Abstract
Background
Recently, we demonstrated that urinary angiotensinogen (AGT) levels are increased and reflect intrarenal renin–angiotensin system (RAS) status in pediatric patients with chronic glomerulonephritis. Therefore, this study was performed to test the hypothesis that urinary AGT (UAGT) levels provide a specific index of intrarenal RAS status associated with RAS blockade treatment in pediatric IgA nephropathy (IgAN) patients.
Methods
We measured plasma and UAGT levels and urinary transforming growth factor beta (TGF-β) levels, after which we performed immunohistochemical analysis of AGT, angiotensin II (Ang II), and TGF-β in 24 pediatric IgAN patients treated with RAS blockades for 2 years. Paired tests were used to analyze the changes from baseline to study end.
Results
Although there was no change in plasma AGT levels, UAGT and TGF-β levels were significantly decreased after RAS blockade, which was accompanied by the expression levels of AGT, Ang II, and TGF-β, as well as the magnitude of glomerular injury. Baseline UAGT levels positively correlated with diastolic blood pressure, urinary protein levels, scores for mesangial hypercellularity, and the expression levels of AGT, Ang II, and TGF-β in renal tissues.
Conclusions
These data indicate that UAGT is a useful biomarker of intrarenal RAS activation, which is associated with glomerular injury during RAS blockade in pediatric IgAN patients.
Keywords: Renin–angiotensin system, Angiotensinogen, IgA nephropathy, RAS blockade, TGF-β
Introduction
Renin–angiotensin system (RAS) activation plays a pivotal role in the pathogenesis of hypertension and chronic kidney disease [1]. Focus on the RAS as the mechanism of renal damage has shifted to the role of tissue RAS in the kidney [2, 3]. Increasing evidence shows that the local RAS in various tissues, including brain [4], heart [5], vasculature [6], and kidneys [3], is independently regulated by the systemic RAS. Angiotensin II (Ang II) is the most powerful biologically active product of the RAS, and abnormal increases in the kidney’s Ang II levels can lead to renal functional damage and renal tissue injury [1]. In the kidney, Ang II is derived from its locally formed substrate, angiotensinogen (AGT) [3]. Experimental studies have demonstrated that AGT levels in renal tissues reflect the activity of intrarenal RAS [1, 7]. A direct quantitative method to measure urinary AGT (UAGT) using human AGT enzyme-linked immunosorbent assays (ELISA) has now been developed [8] that reveals significantly increased UAGT levels in patients with hypertension [9, 10], chronic kidney disease [11, 12], and diabetes [13, 14]. Thus, AGT plays an important role in the development and progression of hypertension and kidney disease.
Although its prevalence varies across different geographical regions, IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. IgAN is diagnosed by kidney biopsy and is defined as dominant or codominant staining with IgA in glomeruli by immunohistology [15]. In pediatric IgAN, a glomerular lesion is characterized by an increased number of mesangial cells accompanied by mesangial extracellular matrix; this pathological change is thought to be an early lesion for IgAN [16]. Our previous study showed an increased expression of AGT in the glomerular endothelial and mesangial cells of IgAN patients [17, 18]. Furthermore, studies also show that treatment with an angiotensin-converting enzyme inhibitor (ACEi) and/or an Ang II type 1 receptor blocker provides antiproteinuric and renoprotective effects in normotensive pediatric patients with IgAN [19, 20]. These results suggest that intrarenal RAS activation plays a critical role in IgAN progression. However, no direct evidence exists showing that UAGT reflects intrarenal RAS status in pediatric IgAN patients. Therefore, we tested the hypothesis that measurements of UAGT level provide a specific diagnostic test for identifying patients with activated intrarenal RAS in IgAN. Additionally, we further hypothesized that UAGT is associated with reduced renal damage in IgAN after RAS blockade.
Material and methods
Participants and study protocol
The experimental protocol for this study was approved by the Institutional Review Board of the University of Tokushima Graduate School. We recruited 24 pediatric patients who were detected by the yearly urine screening programs in Japanese school children and diagnosed with IgAN by clinical course and renal biopsy at the Tokushima University Hospital from 1 April 2008 to 1 November 2013. All study participants with other disorders, including Henoch–Schönlein purpura, systemic lupus erythematosus, chronic liver disease, diabetes mellitus, malignancies, and urinary tract infections were excluded. All participants received and signed a consent form, and when a participant was not capable of providing assent based on age, simple oral explanation of the study was offered and a written parental permission form was obtained. At the time of study enrollment, all patients were asked to undergo repeat renal biopsies for 2 years following the end of RAS blockade (candesartan or benazepril). The dose was 0.2 mg/kg body weight (maximum 4 mg/day) for candesartan and 0.2 mg/kg body weight (maximum 5 mg/day) for benazepril. Clinical parameters, including gender, age, height, body weight, and blood pressure, and laboratory parameters, including serum concentrations of sodium, potassium, creatinine and cystatin C, and urinary concentrations of protein and creatinine were determined at the time of biopsy. Urine collections were combined to calculate the 24-h creatinine clearance (ml/min), which was used as an index of glomerular filtration rate. Plasma renin activity was measured in the blood by radioimmunoassay. Plasma and urinary concentrations of AGT were measured with a human AGT ELISA kit (IBL, Fujioka, Japan), as previously described [12]. Additionally, we performed sandwich ELISA for transforming growth factor beta (TGF-β) with urine in accordance with the manufacturer’s specification (R & D Systems, Minneapolis, MN, USA).
Histological study
For light-microscopic examination, the biopsied tissues were fixed in 10 % buffered formalin and embedded in paraffin. The paraffin sections (3 µm) were stained with periodic acid- Schiff reagent. All glomeruli in each section (usually 10–26) were coded and read by two independent observers blinded to all clinical data. Modifying the previous studies [21, 22], histological changes were scored as follows: Mesangial hypercellularity, 0=none; 1=mild (three to four mesangial cells per peripheral lobule); 2=severe segmental (more than four mesangial cells per peripheral lobule); 3=severe global proliferation);. Mesangial matrix score, 0=none; 1=mild; 2= marked segmental (width of mesangial interspace between capillaries exceeding three mesangial cells; 3=marked global increase). Endocapillary hypercellularity, 0=absence; 1=presence. Interstitial fibrosis, 0=none; 1=<25 %; 2=26–50 %; 3>50 % of the cortical area. Tubular atrophy, 0=none; 1= <25 %; 2=26–50 %; 3=>50 % of the cortical tubules.
Immunohistochemistry
Using formalin-fixed paraffin-embedded renal sections, immunohistochemistry with anti-AGT antibody (H7; IBL), anti- Ang II antibody (H-002-12; Phoenix Pharmaceuticals, Burlingame, CA, USA), or anti-TGF-β antibody (sc-146; Santa Cruz Biotechnology, Dallas, TX, USA) was performed. Sections (3 µm) were incubated with primary antibodies overnight at 4 °C, rinsed, and incubated with biotinylated secondary antibodies (Vector Labs, Burlingame, CA, USA). After rinsing, the sections were incubated with avidin–biotin–peroxidase complex (ABC Elite; Vector Labs), followed by 3,3’-diaminobenzidine (Dojindo, Kumamoto, Japan). Each section was counterstained with Mayer’s hematoxylin (Wako, Tokyo, Japan), dehydrated, and cover-slipped. The fraction of glomeruli occupied by immunoreactive area was determined using the EIS-Elements software (Nikon Corporation, Tokyo, Japan). For each glomerulus, the immunoreactive area (brown) was automatically calculated by the software, and this affected area was, in turn, divided by the total area of the glomerulus. At least six equatorially sectioned glomeruli were examined from each slide.
Statistical analysis
Pearson and Spearman correlation coefficients were used for parametric and nonparametric data, respectively. The Wilcoxon signed-rank test was used to perform paired comparisons before and after RAS blockade. All data are presented as mean ± standard error of the mean (SEM). A value of P<0.05 was considered significant. All computations, including data management and statistical analysis, were performed with JMP software (SAS Institute, Cary, NC, USA) and GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Demographics and laboratory data
Table 1 describes patients’ demographic and baseline laboratory data. Baseline and posttreatment indices in blood pressure and laboratory data are shown in Table 2. Plasma renin activity was significantly increased because RAS blockades suppress the negative feedback inhibition of renin release, and the urinary protein–creatinine ratio (UPro/UCre) was significantly lower at the study end. There was no significant change in either systolic or diastolic blood pressure or in serum concentrations of sodium, potassium or cystatin C, or in creatinine clearance.
Table 1.
Baseline characteristics of 24 patients with immunoglobulin A nephropathy (IgAN)
| Parameters | Data (mean±standard error) |
|---|---|
| Gender (F/M) | 12/12 (n) |
| Age, Year | 12.67±0.82 |
| Height, cm | 149.18±4.25 |
| BW, kg | 43.25±2.76 |
| BMI | 18.82±0.57 |
| Serum Cre, mg/dl | 0.52±0.04 |
F females, M males, BW body weight, BMI body mass index, Cre creatinine
Table 2.
Changes in blood pressure and laboratory data
| Parameters | Baseline N=24 | Posttreatment N=24 | Change | P values |
|---|---|---|---|---|
| SBP, mmHg | 109.67±1.75 | 108.08±1.58 | −1.58±1.91 | 0.1946 |
| DBP, mmHg | 59.54±1.84 | 62.00±1.55 | 2.46±2.15 | 0.2480 |
| Serum Na, mEq/L | 139.83±0.29 | 140.42±0.31 | 0.58±0.33 | 0.0720 |
| Serum K, mEq/ | 4.19±0.08 | 3.97±0.05 | −0.22±0.10 | 0.0584 |
| PRA, ng/ml/h | 2.73±0.30 | 18.18±4.01 | 15.45±4.03 | < 0.0001* |
| UPro/UCre, g/g | 0.47±0.06 | 0.05±0.01 | −0.42±0.06 | < 0.0001* |
| CCr, ml/min | 108.33±3.10 | 113.97±4.70 | 5.63±6.13 | 0.2626 |
| Cystatin C, mg/L | 0.76±0.02 0 | 0.74±0.02 | −0.02±0.01 | 0.1777 |
SBP systolic blood pressure, DBP diastolic blood pressure, Na sodium, PRA plasma renin activity, UPro/UCre urinary protein-creatinine ratio, CCr creatinine clearance
P<0.05
Plasma and urinary AGT, urinary TGF-β levels, and histological findings
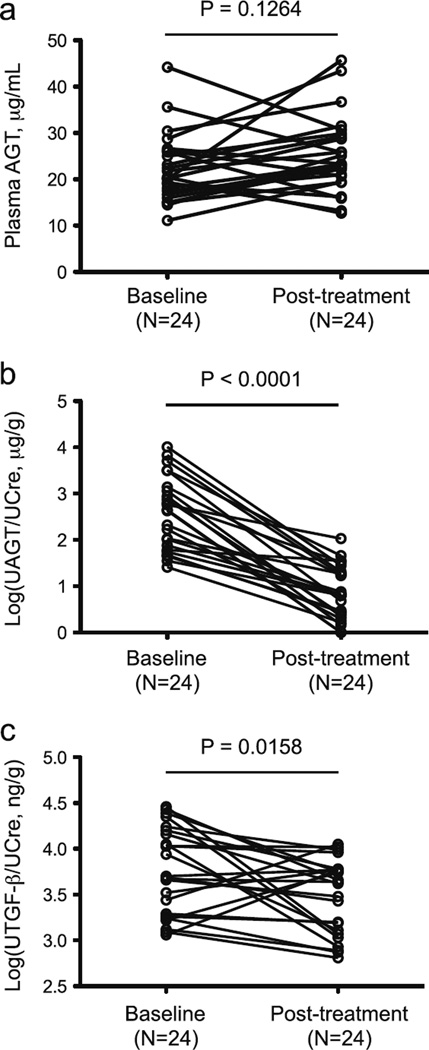
Figure 1 illustrates plasma AGT levels (Fig. 1a), logarithmically transformed UAGT/creatinine ratio (Log[UAGT/UCre]) (Fig. 1b), and logarithmically transformed urinary TGF-β/creatinine ratio (Log[UTGF-β/UCre]) (Fig. 1c). While there was no significant change in plasma AGT between baseline and posttreatment (22.20±1.51 vs. 25.21±1.71 µg/ml), the Log(UAGT/UCre) levels were significantly decreased at the study end (2.57±0.16 vs. 0.82±0.13 µg/g). There were also significant decreases in Log(UTGF-β/UCre) levels in patients treated with RAS blockade (3.76±0.10 vs. 3.48±0.08 ng/g). Scores for mesangial hypercellularity, mesangial matrix, and glomerular expression levels of AGT, Ang II, and TGF-β in renal tissue were lower at the study end compared with baseline (Table 3, Fig. 2).
Fig. 1.
Changes in plasma angiotensinogen (AGT) in pediatric patients with immunoglobulin A nephropathy (IgAN) after renin–angiotensin system (RAS) blockade (a). Changes in logarithmically transformed urinary AGT/urinary creatinine ratio (Log[UAGT/UCre]) in pediatric patients with IgAN after RAS blockade (b). Changes in logarithmically transformed urinary transforming growth factor beta (TGF-β)/creatinine ratio (Log[UTGF-β/UCre]) in pediatric patients with IgAN after RAS blockade (c). Each marker and line represents an individual participant in the study
Table 3.
Changes in histological findings
| Parameters | Baseline N=24 | Posttreatment N=24 | Change | P values |
|---|---|---|---|---|
| Mesangial hypercellularity | 1.07±0.08 | 0.60±0.05 | −0.48±0.09 | < 0.0001 |
| Mesangial matrix score | 1.04±0.07 | 0.80±0.08 | −0.25±0.10 | 0.0205 |
| Endocapillary hypercellularity | 0.13±0.07 | 0.02±0.02 | −0.10±0.06 | 0.0961 |
| Interstitial fibrosis | 0.08±0.06 | 0.15±0.07 | 0.06±0.10 | 0.6875 |
| Tubular atrophy | 0.10±0.06 | 0.25±0.09 | 0.15±0.08 | 0.0625 |
| AGT expression | 2.74±0.40 | 0.63±0.05 | −2.10±0.40 | < 0.0001* |
| Ang II expression | 0.63±0.04 | 0.33±0.02 | −0.30±0.04 | < 0.0001* |
| TGF-β expression | 0.89±0.07 | 0.34±0.03 | −0.54±0.07 | < 0.0001* |
AGT, angiotensinogen, Ang II, angiotensin II, TGF-β, transforming growth factor-β
P<0.05
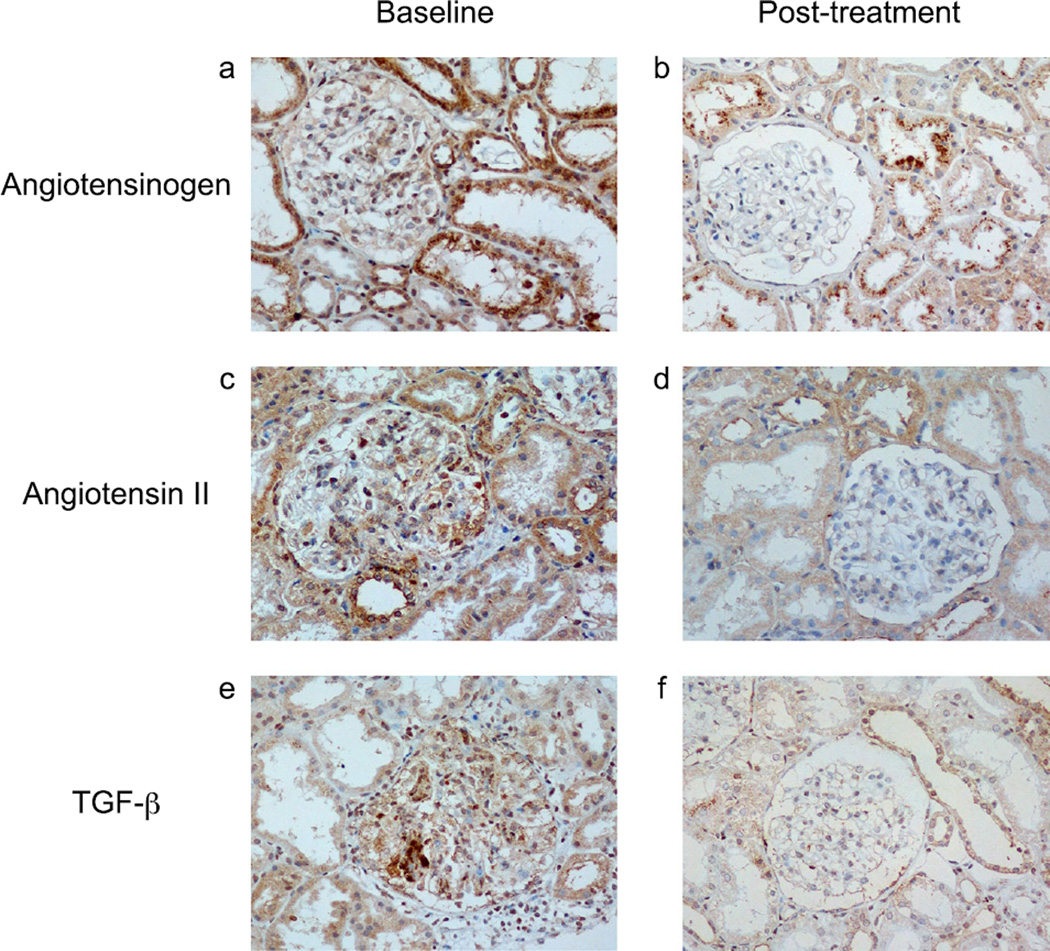
Fig. 2.
Representative images of angiotensinogen (AGT) (a, b), angiotensin II (Ang II) (c, d), and transforming growth factor beta (TGF-β) (e, f) in pediatric patients with immunoglobulin A nephropathy (IgAN) at baseline (a, c, e) vs. posttreatment (b, d, f). Renin–angiotensin system (RAS) blockade decreased immunoreactivity of AGT, Ang II, and TGF-β in renal tissues from pediatric patients with IgAN
Single regression analysis
Table 4 demonstrates the single-regression analyses for Log(UAGT/UCre) with clinical parameters. Log(UAGT/UCre) levels are significantly positively correlated with diastolic blood pressure, UPro/UCre, scores for mesangial hypercellularity, and glomerular expression levels of AGT, Ang II, and TGF-β in renal tissue. However, Log(UAGT/UCre) levels were not correlated with age, height, body weight, body mass index (BMI), systolic blood pressure, serum sodium levels, serum potassium levels, plasma renin activity, creatinine clearance, plasma AGT levels, scores for mesangial matrix, or interstitial fibrosis and tubular atrophy.
Table 4.
Urinary angiotensinogen (UAGN) and correlation in patients with immunoglobulin A nephropathy (IgAN)
| Parameters | R values | P values |
|---|---|---|
| Age | 0.3835 | 0.0843 |
| Height | 0.3084 | 0.1425 |
| BW | 0.1140 | 0.5957 |
| BMI | 0.2483 | 0.2420 |
| SBP | 0.1570 | 0.4638 |
| DBP | 0.5010 | 0.0126* |
| Serum Na | 0.2615 | 0.2171 |
| Serum K | 0.1008 | 0.6392 |
| PRA | 0.0933 | 0.6644 |
| UPro/UCre | 0.4591 | 0.0240* |
| CCr | 0.1197 | 0.5776 |
| Plasma AGT | 0.0873 | 0.6849 |
| Mesangial hypercellularity | 0.4904 | 0.0150* |
| Mesangial matrix score | 0.0195 | 0.9278 |
| Interstitial fibrosis | 0.2858 | 0.1758 |
| Tubular atrophy | 0.3080 | 0.1432 |
| AGT expression | 0.5772 | 0.0031* |
| Ang II expression | 0.6854 | 0.0023* |
| TGF-β expression | 0.5621 | 0.0043* |
BW body weight, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, PRA plasma renin activity, UPro/UCre urinary protein-creatinine ratio, CCr creatinine clearance, AGT angiotensinogen, Ang II angiotensin II, TGF-β transforming growth factor-β
P<0.05
Multiple regression analysis
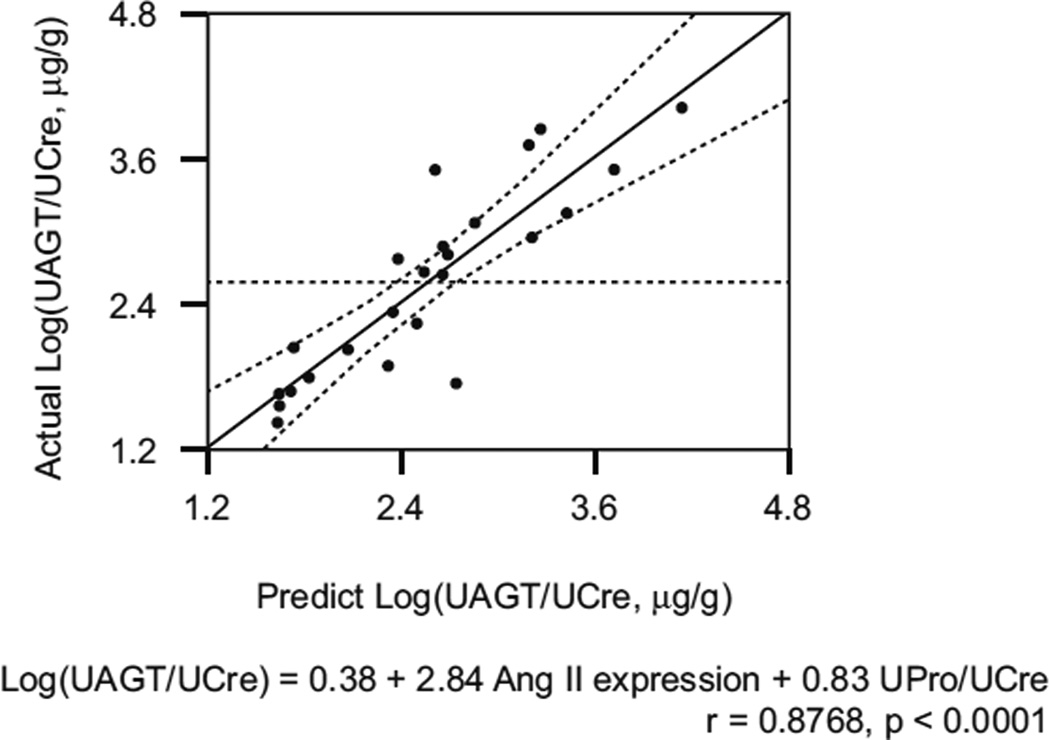
Factors with significant single correlation were adopted as explanatory variables in multiple regression analysis. As shown in Fig. 3, the expression level of Ang II in renal tissue and UPro/UCre ratio can account for 76.88 % of the variation in the Log(UAGT/UCre) (r=0.8768, R2=0.7688, P<0.0001).
Fig. 3.
Multiple regression analysis for logarithmically transformed urinary angiotensinogen (AGT)/urinary creatinine ratio (Log[UAGT/UCre]) in pediatric immunoglobulin A nephropathy (IgAN) patients. Only two parameters—angeotensin II (Ang II) expression levels by immunohistochemical study and urinary protein/UCre ratio [UPro/UCre])—accounted for 76.88 % of the variation in the Log(UAGT/UCre) (r=0.8768, R2=0.7688, P<0.0001)
Discussion
Recent studies have demonstrated the involvement of UAGT levels in RAS activation and the development of cardiovascular and kidney disease [1, 7]. In human studies, a link has been established between UAGT and hypertension/renal damage [1, 7]. However, UAGT excretion and renal AGT expression associated with the pathophysiology of renal injury has not been studied extensively in humans, especially in pediatric patients with IgAN. For the first time, we demonstrated that UAGT and TGF-β levels, as well as renal AGT, Ang II, and TGF-β expression were decreased in pediatric IgAN patients treated with RAS blockade. We also demonstrated that UAGT levels positively correlated with diastolic blood pressure, urinary protein levels, scores for mesangial hypercellularity, and expression levels of AGT, Ang II, and TGF-β in renal tissue.
Intrarenal RAS activation during IgAN progression has been suggested, which is largely based on the ability of RAS blockade to reduce proteinuria [19, 20]. We demonstrated that renal AGT expression is elevated in patients with IgAN [17, 23, 24]. Furthermore, UAGT levels are correlated with the degree of proteinuria and reflect intrarenal AGT expression in patients with IgAN [25, 26]. In the study reported here, UAGT levels and glomerular AGT expression levels decreased after RAS blockade. Additionally, correlation analysis provided some important findings: urinary AGT levels positively correlated with glomerular expression levels of AGT and Ang II in renal tissue, as well as with scores for mesangial hypercellularity. Multiple regression analysis showed that UAGT levels were coupled with expression levels of Ang II and urinary protein excretion. These data are compatible with former reports and indicate that intrarenal RAS is activated through local augmentation of AGT production.
We previously tested the hypothesis that UAGT levels reflect intrarenal RAS status in chronic glomerulonephritis during childhood [12]. We found that UAGT levels were significantly increased in patients with chronic glomerulonephritis who were not treated with RAS blockers compared with control individuals. Notably, patients with glomerulonephritis treated with RAS blockers showed a marked attenuation of this augmentation. Thus, the efficacy of RAS blockade in reducing intrarenal RAS activity can be confirmed by UAGT measurements in patients with chronic glomerulonephritis during childhood [12]. We further reported that UAGT levels were significantly increased in patients with juvenile type 1 diabetes who showed no microalbuminuria, compared with controls, while plasma AGT levels were not increased [13]. These data indicate that increased UAGT levels precede increased urinary albumin levels, which may be one of the earliest predictors of intrarenal RAS activation in juvenile type 1 diabetes. These findings, along with the findings reported here, suggest that UAGT can be a novel biomarker of intrarenal RAS activation in pediatric patients.
Although most circulating AGT is produced and secreted by the liver, it is also produced by the kidneys [7]. Messenger RNA (mRNA) and AGT protein have been localized to proximal tubular cells, and intratubular Ang II can be derived from locally formed and secreted AGT [27]. The AGT produced in proximal tubule cells appears to be secreted directly into the tubular lumen, in addition to producing its own metabolites intracellularly and secreting them into the tubular lumen [28]. Proximal tubular AGT concentrations in anesthetized rats have been reported to range from 300 to 600 nM, which greatly exceeds free Ang I and Ang II tubular fluid concentrations [3]. Because of its molecular size (50–60 kDa), it seems unlikely that much plasma AGT filters across the glomerular membrane, further supporting the concept that proximal tubular cells secrete AGT directly into the tubules [29]. To determine whether circulating AGT is a source of UAGT, glomerular permeability of AGT was examined by multiphoton imaging [30]: Glomerular permeability of injected exogenous AGT was extremely low. By contrast, urinary excretion of endogenous AGT was significantly increased. These results indicate that the majority of UAGT originated from the kidney rather than from glomerular filtration [30]. Consistent with this concept, the study reported here found that plasma AGT levels did not differ between baseline and posttreatment despite significant differences in UAGT levels. Furthermore, we previously revealed that cultured glomerular cells produced both AGT mRNA and protein [17, 31]. Therefore, it seems highly likely that AGT in urine originates from AGT in the kidney, not from AGT in plasma.
It is well known that intrarenal RAS activation is a major mediator in the pathogenesis of progressive glomerular injury [32–35]. Ang II induces mesangial cell activation, and former studies have shown that the degree of mesangial cellularity is associated with a high sensitivity to the beneficial effects of RAS blockade on glomerular structure and proteinuria [36, 37]. Minutolo et al. suggested that high intrarenal Ang II production contributed to the proliferation of mesangial cells [38]. In our study, glomerular Ang II levels were correlated with mesangial hypercellularity score (r=0.4303, P=0.0358). Additionally, we clarified that UAGT levels are associated with glomerular Ang II levels and mesangial hypercellularity score; RAS blockade consistently reduced those levels. Therefore, we believe that UAGT is a useful marker for monitoring changes in intrarenal RAS activity associated with glomerular injury in pediatric IgAN patients.
TGF-β is a major profibrotic factor that plays a key role in the progression of glomerular injury [39]. Moreover, Ang II is the key inducer for TGF-β [40, 41]. In the study reported here, RAS blockade reduced urinary and glomerular expression levels of TGF-β associated with reduced glomerular Ang II and UAGT levels, which suggests the mitigation of intrarenal RAS activation. Based on these findings, it is suggested that overexpression of TGF-β induced by intrarenal RAS activation contributed to the development of glomerular injury in pediatric patients with IgAN.
Our relatively small sample size is a potential limitation to this study. For instance, patients with nephrotic proteinuria were not enrolled because all participants were detected by school urinary screening and were identified in the early stages of the disease. Therefore, it is difficult to subdivide patients according to urinary protein level or pathology pattern. However, this study demonstrated that UAGT levels were statistically decreased after RAS blockade, accompanied by mesangial hypercellularity score, renal AGT, Ang II, and TGF-β. Furthermore, UAGT levels were correlated with those parameters. These data strongly support the hypothesis that UAGT is a powerful tool for determining intrarenal RAS status and associated glomerular injury in pediatric IgAN. However, we recognize that future evaluation of UAGT in prospective studies with a larger number of patients and an extended, long observation period is needed. Subsequently, a prospective study has been projected to clarify the novel mechanism of the RAS, which may provide useful information for optimizing the approach for treating pediatric IgAN.
Acknowledgments
The authors acknowledge technical assistance from Ms. Naomi Okamoto and Ms. Chizuko Yamamoto. This study was supported by JSPS KAKENHI Grant Numbers 23591569 and 26461612.
Footnotes
Conflict of interest None.
Contributor Information
Maki Urushihara, Email: urushihara@tokushima-u.ac.jp, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Takashi Nagai, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Yukiko Kinoshita, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Sato Nishiyama, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Kenichi Suga, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Natsuko Ozaki, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Ariunbold Jamba, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Shuji Kondo, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
Hiroyuki Kobori, Department of Pharmacology, Faculty of Medicine, Kagawa University, Kagawa, Japan.
Shoji Kagami, Department of Pediatrics, Institute of Health Biosciences, University of Tokushima Graduate School, Kuramoto-cho 3-18-15, Tokushima 770-8503, Japan.
References
- 1.Kobori H, Urushihara M. Augmented intrarenal and urinary angiotensinogen in hypertension and chronic kidney disease. Pflugers Arch. 2013;465:3–12. doi: 10.1007/s00424-012-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift. Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 3.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- 5.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, Durand J, Hankes GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danser AH, Admiraal PJ, Derkx FH, Schalekamp MA. Angiotensin I-to-II conversion in the human renal vascular bed. J Hypertens. 1998;16:2051–2056. doi: 10.1097/00004872-199816121-00029. [DOI] [PubMed] [Google Scholar]
- 7.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 8.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study) J Hypertens. 2010;28:1422–1428. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal Renin-Angiotensin system activity. Am J Nephrol. 2010;31:318–325. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawaguchi M, Araki SI, Kobori H, Urushihara M, Haneda M, Koya D, Kashiwagi A, Uzu T, Maegawa H. Association between urinary angiotensinogen levels and renal and cardiovascular prognoses in patients with type 2 diabetes mellitus. J Diabetes Investig. 2012;3:318–324. doi: 10.1111/j.2040-1124.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa N, Iijima K, Maehara K, Yoshiara S, Yoshiya K, Matsuo T, Okada S. Mesangial changes in IgA nephropathy in children. Kidney Int. 1987;32:585–589. doi: 10.1038/ki.1987.248. [DOI] [PubMed] [Google Scholar]
- 17.Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, Kobori H, Kagami S. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–1267. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda M, Urushihara M, Wakamatsu T, Oikawa T, Kobori H. Proximal tubular angiotensinogen in renal biopsy suggests nondipper BP rhythm accompanied by enhanced tubular sodium reabsorption. J Hypertens. 2012;30:1453–1459. doi: 10.1097/HJH.0b013e328353e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Konno Y, Tsuruga K, Ito E, Waga S. Combined therapy of enalapril and losartan attenuates histologic progression in immunoglobulin A nephropathy. Pediatr Int. 2004;46:576–579. doi: 10.1111/j.1442-200x.2004.01955.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Ohta K, Shimizu M, Nakai A, Kasahara Y, Yachie A, Koizumi S. Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol. 2005;64:35–40. doi: 10.5414/cnp64035. [DOI] [PubMed] [Google Scholar]
- 21.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 22.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 23.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YG, Song SB, Lee SH, Moon JY, Jeong KH, Lee TW, Ihm CG. Urinary angiotensinogen as a predictive marker in patients with immunoglobulin A nephropathy. Clin Exp Nephrol. 2011;15:720–726. doi: 10.1007/s10157-011-0475-4. [DOI] [PubMed] [Google Scholar]
- 26.Jang HR, Kim SM, Lee YJ, Lee JE, Huh W, Kim DJ, Oh HY, Kim YG. The origin and the clinical significance of urinary angiotensinogen in proteinuric IgA nephropathy patients. Ann Med. 2012;44:448–457. doi: 10.3109/07853890.2011.558518. [DOI] [PubMed] [Google Scholar]
- 27.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 29.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 30.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species–ERK/JNK pathways. Hypertens Res. 2010;33:1174–1181. doi: 10.1038/hr.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner HR. ACE inhibitors in renal disease. Kidney Int. 1992;42:463–479. doi: 10.1038/ki.1992.311. [DOI] [PubMed] [Google Scholar]
- 33.Lafayette RA, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90:766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohan DE. Angiotensin II and endothelin in chronic glomerulonephritis. Kidney Int. 1998;54:646–647. doi: 10.1046/j.1523-1755.1998.00038.x. [DOI] [PubMed] [Google Scholar]
- 35.Urushihara M, Kinoshita Y, Kondo S, Kagami S. Involvement of the intrarenal renin-angiotensin system in experimental models of glomerulonephritis. J Biomed Biotechnol. 2012;2012:601786. doi: 10.1155/2012/601786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Garre D, Ruiz-Ortega M, Ortego M, Largo R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J. Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension. 1996;27:885–892. doi: 10.1161/01.hyp.27.4.885. [DOI] [PubMed] [Google Scholar]
- 38.Minutolo R, Balletta MM, Catapano F, Chiodini P, Tirino G, Zamboli P, Fuiano G, Russo D, Marotta P, Iodice C, Conte G, De Nicola L. Mesangial hypercellularity predicts antiproteinuric response to dual blockade of RAS in primary glomerulonephritis. Kidney Int. 2006;70:1170–1176. doi: 10.1038/sj.ki.5001732. [DOI] [PubMed] [Google Scholar]
- 39.Border WA, Noble NA. Transforming growth factor-beta in glomerular injury. Exp Nephrol. 1994;2:13–17. [PubMed] [Google Scholar]
- 40.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H. Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension. 2011;57:586–593. doi: 10.1161/HYPERTENSIONAHA.110.165704. [DOI] [PMC free article] [PubMed] [Google Scholar]