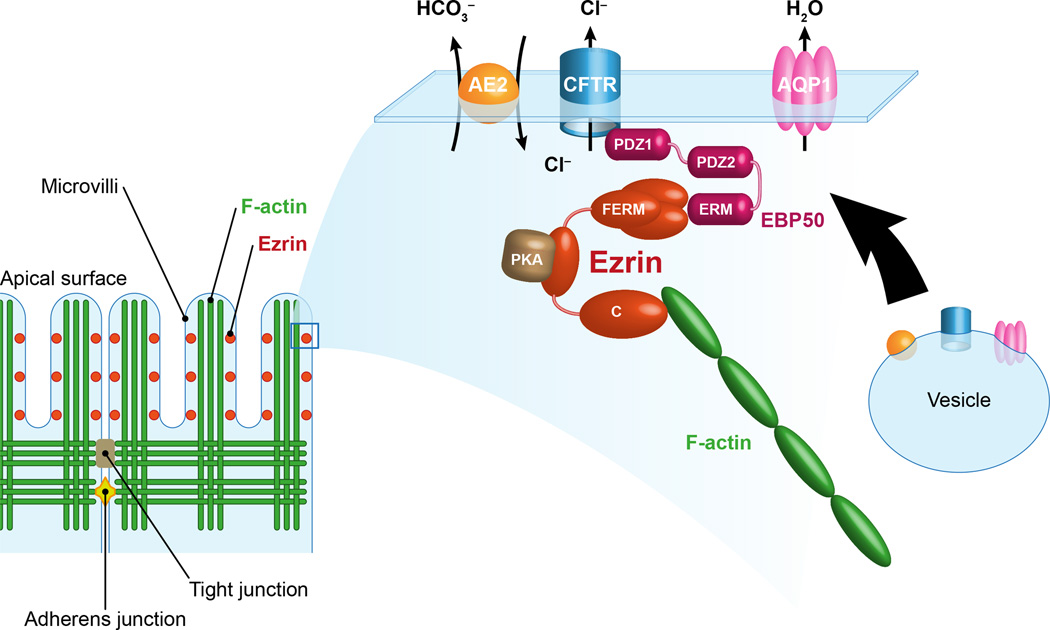
Figure 1. Proposed model for the regulation of bile secretion by ezrin in cholangiocytes.
Ezrin is localized at the apical actin-rich region of cholangiocytes where it contributes to the organization of multiprotein complexes. Ezrin regulates the membrane localization and the activation of CFTR. The FERM domain of ezrin anchors CFTR indirectly through the PDZ protein EBP50, whereas the C-term domain interacts with F-actin promoting the stabilization of the channel at the membrane. Ezrin can bind additional proteins such as PKA, thereby contributing to the regulation of CFTR. By acting as a protein kinase A anchoring protein (AKAP), ezrin positions the PKA near CFTR to be phosphorylated and activated. Furthermore, the activation of CFTR is spatially and functionally coordinated with the activation of AE2 and AQP1. This secretory complex already co-localizes in intracellular vesicles whose trafficking and membrane insertion are regulated through the interaction with actin cytoskeleton and by PKA activation. The involvement of ezrin in the insertion/stabilization and regulation of AE2 and AQP1 is currently undetermined. Thus, the architecture of the subcortical cytoskeleton and the distribution and retention of proteins at the apical membrane are important signals to maintain the apical polarity and the secretory functions of the epithelium.
Acknowledgments
This work was supported, in part, by Fondation de France, Fondation ARC pour le Recherche sur le Cancer, La Ligue National contre le Cancer and GEFLUC (to LF) and the National Institute of Health (DK096096), Fondazione Fibrosi Cistica (Grant #18-2012), Telethon (Grant #GGP12133) and PSC Partners Seeking a Cure Foundation (to RF).
The authors thank Yves Chrétien for the graphic support.
Abbreviations
- ERM
ezrin-radixin-moesin
- EBP50
ERM-binding phosphoprotein 50
- NHERF1
Na+/H+ exchanger regulatory factor 1
- CFTR
cystic fibrosis conductance transmembrane regulator
- AE-2
Anion exchanger 2
- PKA
protein kinase A
- cAMP
cyclic-adenosine-monophosphate
- CF
cystic fibrosis
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- BDL
bile duct ligation
- DSS
dextran sulfate sodium
- PDZ
Post synaptic density protein (PSD95)-Drosophila disc large tumor suppressor (Dlg1)-Zonula occludens-1 protein (ZO-1)
Footnotes
Potential conflict of interest: Nothing to disclose.
References
- 1.Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981;24:741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- 3.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 7.Claperon A, Debray D, Redon MJ, Mergey M, Ho-Bouldoires TH, Housset C, et al. Immunohistochemical profile of ezrin and radixin in human liver epithelia during fetal development and pediatric cholestatic diseases. Clin Res Hepatol Gastroenterol. 2013;37:142–151. doi: 10.1016/j.clinre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Hatano R, Akiyama K, Tamura A, Hosogi S, Marunaka Y, Caplan MJ, et al. Knockdown of ezrin causes intrahepatic cholestasis by the dysregulation of bile fluidity in the bile duct epithelium. Hepatology. 2014 doi: 10.1002/hep.27565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003;124:737–753. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 10.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 11.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, et al. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. Embo J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun F, Hug M, Bradbury N, Frizzell R. Protein Kinase A associates with CFTR via an interaction with ezrin. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- 13.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, et al. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33:166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, et al. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem. 2010;285:19299–19307. doi: 10.1074/jbc.M109.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouassier L, Rosenberg P, Mergey M, Saubamea B, Claperon A, Kinnman N, et al. Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation. Am J Pathol. 2009;174:869–880. doi: 10.2353/ajpath.2009.080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 17.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. 1508, e1491–e1495. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorotto R, Villani A, Kourtidis A, Scirpo R, Spirli C, Anastasiadis PZ, et al. The Cystic Fibrosis Conductance Regulator (CFTR) controls c-Src tyrosine kinase signaling and regulates innate immunity and epithelial polarity in cholangiocytes. Hepatology. 2014;60:299A–299A. [Google Scholar]
- 19.Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, et al. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413–20419. doi: 10.1074/jbc.M302108200. [DOI] [PubMed] [Google Scholar]