Abstract
Objectives
A simple measure of activities of daily living (ADL), wounds, and indwelling devices (urinary catheter and/or feeding tube) has been developed by Arling et al as a relative measure of NH residents’ need (time-intensity) for nursing resources. We used the Arling scale to predict prevalent, new and intermittent multi-drug resistant organism (MDRO) acquisition in nursing home (NH) residents.
Design
Secondary analysis, prospective cohort study
Setting
15 Southeast Michigan NHs
Participants
111 NH residents with ≥ 2 monthly visits (729 total)
Measurements
Monthly microbiologic surveillance for MDROs from multiple anatomic sites from enrollment until discharge or one year. We used the Arling scale to predict prevalent and time-to-new or intermittent acquisition (months) of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and resistant Gram-negative bacteria (R-GNB) colonization using multiple-failure accelerated time-factor survival analysis, controlling for comorbidity, hospitalization, and antibiotic use in the prior month.
Results
Mean age was 81 years. One-fifth had a wound and one-third had a device. There were 60 acquisitions of MRSA, 56 of R-GNB, and 15 of VRE. Expected (median) time-to-acquisition was < 1 year for MRSA (6.7 month) and R-GNB (4.5 months), and more than 1 year for VRE (40 months). The Arling score was associated with earlier new MRSA and VRE acquisition. A resident with only mild functional impairment and no device/wound would be expected to acquire MRSA in 20 months, versus 5 months for someone needing the most intense nursing contact.
Conclusion
MDRO acquisition is common in community NHs. Need for nursing care predicts new MDRO acquisition in NHs, suggesting potential mechanisms for MDRO acquisition and strategies for future interventions for high-risk individuals, for example, enhanced barrier precautions.
INTRODUCTION
Multi-drug resistant organisms (MDROs) are endemic in nursing homes (NHs) with prevalence rates surpassing those in hospitals.1–4 Indwelling device use, prior antibiotic exposure, presence of a wound or pressure ulcer, and prior hospitalization are considered to be individual risk factors for colonization with MDROs.5 Retrospective and cross-sectional studies have shown that geriatric patients with greater functional disability are at increased risk for symptomatic infection6, 7 and asymptomatic colonization with MDROs whether they reside in NHs or have been transferred from a NH to acute care.8–10 In a cross-sectional study, active surveillance for MDROs showed a dose-response relationship between a NH resident’s overall functional disability burden and MDRO colonization.8 Subsequently, a prospective study identified functional disability as an independent risk factor for NH residents to acquire new MDROs over one year of care.9, 11
In this study, we hypothesized that functional disability is related to MDRO acquisition due to greater need for patient contact with health care professionals. We measured how many months, on average, it took to acquire an MDRO colonization for NH residents requiring increasing intensity of nursing care due to functional impairment, as well as needing other skilled nursing assistance.
METHODS
We analyzed data from a prior prospective microbial study involving 15 community-based NHs in Southeast Michigan. Details of the study design have been reported.12 Briefly, this prospective observational study was conducted from October 2005 to January 2010. The participating NH facilities accepted people from local hospitals, but none were academic or hospital-based NHs. Bed size ranged from 71 to 161. Four sites were either non-for-profit or operated by the County Government and the remaining 11 were for-profit facilities. All residents with an indwelling device (urinary catheter and/or enteral feeding tube) were approached for recruitment. Upon enrollment of a resident with an indwelling device, a device-free resident was randomly approached for recruitment as a control. All participating residents were followed for a maximum of 12 months or until loss to follow-up, which occurred due to death, culture refusal or device removal. This study was approved by the University of Michigan and Veterans Affairs Ann Arbor Health Care System Institutional Review Boards. Written informed consent was obtained from all participants or appropriate proxies for enrollment.
Demographic data were recorded at enrollment and clinical and microbiologic data were obtained at baseline and every month thereafter until death or discharge. We collected monthly samples from the nares, oropharynx, groin, perianal area, and any wounds or device sites.13 We used standard microbiologic methods to identify methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and/or resistant Gram-negative bacteria (R-GNB, either to ciprofloxacin or ceftazadime) organisms. In order to test our hypothesis that higher nursing resource utilization would lead to shorter time to acquisition of MDROs, we included only residents with at least a baseline and one follow-up culture for this study.
Participant data such as age at the time of enrollment, gender, functional status, presence of indwelling device (urinary catheter and/or enteral feeding tube), and comorbid illnesses were attained from medical records by trained research personnel. Baseline function was assessed for severity of impairment in six activities of daily living (ADLs: bathing, toileting, dressing, feeding, walking, and grooming) using the Lawton-Brody Physical Self-Maintenance Scale (PSMS, range: 1–5)14 indicating: (1) independent with the ADL, (2) needs reminders, (3) moderate assist, (4) extensive assistance, and (5) full assist/resistance of care. Comorbidity was calculated using the Charlson Comorbidity Index (CCI).15 In addition, we collected data on time-varying variables such as hospitalization, antibiotic use, and presence of wounds or pressure ulcers in the prior 30 days.
We adapted research by Arling and associates16 to estimate each participant’s relative need for nursing care, based on ADL impairment severity, device presence, and wounds. We elected to employ the Arling criteria because of its relative simplicity, having reduced the variables employed by Medicare for determining NH case-mix17 to a simpler set of variables that were subsequently validated in a time-motion study of nursing intensity of care.16 The scale has excellent fit with relative observed nursing utilization time (53% of variance).16 The method classifies patients into six groups, using feeding dependence, overall ADL impairment, devices, and wounds to determine the groups. Each group is associated with a relative weight representing the ratio between the average nursing time spent on care for that group compared to a normative group of residents in the same NH.
To convert our PSMS-based ADL impairments, disability of 4 or 5 (extensive or greater assistance) converted to 2 ADL-points in the Arling method, and disability of 2–3 (some assistance) were converted to 1 ADL-point. A patient’s ADL score using the Arling method is the sum of all ADL-points, ranging from 0–12.
The Arling groups (with their corresponding relative nursing utilization weights) were distinguished by:
Group 1 (1.79 weight): Severe functional impairment (9+ ADL-points) AND (Device OR wound)
Group 2 (1.49 weight): Severe functional impairment (9+ ADL-points), AND absence of device/wound.
Group 3 (1.25 weight): Moderate-severe impairment (7+ ADL-points) AND completely dependent for feeding
Group 4 (0.95 weight): Moderate-severe impairment (7+ ADL-points) but NOT completely dependent for feeding
Group 5 (0.77 weight): Mild-moderate impairment (5–6 ADL-points)
Group 6 (0.46 weight): Mild or no impairment (< 5 ADL-points)
We simplified criteria for Groups 1 and 2, e.g., presence of any wound, whereas the original criteria specified daily wound care.16 Thus, we first assigned a participant to a group based on the modified Arling criteria at baseline, then assigned the mean relative resource points for that patient’s group.
Our outcome variables were based on the results of MDRO cultures obtained at baseline and all follow-up visits. Positive colonization for any of the three MDROs studied (MRSA, R-GNB, or VRE) was defined as a positive culture at any anatomic site (nares, oropharynx, groin, perianal area, and any wounds or device) for that organism. Prevalent colonization was defined as positive culture at the baseline visit. A new acquisition was defined as positive colonization at a follow-up visit and negative at all sample sites the time before. Thus, we could measure time (in months) to new asymptomatic colonization with MRSA, R-GNB, or VRE as three separate events.
Statistical Analysis
First, we performed a patient-level cross-sectional observational analysis of prevalent MDROs (i.e., positive MRSA, R-GNB, or VRE culture found at the baseline as separate outcomes) using unadjusted logistic regression, using all candidate variables, including the Arling scale, as univariate predictors.
Next, we used monthly surveillance samples to perform a longitudinal survival analysis to assess time (in months) to MDRO acquisition. Rather than the standard methods of Cox regression, we used parametric survival analysis, which requires determination of the functional form of the hazard of event. A parametric analysis allows for an estimate of the mean time-to-event, or “life expectancy” in MDRO-free months, representing when half of the sample is expected to acquire an MDRO.
In addition, we applied a repeated-failure model, which allowed us to estimate time to re-colonization in participants who were colonized transiently. 18, 19 Application of this methodology is novel to the infection prevention literature. The approach captures the dynamic nature of MDRO transmission (transient versus persistent) in the NH setting. In traditional time-to-first event survival analysis, valuable surveillance data for patients who are intermittently colonized are discarded after the first occurrence. In our study, participants who lose positive MDRO status across all of their cultured anatomic locations for two consecutive months can re-enter the analysis and be at risk for intermittent (i.e., recurrent) colonization.
To assure that we did not misclassify a low-level of colonization as intermittent colonization, we employed two additional criteria as safeguards. First, participants with MDROs at baseline (who were not eligible for initial acquisition) could become eligible for recurrent acquisition after we obtained negative cultures at all anatomic sites for two consecutive visits. Second, we conservatively considered participants as persistently colonized (ineligible for re-colonization) if we found an isolated negative culture between two positive cultures or if the last culture prior to study exit was an isolated negative culture.
We first examined, and found, that the cumulative baseline hazard monotonically increased for the acquisition of MDROs over time, thus allowing for parametric survival analysis. Our data best fit a log-logarithmic distribution using the lowest Akaike information criterion. This allowed us to express results as a accelerated failure time survival model20:
where T is time in months to MDRO acquisition (MRSA, R-GNB, or VRE), α1 is the acceleration time factor (a multiplicative factor of time-to-event per unit of X), and ε is the error term with the log-logarithmic distribution. Like a hazard ratio, an acceleration time factor (or TF) less than 1 represents a shorter time-to-event (greater risk). The cumulative baseline hazard of acquiring a recurrent MDRO (second, third, or fourth) acquisition was greater than and steeper over time than for initial MDRO acquisition. Therefore, our model considered a different baseline hazard by initial versus recurrent acquisition.
For all three MDROs, we tested the unadjusted effect of severity of impairment (six specific ADLs and the Arling scale), baseline age, gender, presence of indwelling device, and time-varying predictors (hospitalization, recent antibiotic use, and presence of wound/pressure ulcer within 30 days) on prevalent MDRO and time to MDRO acquisition. We focused on the Arling scale as the composite measure of interest, introducing multivariable controls for the presence of antibiotics, hospitalizations, and comorbidity based on prior evidence.1, 8, 10
RESULTS
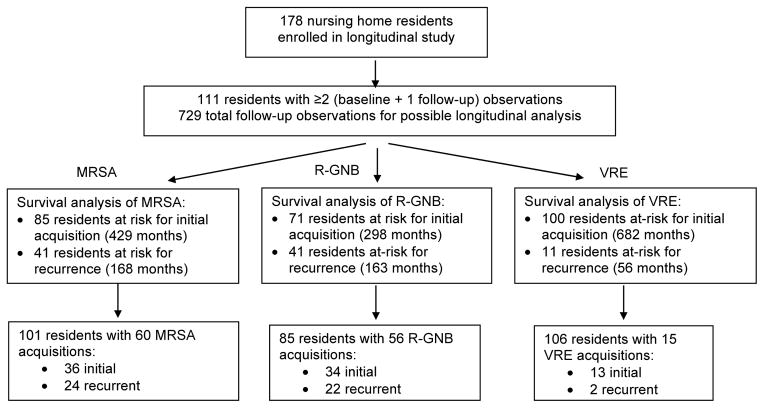
Of the 178 NH participants in our original study, we conducted this longitudinal analysis on the 111 participants with ≥ 2 visits (729 visits total). The number of visits (baseline plus monthly visits) per participant ranged from 2 to 13, and the mean number of samples collected was 6 (Table 1). The mean participant age for the analytic sample was 81 years; 20% had a wound. The baseline ADLs of the participants, along with the nursing resource utilization information is detailed in Table 1. The flow of data is shown in Figure 1.
Table 1.
Baseline characteristics of the longitudinal analysis sample (n=111)
| Characteristic | Mean (±SD) or number of residents (% of sample) | Range | |
|---|---|---|---|
| Age, in years | 82 (±11) | 43 - 103 | |
| Male | 32 (29%) | ||
| Number of specimens collected per resident | 6.1 (±5.9) | 2–13 | |
| Number of residents MDRO positive at baseline (prevalent MDRO) | MRSA | 26 (23%) | |
| R-GNB | 40 (36%) | ||
| VRE | 11 (10%) | ||
| Number of residents acquiring ≥ 1 new colonization within 12 month study | MRSA | 48 (43%) | |
| R-GNB | 43 (39%) | ||
| VRE | 14 (13%) | ||
| Mean number of MDRO acquisitions per resident during the 12-month study | MRSA | 0.54 (±.71) | 0–3 |
| R-GNB | 0.50 (±.71) | 0–3 | |
| VRE | 0.14 (±.37) | 0–2 | |
| Number of residents with at least one device (feeding or urinary tube) at baseline | 39 (35%) | ||
| Number of residents with wounds or pressure ulcer (< 30 days) at baseline | 22 (20%) | ||
| Number of residents with recent (< 30 day) use of any antibiotic at baseline | 48 (43%) | ||
| Number of residents with a recent hospitalization (< 30 days) at baseline | 27 (24%) | ||
| Charlson Comorbidity Index | 2.5 (1.5) | 0–8 | |
| Mean ADL impairment at baseline, defined as in the PSMS (range: 1–5, higher = more severe) | Bathing | 3.6 (±.8) | 2–5 |
| Toileting | 3.8 (±1.3) | 1–5 | |
| Feeding | 2.7 (±1.4) | 1–5 | |
| Dressing | 3.6 (±1.0) | 1–5 | |
| Grooming | 3.4 (±1.0) | 1–5 | |
| Ambulation | 3.6 (±0.8) | 1–5 | |
| Relative need for nursing care (greater points16 indicate greater need, relative to a “standard” patient with a weight of 1)* | Group 6 (0.46 weight) | 9% (8%) | |
| Group 5 (0.77 weight) | 17% (15%) | ||
| Group 4 (0.95 weight) | 41 (37%) | ||
| Group 3 (1.25 weight) | 8 (7%) | ||
| Group 2 (1.49 weight) | 0 (0%) | ||
| Group 1 (1.79 weight) | 36 (32%) | ||
PSMS = Lawton and Brody Physical Self-Maintenance Scale
ADL = Activity of Daily Living
The criteria for group 1 and 2 were simplified from the original work by Arling and associates.
Figure 1. Flow of data from original monthly culture dataset to survival analysis datasets.
This figure describes the flow of data from the 178 NH residents in our original study to our longitudinal analysis on 111 residents. The analytic dataset for the survival analysis is specific to each organism. Residents who are persistently colonized throughout the entire study with that organism cannot be at risk for acquisition of that organism. Once positive for an organism, a resident with continued positivity also stops contributing time at risk. If a resident becomes negative for at least 2 consecutive cultures, he/she can re-enter the study and contribute to time at risk for recurrence.
MRSA= methicillin-resistant Staphylococcus aureus
R-GNB = antibiotic-resistant (ceftazidime or ciprofloxacin) Gram-negative bacteria
VRE = vancomycin-resistant enterococci
Predictors of Prevalent MDRO Colonization
All participants regardless of baseline colonization status were included in the initial analysis of prevalent MDRO colonization. Several characteristics predicted prevalent MDRO colonization in our NH setting: ADL dependence, presence of indwelling device, recent wound or pressure ulcer, recent antibiotic use, and recent hospitalization at baseline. More specifically and as expected, the 10 participants with prevalent MRSA colonization (compared to the 101 who were not colonized at baseline) were more likely to have a higher score on the Arling scale (1.6 vs. 1.1, P<. 01), an indwelling device (70% vs. 32%, P<.02), recent hospitalization (70% vs. 20%, P<.01), and have a wound/pressure ulcer (50% vs. 17%, P<.02), They also needed greater help with bathing (severity score 4.2 vs. 3.5, P<.02), enteral feeding tube (severity score 3.8 vs. 2.6, P<.02), dressing (severity score 4.3 vs. 2.6, P<.02), and grooming (severity score 4.1 vs. 3.4, P<.05). When comparing 26 participants with prevalent R-GNB colonization (compared with the 85 not colonized at baseline), participants with prevalent R-GNB colonization were more likely to have an indwelling device (58% vs. 28%, P<.01), higher Arling score (1.4 vs. 1.1, P<. 01), greater need for help with walking (severity score 4.0 vs. 3.5, P<.01), recent antibiotic use (65% vs. 36%, P<.01), a recent hospitalization (54% vs. 15%, P<.001), and presence of wound/pressure ulcer (35% vs. 15%, P<.05). Similarly, five participants with prevalent VRE colonization (compared to the 106 residents without) were more likely to have an indwelling device (80% vs. 30%, P<.05), recent antibiotic use (100% vs. 41%, P<.01), and recent hospitalization (100% vs. 21%, P<.01). These results demonstrated that individual risk factors as well as the composite Arling scale were consistently predictive of prevalent MDRO colonization.
Time to New and Recurrent MDRO Acquisition
There were 60 instances of MRSA acquisition in the participants, 56 of R-GNB and 15 of VRE (Figure 1). The predicted time-to-acquisition (50th percentile residents to become colonized) for both MRSA and R-GNB was less than one year: initial MRSA 7.6 months, recurrent 4.6, overall (either initial or recurrent acquisition) 6.7 months; initial R-GNB 5.1, recurrent 4.5, and overall 4.9 months. Because there were so few acquisitions of VRE, the median predicted time-to-acquisition exceeded 12 months: 42.0 months for initial, 13.2 months for recurrence, and 39.7 months overall.
The unadjusted analyses (Table 2) showed that severity of walking, feeding, and toileting disability is associated with shorter time to initial R-GNB acquisition. In addition, walking disability is associated with shorter time to initial VRE acquisition. Hospitalization, wounds/pressure ulcers, and antibiotic use in the past 30 days are also associated with shorter time to initial VRE acquisition. A hospitalization had occurred within 30 days of only 3%, 6%, and 23% of the new acquisitions of MRSA, R-GNB, and VRE, respectively (not shown). On univariate analysis of time to recurrent acquisition (not displayed), none of the variables (including the Arling score) were significant except for age (greater time to MRSA acquisition, P<.04), comorbidity (less time to R-GNB acquisition, P<.05), and feeding dependency (less time to VRE acquisition, P<.02).
Table 2.
Unadjusted acceleration time factor survival models predicting months to initial MDRO acquisition
| Outcome (Time to event) | Initial MRSA acquisition | Initial R-GNB acquisition | Initial VRE acquisition | ||||
|---|---|---|---|---|---|---|---|
| Time at risk: 429 months | Time at risk: 298 months | Time at risk: 682 months | |||||
| Residents: 85 | Residents: 71 | Residents: 100 | |||||
| New Initial Acquisition events: 36 | New Initial Acquisition events: 34 | New Initial Acquisition events: 13 | |||||
| Predictors (unit of analysis) | TF | 95%CI | TF | 95% CI | TF | 95% CI | |
| Age (per year) | 1.00 | (0.97, 1.03) | 0.97 | (0.93, 1.00) | 0.99 | (0.94, 1.04) | |
| Gender (male) | 1.51 | (0.69, 3.31) | 3.18 | (1.20, 8.39) | 0.77 | (0.23, 2.55) | |
| Arling score (need for nursing care, per point in relative weight) | 0.33 | (0.17, 0.64) | 0.52 | (0.21, 1.27) | 0.24 | (0.07, 0.87) | |
| Individual ADLs (level of impairment, range 1–5) | Feeding | 0.81 | (0.64, 1.02) | 0.75* | (0.58, 0.98) | 0.73 | (0.50, 1.09) |
| Bathing | 0.80 | (0.55, 1.15) | 0.64 | (0.41, 1.00) | 0.62 | (0.30, 1.30) | |
| Dressing | 0.74 | (0.54, 1.01) | 0.71 | (0.50, 1.02) | 0.58 | (0.28, 1.23) | |
| Grooming | 0.81 | (0.61, 1.07) | 0.77 | (0.55, 1.10) | 0.75 | (0.41, 1.39) | |
| Walking | 0.77 | (0.52, 1.15) | 0.48* | (0.30, 0.77) | 0.5 | (0.23, 1.00) | |
| Toileting | 0.79 | (0.62, 1.00) | 0.65* | (0.50, 0.84) | 1.00 | (0.67, 1.50) | |
| Device (any vs. none) | 0.33 | (0.18, 0.59) | 0.96 | (0.38, 2.41) | 0.58 | (0.18, 1.90) | |
| Wound/ulcer (any in 30 days vs. none) | 0.43 | (0.17, 1.08) | 2.18 | (0.27, 17.86) | 0.15* | (0.03, 0.69) | |
| Comorbidity (0–8 points) | 1.01 | (0.82, 1.25) | 0.99 | (0.76, 1.30) | 0.75 | (0.52, 1.07) | |
| Hospitalization (any in 30 days vs. none) | 1.18 | (0.22, 6.23) | 0.43 | (0.10, 1.87) | 0.11* | (0.02, 0.55) | |
| Antibiotics (any in 30 days vs. none) | 0.85 | (0.40, 1.83) | 0.61 | (0.23, 1.61) | 0.20* | (0.05, 0.74) | |
TF = acceleration time factor, interpreted similarly to a hazard ratio in that a value < 1 is protective, while > 1 represents increased risk.
95% CI = 95% confidence intervals for acceleration time factor
MDRO=multi-drug resistant organism
MRSA= methicillin-resistant Staphylococcus aureus
R-GNB = antibiotic (ceftazidime or ciprofloxacin) resistant Gram-negative bacteria
P<.05
Example interpretation of acceleration time factor: The time to acquisition of R-GNB is reduced by 0.48 times for a resident with complete dependence in walking (level 4) compared to moderate dependence in walking (level 3).
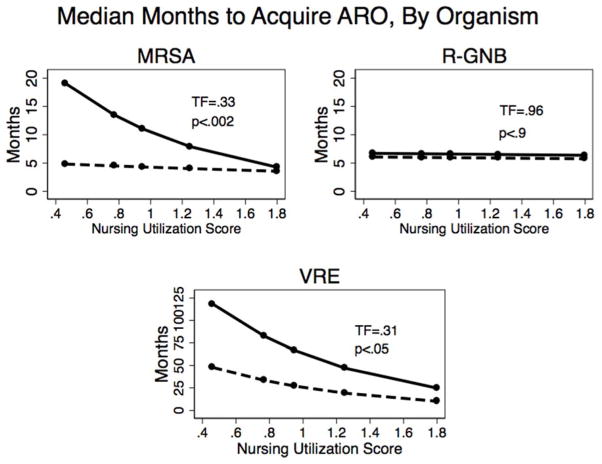
As hypothesized, we found that higher nursing resource utilization is correlated with a shorter time to MRSA and VRE acquisition. On multivariable analysis, greater need for overall nursing by the Arling score was independently associated with earlier MRSA and VRE acquisition, independent of antibiotic use, hospitalization, or co-morbidity. Our predicted time to MDRO acquisition per additional point on the scale was TF=.33 (95% CI=.16– .66) for MRSA acquisition and TF=.31 (95% CI = .10– .95) for VRE acquisition (Figure 2). Our predicted time to initial MRSA acquisition for the lowest versus highest need for nursing care was 20 months versus 5 months, whereas the time to a re-acquisition event did not vary by the Arling score and was, on average, 5 months (Figure 2). We could not predict the time to R-GNB colonization using the Arling score.
Figure 2. Greater need for nursing care predicts shorter time to acquisition of MRSA and VRE but not R-GNB.
Graphical display of expected time to MDRO acquisition for MRSA (methicillin-resistant) Staphylococcus aureus, R-GNB (resistant gram-negative bacteria) and VRE (vancomycin-resistant enterococcus) using accelerated failure time model stratified by initial versus recurrent acquisition event. The y-axis displays the predicted number of months to acquisition for initial (solid line) and recurrent (dashed line) MDRO colonization. Residents’ baseline need for nursing resources16 (an ordinal variable assigned with relative weights, where a higher score = more need for care), expressed as a continuous variable in this analysis, is indicated on the x-axis. Solid circles represent the predictions for nursing resource utilization groups. All predicted time to events are controlled for wounds/pressure ulcers (within 30 days, time-varying), hospitalizations (within 30 days, time-varying), antibiotic use (within 30 days, time-varying), and comorbidity. For example, a resident with the least intense nursing requirement (0.46 relative weight) would be expected to acquire a new MRSA colonization in 20 months, versus 5 months for residents needing the most intense nursing (1.8 relative weight).
DISCUSSION
In this prospective longitudinal study of NH residents under active surveillance for MDRO acquisition, we found that the Arling scale, a simple and validated clinical assessment of nursing need based on severity of ADL impairment, and presence of indwelling devices and wounds/pressure ulcers16 predicted residents with pre-existing MDRO colonization. Second, we showed that the mean time to new MDRO acquisition was less than one year for MRSA and R-GNB organisms. Third, after controlling for known risk factors, such as recent hospitalizations and antibiotic use, we found that residents with greater nursing need, which we quantified using the Arling scale, had shorter time to MRSA and VRE acquisition.
Colonization by MRSA21, 22 or VRE22–24 is associated with infection by that organism, although less so for R-GNB.22, 25 Our results suggest potential strategies to prevent MRSA and VRE acquisition in NHs. From a patient-perspective, we should target residents at greater risk, whenever that risk arises. From the facility perspective, our results suggest acquisition occurs within 6 months of observation, therefore underscoring the urgency of implementing surveillance and prevention efforts by identifying high-risk patients early in the NH stay.
A prior study of NH residents has shown that limited mobility and functional disability are associated with higher asymptomatic MRSA prevalence.11 Functional disability has also been associated with high-level gentamicin-resistant enterococcal colonization.10 Furthermore, it has been shown that assistance in more than three ADLs increases the risk for MRSA surgical site infections among older hospitalized adults.6 Using active surveillance in a cross-sectional study of NH residents, we have shown a dose-response relationship between functional disability and R-GNB colonization.8
While prior research has identified separate risk factors, we used a parsimonious composite measure (the Arling scale) that reflects both risk factors for MDROs and assigns greater weight to individuals who require more nursing contact time. Our results suggest that greater and more intense contact with healthcare providers, especially NH residents with severe ADL impairment and with potential entry points such as indwelling devices and wounds,16 increases the risk of MDRO colonization. There residents are at heightened risk of MDRO colonization, and therefore should be targeted for enhanced barrier precautions.
While we were not adequately powered to study whether certain individual ADLs predispose to MDRO acquisition, we found that dressing and toileting, ADL care that requires both skin-to-skin contact with the care provider and contact with residents’ bacteria-rich skin regions, are predictors of new MRSA acquisition. In older hospitalized adults with surgical site infections, it has been shown that assistance in bathing or dressing increases the risk for MRSA surgical site infections.6 Although it is probable that contact-intense ADL dependency reflects underlying severity of illness, we speculate that specific ADL dependency increases opportunity for pathogen transmission from healthcare workers to residents and vice versa.
The Arling scale did not differentiate time-to-acquisition for R-GNB. Perhaps one mechanism may be that R-GNB is more limited to the genitourinary tract, and therefore, the reason its acquisition was associated with only severe toileting dependency rather than the Arling scale, which represents overall nursing contact intensity. Also, we speculate that combining different organisms and patterns of resistance as a single R-GNB may have weakened relationships between risk factors with transmission in comparison to VRE and MRSA. Future studies should be designed to identify epidemiologically significant R-GNB species that are likely to be transmitted to other frail NH residents or cause infections in this population.
Our prior research8, 11 considered poor function and MDRO acquisition in a cross-sectional manner and reported time-to-colonization among only colonized residents. In contrast, this research uses survival analysis to predict each resident’s MDRO-free time at enrollment, or the number of months expected for the 50th percentile resident to acquire an MDRO. In future surveillance research, a delay in time-to-acquisition should be considered as an outcome measure of successful infection prevention.
We identified several avenues for future research. We found less contribution of recent hospitalization to acquisition of MRSA and R-GNB (<10% of new cases) than expected, which leads us to speculate that the facility is the source of the MDROs, facilitated by patient characteristics and need for contact-intense nursing. However, the role of healthcare workers’ assistance, environmental contamination, and interaction with resident-level factors that can lead to new MDRO acquisitions requires further study. Second, future studies should also address whether current infection prevention interventions such as active surveillance and feedback, as well as use of barrier precautions when providing help with contact-intense ADLs leads to reduced MDRO transmission in the NH setting. A single NH-study 13 found that pre-emptive gowning and gloving of all residents prevents transmission of Klebsiella pneumonia from resident-to-resident. A trial including preventive barrier intervention for residents with indwelling devices at 12 NHs is now underway.26, 27 A third future direction would be to use the Resource Utilization Group (RUG) scores, which are routinely collected by NH, rather than research, staff as a way to stratify contact-intense patients who should receive enhanced MDRO and infection prevention efforts.
One weakness of this study was that new VRE acquisition was uncommon, thus limiting our power to detect an effect of most of the predictor variables on new VRE acquisition. Limitations to the current active surveillance methods include potential false-positive errors due to fluctuating levels of colonization rather than true re-colonization. We also did not quantify the actual time spent by healthcare workers when providing assistance with ADLs. Third, our data lacked enough power to include facility-level variables for any of the MDRO models. Thus, our analysis only addressed how patient-level needs contributed to MDRO acquisition. Third, we lacked the sample size to test for the effect of individual ADLs on MDRO acquisition. Future research is needed to examine whether there is facility-level variation in the link between specific ADLs and new MDRO acquisition in NH residents, which could lead to more targeted preventive interventions. Last, the newer RUG scores include other variables than the Arling scale that more accurately predict nursing utilization.17, 28 More detailed variables, e.g., specific rehabilitation services and behavior, that marginally contribute to nursing utilization beyond ADLs (although none more important than ADLs) could be explored in future studies of MRDO acquisition.
The strength of this study is our use of prospective comprehensive monthly collection and microbiological testing of specimens from NH residents for the presence of MDROs, which is crucial for detecting new asymptomatic MDRO colonization. To our knowledge, this is the first report of a comprehensive evaluation of nursing resource utilization, as well as the severity of functional disability and its impact on new MDRO acquisition. The current practice of identifying resistance among NH residents using passive surveillance methods represent just the “tip-of-the-iceberg” of all colonized residents. With our sensitive multi-site, multi-visit surveillance, we were able to show the underlying magnitude of new MDRO colonization in these residents. We also tested for multiple resistance patterns for R-GNB, increasing sensitivity for new MDRO acquisition. Last, we utilized a network of multiple NHs in Southeast Michigan with varying bed size and facility characteristics, thus broadening the potential generalizability of our results.
In conclusion, we identified contact-intense ADLs that were associated with shorter time to MDRO colonization, thus presenting an opportunity for future interventions to prevent MDROs in NH residents.
Acknowledgments
This work was supported by the University of Michigan Claude D. Pepper Center (Min, Mody, Galecki), the VA Healthcare System Geriatric Research Clinical Care Center (Min and Mody), Hartford Foundation (Min), NIH R01AG032298 (Mody, Galecki), and AHRQ R18 HS019979 (Mody), NIH-LRP (Min), T Franklin Williams Scholars Program (Mody).
Sponsor’s Role: None.
Footnotes
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts | * Author 1 | Author 2 | Author 3 | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||
| Grants/Funds | X | X | X | |||
| Honoraria | X | X | X | |||
| Speaker Forum | X | X | X | |||
| Consultant | X | X | X | |||
| Stocks | X | X | X | |||
| Royalties | X | X | X | |||
| Expert Testimony | X | X | X | |||
| Board Member | X | X | X | |||
| Patents | X | X | X | |||
| Personal Relationship | X | X | X | |||
Author Contributions: All authors contributed to this paper. Dr. Min had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mody L, Kauffman CA, Donabedian S, et al. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46:1368–1373. doi: 10.1086/586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds C, Quan V, Kim D, et al. Methicillin-resistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect Control Hosp Epidemiol. 2011;32:91–93. doi: 10.1086/657637. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infect Control Hosp Epidemiol. 2010;31:1230–1235. doi: 10.1086/657335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 5.Bonomo RA. Multiple antibiotic-resistant bacteria in long-term-care facilities: An emerging problem in the practice of infectious diseases. Clin Infect Dis. 2000;31:1414–1422. doi: 10.1086/317489. [DOI] [PubMed] [Google Scholar]
- 6.Chen TY, Anderson DJ, Chopra T, et al. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J Am Geriatr Soc. 2010;58:527–532. doi: 10.1111/j.1532-5415.2010.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mody L, Sun R, Bradley SF. Assessment of pneumonia in older adults: effect of functional status. J Am Geriatr Soc. 2006;54:1062–1067. doi: 10.1111/j.1532-5415.2006.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dommeti P, Wang L, Flannery EL, et al. Patterns of ciprofloxacin-resistant gram-negative bacteria colonization in nursing home residents. Infect Control Hosp Epidemiol. 2011;32:177–180. doi: 10.1086/657946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flannery EL, Wang L, Zollner S, et al. Wounds, functional disability, and indwelling devices are associated with cocolonization by methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in southeast Michigan. Clin Infect Dis. 2011;53:1215–1222. doi: 10.1093/cid/cir733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenoweth CE, Bradley SF, Terpenning MS, et al. Colonization and transmission of high-level gentamicin-resistant enterococci in a long-term care facility. Infect Control Hosp Epidemiol. 1994;15:703–709. doi: 10.1086/646841. [DOI] [PubMed] [Google Scholar]
- 11.Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol. 2012;50:1698–1703. doi: 10.1128/JCM.06469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Lansing B, Symons K, et al. Infection rate and colonization with antibiotic-resistant organisms in skilled nursing facility residents with indwelling devices. Eur J Clin Microbiol Infect Dis. 2012;31:1797–1804. doi: 10.1007/s10096-011-1504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trick WE, Weinstein RA, DeMarais PL, et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 2004;52:2003–2009. doi: 10.1111/j.1532-5415.2004.52555.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Arling G, Nordquist RH, Brant BA, et al. Nursing home case mix. Patient classification by nursing resource use. Med Care. 1987;25:9–19. doi: 10.1097/00005650-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Fries BE, Schneider DP, Foley WJ, et al. Refining a case-mix measure for nursing homes: Resource Utilization Groups (RUG-III) Med Care. 1994;32:668–685. doi: 10.1097/00005650-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cleves M. Analysis of multiple failure-time survival data. Stata Technical Bulletin. 1999;49:30–33. [Google Scholar]
- 19.Anderson PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Statistics. 1982;10:1100–1120. [Google Scholar]
- 20.Coppini DV, Bowtell PA, Weng C, et al. Showing neuropathy is related to increased mortality in diabetic patients - a survival analysis using an accelerated failure time model. J Clin Epidemiol. 2000;53:519–523. doi: 10.1016/s0895-4356(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 21.Davis KA, Stewart JJ, Crouch HK, et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39:776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 22.Pacio GA, Visintainer P, Maguire G, et al. Natural history of colonization with vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and resistant gram-negative bacilli among long-term-care facility residents. Infect Control Hosp Epidemiol. 2003;24:246–250. doi: 10.1086/502201. [DOI] [PubMed] [Google Scholar]
- 23.Zaas AK, Song X, Tucker P, et al. Risk factors for development of vancomycin-resistant enterococcal bloodstream infection in patients with cancer who are colonized with vancomycin-resistant enterococci. Clin Infect Dis. 2002;35:1139–1146. doi: 10.1086/342904. [DOI] [PubMed] [Google Scholar]
- 24.Salgado CD, Farr BM. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect Control Hosp Epidemiol. 2003;24:690–698. doi: 10.1086/502271. [DOI] [PubMed] [Google Scholar]
- 25.Lee YL, Cesario T, McCauley V, et al. Low-level colonization and infection with ciprofloxacin-resistant gram-negative bacilli in a skilled nursing facility. Am J Infect Control. 1998;26:552–557. doi: 10.1053/ic.1998.v26.a88774. [DOI] [PubMed] [Google Scholar]
- 26.Mody L, Krein SL, Saint S, et al. TIP Intervention to Reduce Multi-drug Resistance and Infections in Nursing Home Residents with Indwelling Devices: A Cluster-Randomized Trial. J of Am Geriatr Soc. 2014:62. [Google Scholar]
- 27.Mody L, Bradley SF, Galecki A, et al. Conceptual model for reducing infections and antimicrobial resistance in skilled nursing facilities: focusing on residents with indwelling devices. Clin Infect Dis. 2011;52:654–661. doi: 10.1093/cid/ciq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iowa Foundation for Medical Care. Staff Time Resource Intensity Verification (STRIVE) Final Report. 2007 available at https://http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/downloads/STRIVE_Final_Report_Phase_I_Sampling_Methodology.pdf.