Abstract
Recent advances in high density surface electromyogram (EMG) decomposition have made it a feasible task to discriminate single motor unit activity from surface EMG interference patterns, thus providing a noninvasive approach for examination of motor unit control properties. In the current study we applied high density surface EMG recording and decomposition techniques to assess motor unit firing behavior alterations post-stroke. Surface EMG signals were collected using a 64-channel 2-dimensional electrode array from the paretic and contralateral first dorsal interosseous (FDI) muscles of nine hemiparetic stroke subjects at different isometric discrete contraction levels between 2 N to 10 N with a 2 N increment step. Motor unit firing rates were extracted through decomposition of the high density surface EMG signals, and compared between paretic and contralateral muscles. Across the nine tested subjects, paretic FDI muscles showed decreased motor unit firing rates compared with contralateral muscles at different contraction levels. Regression analysis indicated a linear relation between the mean motor unit firing rate and the muscle contraction level for both paretic and contralateral muscles (p < 0.001), with the former demonstrating a lower increment rate (0.32 pulses per second (pps)/N) compared with the latter (0.67 pps/N). The coefficient of variation (CoV, averaged over the contraction levels) of the motor unit firing rates for the paretic muscles (0.21 ± 0.012) was significantly higher than for the contralateral muscles (0.17 ± 0.014) (p < 0.05). This study provides direct evidence of motor unit firing behavior alterations post-stroke using surface EMG, which can be an important factor contributing to hemiparetic muscle weakness.
Keywords: Hemiparetic stroke, muscle weakness, high density surface EMG, decomposition, motor unit firing rate
I. Introduction
Stroke is the leading cause of adult disability and the second leading cause of death worldwide [1]. According to the World Health Organization, 15 million people suffer stroke each year. Of these, 5 million die and another 5 million are permanently disabled [2]. Studies have shown that stroke has a detrimental effect on health-related quality of life [3]. Following a hemispheric stroke, many patients suffer a variety of disabling physical symptoms on the contralesional side of the body, such as spastic hypertonia, muscular weakness, and impaired movement coordination [4].
Restoration of impaired function after stroke should focus on treating each of the suffered symptoms with appropriate strategies. Compared with considerable efforts that have been developed in treating spasticity for stroke survivors [5], there are relatively few effective treatments available for paresis or muscle weakness [4]. This may be attributed to the fact that the mechanisms of paresis in stroke are largely unclear and might include muscle atrophy [6] [7], loss of motor units [8-12], uncoordinated muscle activation [13], and impairment in motor unit pool activation [4]. In the current study, we focus on the last factor and investigate motor unit control property alterations that may cause insufficient motor unit activation.
In normal muscles, an optimal combination of motor unit recruitment and rate modulation produces a maximal level of force [14]. There is cumulative evidence to suggest that disruption of the functional relation between motoneuron firing and motor unit contractile property may contribute to muscular weakness in stroke. The evidence is still incomplete and mainly comes from indirect or elusive measurement of global surface electromyogram (EMG) parameters [15-20], or from selective intramuscular EMG decomposition [4] [21-24]. For example, to reach the same force, an increase in paretic muscle surface EMG activity (as measured by EMG amplitude) with respect to the contralateral muscle was observed, suggesting that motor unit activation patterns become disorganized with an abnormally low motor unit firing rate (which requires recruitment of higher threshold motor units to reach the target force) [15] [18] [20]. Reduced motor unit firing rates in paretic muscles of stroke subjects were further confirmed by direct measurement of single motor unit behavior through intramuscular EMG decomposition. Relying on fine wire EMG recording, Gemperline et al. [4] reported that all the six tested stroke subjects showed a failure to increase motor unit firing rate during voluntary force increments in paretic (hypertonic) muscles, with compression of the motoneuron recruitment range. In half of the subjects, there were significant reductions in motor unit mean firing rate in the paretic muscle (while the other half showed similar mean firing rates), when compared to the contralateral muscle. Reduced motor unit firing rates are expected to contribute to weakness by altering the normally precise match between motoneuron property and the mechanical property of the innervated muscle fibers, which will reduce the efficiency of muscle contraction, and in turn lead to increased effort and more rapid fatigue.
So far direct evidence of motor unit firing behavior disruption from surface EMG is lacking due to great difficulties in extracting motor unit firing behavior from interference surface EMG patterns. Furthermore, previous studies on motor unit firing rate alteration in stroke were mainly performed in proximal muscles [4] [22]. It is presently unknown whether or how the motor unit firing behavior may be altered in distal muscles. In light of this, the current study investigated the behavior of single motor units of a hand muscle over an extended range of force, to determine directly the nature and extent of motor unit firing abnormalities using the state of the art high density surface EMG techniques. Understanding the single motor unit behavior in stroke survivors will help classify the complicated nature of the functional impairment, which is important for development of possible therapeutic interventions.
In this study, the first dorsal interosseous (FDI) muscle was examined. The rationale for this choice was that the FDI muscle is the only agonist of the thumb-index finger joint so that a correlation between force and EMG feature is explicit. In contrast, for other joints, multiple muscles are activated to produce flexion or extension, thus compromising the accurate relationship between force (generated by multiple muscles) and surface EMG (generated mostly by one muscle). Part of the study was presented in abstract form at the Annual Conference of Society for Neuroscience in 2013 [25].
II. Methods
A. Subjects
Nine subjects with chronic hemiparetic stroke (6 male, 3 female, 59 ± 12 years) were recruited using the Clinical Neuroscience Research Registry at the Rehabilitation Institute of Chicago. The study was approved by the Institutional Review Board of Northwestern University (Chicago, USA). All subjects gave their written consent before the experiment. A screening examination and clinical assessment were performed by a physical therapist. All the subjects had first time stroke. The duration of their stroke condition was 5.4 ± 3.8 years. They had no history of spinal cord injury or traumatic brain damage, serious medical illness such as cardiovascular or pulmonary complications, or severe motion sickness. The index finger function of the paretic hand was examined, and subjects who were unable to perform voluntary index finger abduction were excluded from the study. Across all the subjects, the Fugl-Meyer scale [26] was 50/66 ± 15/66, and the Chedoke-McMaster assessment [27] was 5.3 ± 1.3. A summary of subject information and clinical assessment is presented in Table 1.
Table 1.
Characteristics for the nine tested stroke subjects
| Subject ID | Gender | Age (years) |
Duration
(years) |
Paretic
side |
Chedoke |
Fugl-
Meyer |
MVC_p (N) |
MVC_c
(N) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | 3.3 | right | 7 | 64/66 | 22.3 | 32 |
| 2 | M | 49 | 1.9 | right | 6 | 48/66 | 20.2 | 47.5 |
| 3 | M | 46 | 6 | right | 5 | 58/66 | 31.5 | 52.5 |
| 4 | M | 76 | 5.7 | left | 3 | 37/66 | 16.8 | 40.9 |
| 5 | M | 57 | 8.1 | right | 5 | 51/66 | 34.6 | 44.5 |
| 6 | M | 70 | 1.4 | left | 7 | 56/66 | 21.7 | 28.1 |
| 7 | F | 53 | 3.4 | right | 6 | 64/66 | 24 | 54 |
| 8 | F | 45 | 13.9 | left | 4 | 16/66 | 10.3 | 35.8 |
| 9 | F | 68 | 5.3 | right | 5 | 53/66 | 6.2 | 18.4 |
MVC_p: maximum voluntary contraction for the paretic FDI muscle;
MVC_c: maximum voluntary contraction for the contralateral FDI muscle
B. Experiments
The examination of the FDI muscle was performed bilaterally for each stroke subject. FDI is a multifunctional muscle that generates torque about the 2nd metacarpophalangeal (MCP) joint. FDI muscle activation was measured during the isometric abduction of the index finger based on the fact that the abduction force produced at the MCP joint is generated primarily by the FDI muscle [28].
Subjects were seated upright in a height-adjustable Biodex Chair and a standard six degree-of-freedom load cell (FT 3226, ATI Inc, Apex, NC) setup was used to accurately record the isometric contraction force of the FDI muscle (Figure 1). The load cell can measure up to 65 N in the x or y axis with a maximum output of 10 volts. To minimize spurious force contributions from muscles other than FDI, the following procedures have been adopted: (1) the shoulders and waist of the subject were tightly strapped to the chair to limit trunk and shoulder movements ; (2) the forearm and wrist were immobilized on a plastic platform inside a fiberglass cast; (3) a ring-mount interface was used to strap the wrist in a partial pronation position; and (4) the proximal phalanx of the index finger was casted and fixed to a small ring-mount interface attached to the load cell.
Fig. 1.

(a) Experimental setup; (b) Channel index of the flexible high density surface electrode array used in this study. The array has 64 recording electrodes arranged in an 8 × 8 square matrix, with diameter of each electrode of 1.2 mm and the center-to-center electrode distance of 4 mm for both horizontal and vertical directions.
Surface EMG signals were recorded from the FDI muscle using a flexible 2-dimensional electrode array (TMS International BV, The Netherlands) (Figure 1b). The skin over the tested muscle was first wiped with electrode preparation pads saturated with 70% v/v isopropyl alcohol and pumice. Then a small amount of absorbable conductive cream was rubbed on the skin and then removed with great care to avoid short-circuiting the electrodes. A compatible double-sided adhesive sticker was used to attach the flexible electrode array to the tested muscle, after each of its cavities being filled with conductive paste. A stable skin-electrode contact was further secured with medical tapes. A Refa amplifier (128-channel regular plus 8 auxiliaiy channels, TMS International BV, The Netherlands) was used to record surface EMG and force signals. Both surface EMG and force signals were sampled at 2 kHz per channel, with a bandpass filter (bandwidth 10 - 500 Hz) for surface EMG and a low pass filter (cutoff frequency 100 Hz) for force measurement.
For each subject, the paretic and contralateral FDI muscles were examined in two separate sessions on different days. At the beginning of the experiment, the isometric maximum voluntary contraction (MVC) of the FDI muscle for index finger abduction was determined. Then the subject was asked to abduct the index finger and generate an isometric contraction force of the FDI muscle to match the target force of 2 N, 4 N, 6 N, 8 N, and 10 N, in different trials. A computer monitor in front of the subject displayed the target and exerted forces. The target force was displayed as a constant red trapezoid trajectory (16 s in duration including 3 s for the rising stage, 10 s for the constant stage, and 3 s for tlte falling stage), and the trace of the tested muscle’s voluntary contraction force was updated in real time with a different color. Overlapping of the volunta1y trace and the target trajectory in the visual display indicated a good match between the desired and actual forces. Before data recording, practice was given to help subject familiarize with the task and making appropriate adjustments to match the target force (using force visual feedback). Then, the subject was instructed to match the target force trajecto1y and maintain the force as stable as possible during the 10 s constant force stage. The protocol for each target force was performed twice. The order of target forces was randomized for each subject. A rest period of at least 2 minutes between trials was provided to prevent potential mental and muscle fatigue during the experiment.
C. Data Analysis
All the data processing was performed offline. Surface EMG and force signals during the constant force period (for at least 6 seconds) were selected from visual examination of the force signals. Force deviation from pure abduction, defined as the arctangent of the ratio between flexion and abduction forces (Fy/Fx), was examined for all the trials before processing. Only trials with flexion deviation less than 18 degrees were analyzed in this study. The surface EMG signals recorded from the electrode array were decomposed to obtain single motor unit contributions. The Convolution Kernel Compensation (CKC) technique [29] [30] was used for this purpose. This decomposition algorithm has been validated extensively with both simulated and experimental approaches, and the procedures have been described in previous studies [31] [32]. Single motor units with consistent firing patterns during the processed constant force period were selected for further analysis. For each motor unit, instantaneous firing rates were calculated as the inverse of the inter-discharge intervals. During this calculation, we excluded abnormally short (shorter than 33.3 ms) and long (larger than 250 ms) inter-spike intervals. The mean firing rate for each motor unit was calculated as the average of the instantaneous firing rates during the constant force period. The global (or average) mean firing rate for each force level was obtained by averaging the mean firing rates of all the individual motor units processed at this force level. The coefficient of variation (CoV) of each motor unit’s firing rate, defined as the ratio of its standard deviation to the mean, was computed. The CoV of the abduction force for each contraction was calculated to quantify force dispersion. For each tested subject, the CoV of the motor unit firing rate was averaged from all the available motor units. The CoV of the abduction force was also averaged across different levels of contraction. This was performed for the paretic and contralateral muscles, respectively.
Regression analysis was performed to examine any linear relation between the global mean firing rate and contraction force (varying from 2 N to 10 N) for the paretic and contralateral FDI muscles, respectively. Paired T test was applied to assess the difference of slope coefficients (obtained from the linear regression), and CoV of motor unit firing rate or force between the paretic and contralateral muscles. Correlation analysis was also performed to examine the relation between subject characteristics and motor unit firing behavior parameters. Statistical significance was determined as p < 0.05.
III. Results
Across the tested subjects, the maximum strength of the FDI muscle during isometric index finger abduction (mean ± standard deviation) was 20.8 ± 9.1 N (range: 6.2 - 34.6 N) for the paretic side, and 39.3 ± 11.8 N (range: 18.4 – 54 N) for the contralateral side. In this study, the maximum level of force examined for each muscle varied from 29% to 97% MVC with a mean of 53% MVC for paretic muscles, and varied from 19% to 54% MVC with a mean of 28% MVC for contralateral muscles. All subjects were able to complete the required trials except Subject 8 and 9 who were unable to maintain sustained contractions at 8 N or 10 N for 10 s using their paretic FDI muscles. Table 2 summarizes the number of motor units extracted from surface EMG decomposition, the number of motor units (with consistent firing) used for firing behavior analysis, and the number of processed trials for each force level. On average, after decomposition 12.9 and 16.4 motor units per trial were extracted from paretic and contralateral muscles, respectively. With further assessment, on average 7.1 and 10.7 motor units (with consistent and stable firing patterns) per trial were used for firing behavior analysis for paretic and contralateral muscles, respectively.
Table 2.
A summary of extracted motor units
| 2N | 4N | 6N | 8N | 10N | ||
|---|---|---|---|---|---|---|
| Number of motor units from decomposition |
P | 189 | 203 | 215 | 234 | 163 |
| C | 195 | 273 | 304 | 235 | 291 | |
|
| ||||||
| Number of motor units for firing behavior analysis |
P | 108 | 128 | 136 | 98 | 83 |
| C | 129 | 221 | 202 | 150 | 143 | |
|
| ||||||
| Number of trials | P | 16 | 18 | 17 | 15 | 12 |
| C | 15 | 16 | 17 | 15 | 16 | |
P: paretic; C: contralateral
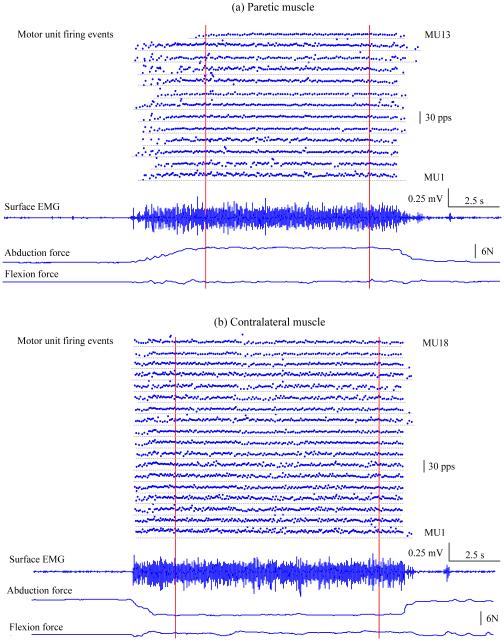
Figure 2 displays an example of a stroke subject’s motor unit firing events, a representative channel of surface EMG, and force signals in abduction and flexion, respectively. The high density surface EMG signals were recorded from the paretic or contralateral FDI muscle when the subject maintained a force level (abduction of the index finger) of approximately 6 N. The mean firing rate of individual motor units and mean force in abduction and flexion were calculated during a constant force period delimited by the two dashed vertical lines. Force deviation from pure abduction was assessed for each trial. In this example, the arctangent of the ratio between flexion and abduction forces (Fy/Fx) was 0.5 degree for the paretic muscle and 5.1 degrees for the contralateral muscle. Thirteen (out of 17 extracted) motor units from the paretic muscle and eighteen (out of 23 extracted) motor units from the contralateral muscle were used for firing behavior analysis, respectively. A lower global (or average) mean motor unit firing rate was observed in the paretic muscle (mean ± standard error: 12.16 ± 0.42 pulses per second (pps)) compared with the contralateral muscle (13.74 ± 0.44 pps) (p < 0.05).
Fig. 2.
An example of a stroke subject’s individual motor unit firing events, surface EMG recording and force signals in abduction and flexion. Individual motor unit firing events were obtained from decomposition of the high density surface EMG recorded on the FDI muscle. A representative channel of the electrode array recordings is displayed. Both hands maintained a constant force of approximately 6 N in abduction. Note that abduction force was positive for paretic muscle and negative for contralateral muscle because the contraction directions were opposite (or towards each other). The vertical lines delimit the constant force period we used for motor unit firing behavior analysis. (a) Paretic side: thirteen motor units with consistent firing patterns were obtained in this trial. (b) Contralateral side: eighteen motor units with consistent firing patterns were obtained in this trial.
In general, eight subjects showed decreased motor unit firing rate in their paretic FDI muscles compared with the contralateral muscles across all force levels. One subject (Subject 3) showed higher mean firing rate at forces from 2 N to 6 N and lower mean firing rate at 8 N and 10 N in his paretic muscle. Pooling all nine stroke subjects’ data, we found that both paretic and contralateral muscles showed an increment of the mean motor unit firing rate with increasing of contraction level. The global (or average) mean motor unit firing rates (mean ± standard error) in the paretic muscle were 11.89 ± 0.48 pps, 13.33 ± 0.84 pps, 13.43 ± 0.7 pps, 14.1 ± 1.7 pps, and 14.73 ± 1.08 pps as force varied from 2 N to 10 N at 2 N increment. Likewise, the global mean firing rate in the contralateral muscle increased from 13.47 ± 0.84 pps to 15.37 ± 0.56 pps, 17.37 ± 0.7 pps, 18.56 ± 1.3 pps, and 18.64 ± 0.66 pps as the force increased from 2 N to 10N at 2 N increment.
Examination of the relation between global mean motor unit firing rate and contraction force was performed in the paretic and contralateral hands for all subjects. An example of one representative subject's data (the same subject as shown in Figure 2) is presented in Figure 3a, which indicates a strong linear relation between the global mean motor unit firing rate and muscle contraction level. For this subject, the ranges of individual mean firing rates in the paretic side were: from 6.93 to 19.73 pps at 2 N (mean ±standard error, 10.95 ±0.68 pps), from 7.55 to 21.26 pps at 4 N (11.7 ± 0.37 pps), from 8.56 to 22.64 pps at 6 N (12.41 ±0.34 pps),from 6.77to 18.95 pps at 8 N (12.72 ± 0.54 pps), and from 8.07 to 20.76 pps at 10N (12.91 ± 0.43 pps). The ranges of individual mean motor unit firing rates in the contralateral muscle were: from 6.43 to 17.38 pps at 2N (11.29 ± 0.78 pps), from 7.56 to 18.15 pps at 4N (12.74 ± 0.26 pps),from 9.55 to 18.88pps at 6N (14.93 ± 0.28 pps), from 8.56 to 18.67 pps at 8 N (13.84 ± 0.42 pps), and from 11.15 to 20.19 pps at 10N (16.71 ± 0.41 pps). The global mean firing rate in the paretic (pmfr) or contralateral (cmfr) muscles can be estimated from the force (f) as: pmfr= 10.66 + 0.25·f (r2 = 0.94, p < 0.01), and cmfr = 10.32 + 0.6·f (r2 = 0.83, p < 0.05), respectively. The contralateral muscle showed a higher increment of firing rate with increasing force (0.6 pps/N) compared with that (0.25 pps/N) of the paretic muscle. Similar findings were observed in other five subjects showing a significant linear regression for both paretic and contralateral muscles, with the latter showing a higher slope coefficient compared with the former. Two of the nine subjects demonstrated a significant linear regression in the contralateral but not in the paretic muscles; note that regression analysis was not applied in one subject’s (Subject 9) paretic muscle due to lack of valid data at 8 N and 10 N. Among the nine subjects, only one demonstrated insignificant and similar slopes for both paretic and contralateral muscles. Statistical analysis based on eight subjects’ (excluding Subject 9) firing rate-force slopes indicated a significant reduced slope coefficient in the paretic FDI compared with the contralateral side (Figure 3b, paretic: 0.32 ± 0.11; contralateral: 0.67 ± 0.11; p < 0.05).
Fig. 3.
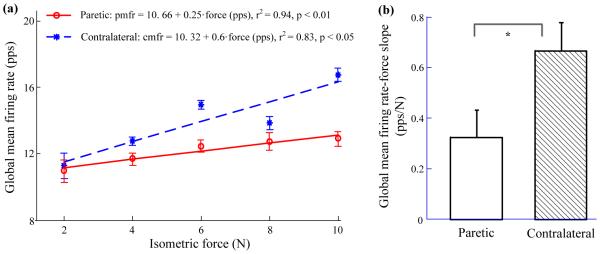
(a) A representative subject’s global mean firing rate (and standard error) at each contraction level. A linear relation was found in both paretic and contralateral sides. Paretic: global mean firing rate (pmfr) = 10.66 + 0.25·f (r2 = 0.94, p < 0.01); Contralateral: global mean firing rate (cmfr) = 10.32 + 0.6·f (r2 = 0.83, p < 0.05). (b) Slope comparison of global mean motor unit firing rate-force relation for nine tested subjects. Paretic, mean ± standard error: 0.32 ± 0.11 pps/N; Contralateral: 0.67 ± 0.11 pps/N. The asterisk (*) represents statistical significance (p < 0.05).
Figure 4 shows a comparison of the coefficient of variation (CoV) of the motor unit firing rates and the contraction forces for the paretic and contralateral muscles, respectively. The CoV (mean ± standard error) of motor unit firing rates from the paretic muscle of all the tested subjects was 0.21 ± 0.012, significantly higher than that (0.17 ± 0.014) of the contralateral muscle (p < 0.05) (Figure 4a). However, examination of the standard deviation of individual motor unit firing rates did not reveal a significant difference between the paretic and contralateral muscles. The average standard deviation of motor unit firing rates was 2.78 ± 0.14 pps and 2.83 ± 0.26 pps for the paretic and contralateral muscles, respectively (p > 0.05). Averaged from all the subjects, the CoV of contraction force in abduction was 0.092 ± 0.008 for the paretic muscle, significantly higher than that (0.055 ± 0.004) of the contralateral muscle (p < 0.01) (Figure 4b). No significant relation was found between subject assessment (weakness, post-stroke duration, Fugl-Meyer and Chedoke scores) and motor unit firing parameters (motor unit firing rate, slope of motor unit firing rate-force regression, slope ratio between paretic and contralateral muscles) (p > 0.1).
Fig. 4.
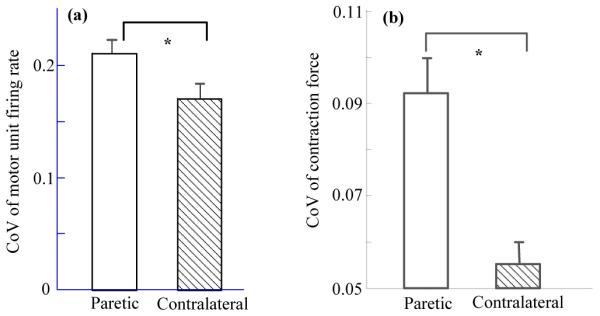
(a) A comparison of coefficient of variation (CoV) of the motor unit firing rates from the paretic and contralateral muscles. Paretic (mean ± standard error): 0.21 ± 0.012; Contralateral: 0.17 ± 0.014. (b) A comparison of CoV of contraction forces from the paretic and contralateral muscles. Paretic: 0.092 ± 0.008; Contralateral: 0.055 ± 0.004. The asterisk (*) represents statistical significance (p < 0.05).
IV. Discussion
A. Importance of Motor Unit Examination Post-stroke
Following a stroke, paretic-spastic muscles often undergo progressive changes in intrinsic mechanical properties, resulting in muscle contractures and alterations in muscle structure, such as muscle fiber loss and connective tissue overgrowth. These phenomena could be the result of one or more mechanisms, including disuse, autonomic vascular changes, pressure neuropathy, a loss of central motoneuron trophic influences, and trans-synaptic spinal motoneuron degeneration [8]. Regardless of the origins, it is very important to determine whether and how stroke may adversely affect motor units’ survival or function. This information can guide development of effective treatments for weakness and other associated muscle property changes in stroke. Knowledge derived from analysis of different motor unit components will help identify factors that contribute to deterioration in muscle strength and ultimately inform intervention strategies that will allow stroke patients to remediate and enhance quality of life. For example, for a specific stroke subject, if research findings indicate that muscle fiber atrophy is the major factor contributing to muscle weakness and other pathological changes, active therapies or targeted exercises are required to prevent further atrophy and regain muscle strength [33]. If research findings confirm motor unit loss and associated muscle weakness post-stroke, treatment strategies can be directed to develop ways to prevent motor unit loss (e.g., growth factor interventions) during acute phase of the stroke [9]. However, given that some motor unit loss may be unavoidable, enhancing the natural reinnervation process through electrical stimulation and other treatments may be necessary [34]. Related to the focus of the current study, if research findings demonstrate impairment in motor unit control properties, a different intervention strategy targeting motor unit activation capacity should be used (see below).
B. Mechanisms of Motor Unit Firing Rate Reduction and Clinical Implications
Our findings in FDI are consistent to those from proximal muscles [4]. There was a significant motor unit firing rate reduction in paretic FDI when compared with the contralateral muscle. However, no significant correlation was found between motor unit firing parameters and clinical assessment. This could be due to several factors. For example, the Chedoke or Fugl-Meyer scores evaluate the general hand and upper limb functions, which may not be directly related to a single muscle. Moreover, motor unit firing parameters were not calculated at the muscle’s maximal strength. It should also be noted that in addition to firing rate, complex changes in other motor unit properties post-stroke might also influence clinical measurements.
As discussed in the previous studies [4] [24], different mechanisms may cause deficient motor unit rate coding in a paretic muscle. The most common explanation is that the motor unit firing rate reduction could be produced as a result of decreasing descending excitation or synaptic input to the motoneuron pool, due to upper motoneuron lesions or interruption of commands from the affected hemisphere to motor units [35-37]. The motor unit firing rate reduction could also be produced as a result of changes in intrinsic spinal motoneuron properties which may influence their sensitivity to neural activation of the muscle [38] [39]. For example, it was demonstrated that the estimated time course of the afterhyperpolarization (AHP) in motoneurons innervating paretic muscles of stroke subjects was significantly longer than those innervating contralateral muscles. The motoneuron AHP time course changes post-stroke may play a role in the reduction of motor unit firing rates of paretic muscles during voluntary contraction [40] [41].
For stroke patients with confirmed impairment in motor unit control properties, the treatment may be oriented toward modifying regional synaptic input to motoneurons or changing their chemical environment, which may help recover motor unit rate coding capacities. We also note that maximum motor unit firing rates are reported to be adaptable to standard resistance training practices [42-44]. It is presently unknown whether or how such training may enhance motor unit pool activation in stroke patients. This is a question worth further investigation.
C. Intramuscular EMG vs. Surface EMG Decomposition
One primary feature of this study was the application of the state of the art surface EMG decomposition techniques. Electrophysiological studies have played an important role in assessing post-stroke changes in muscle and motor unit function. Most of the methods involved invasive EMG recordings (e.g., single-fiber EMG, concentric needle EMG, and macro-EMG) [45-52] or electrical stimulation (compound muscle action potential and motor unit number estimation) [8-12]. Traditionally, intramuscular EMG that relies on invasive needle or fine wire electrodes has been used to obtain physiological and diagnostic information about single motor unit properties [53-55]. Surface EMG decomposition has been a research focus in the past decade, and progress has been made in both surface EMG signal acquisition and processing techniques [56-58]. For example, high density surface electrode arrays with tiny skin-electrode contact area and small inter-electrode distance provide spatial information across a muscle, which provides increased motor unit discrimination capacity and makes extraction of single motor units from surface EMG a feasible task with appropriate signal processing methods [29-32, 59]. Among the surface EMG decomposition studies, two recently reported developments, by Holobar and colleagues using the CKC technique that relies on high density EMG recording [29] [30], and by De Luca and colleagues using an enhanced artificial intelligence algorithm with a small 5-pin sensor [60] [61], have attracted attention (and triggered debates [62] [63]).
Based on high density surface EMG recordings, the CKC technique was applied in this study to extract single motor unit activity. The technique has been demonstrated to cope successfully with the irregular motor unit firing patterns before, such as in the case of pathological tremor [64]. In the current study, it has been for the first time applied to post-stroke patients, providing direct evidence of motor unit behavior alterations after stroke using surface EMG, which supports and supplements the previous findings obtained using intramuscular EMG or conventional single-channel interference surface EMG analysis [4] [15-24].
The advantage of noninvasive surface EMG recording is obvious. It overcomes pain and other discomfort and inconvenience associated with needle insertion. From our experience and other reports [65], it is not uncommon that subjects may feel stress and discomfort with needle insertion and may even withdraw from the study. In contrast, all subjects finished the protocols in this study. Compared with needle or fine wire EMG (with quite limited detection volume), high density surface EMG recording and decomposition techniques can be used to extract activity of a relatively large number of motor units in a large portion of a muscle, thus providing solid and comprehensive information about single motor unit behavior after stroke.
D. Surface EMG Decomposition vs. Interference Pattern Analysis
Measurement based on interference surface EMG analysis has been used to examine neuromuscular changes after stroke. For example, previous investigators reported that almost half of their examined hemiparetic subjects showed substantially elevated EMG-amplitude versus force slopes (measured over the biceps brachii and brachioradialis muscles) in paretic muscles compared with contralateral muscles [4] [15]. In a recent study of the FDI muscle [16], it was reported that there were diverse changes in the slope of the EMG-force relations in paretic muscles compared with contralateral muscles (i.e., with significant increases or decreases of the slopes being observed relative to the contralateral side for different subjects). Similarly, when examining the alterations in the amplitude distribution of the FDI muscle surface EMG post-stroke at matched force levels, different patterns of the distribution (a shift toward both larger and smaller peak amplitude values) were observed in the paretic muscles compared with the contralateral muscles [17]. These interference surface EMG findings, in combination with our previous simulation studies [20], suggest that there appear to be different types of processes (e.g., motor unit control property alteration, muscle fiber atrophy, spinal motoneuron degeneration, muscle fiber denervation and reinnervation, etc., which can also be examined by needle EMG) at work which may be present in different degrees in a given stroke patient. Extraction and examination of motor unit firing behavior (such as performed in the current study) and quantitative motor unit action potential analysis [66] can further help confirm or quantify the specific mechanisms underlying the global interference surface EMG changes post-stroke.
E. Experimental Protocols, Technical Aspects and Study Limitations
We chose to compare motor unit firing rates of paretic muscles to contralateral muscles of the same stroke subjects, rather than muscles from neurologically intact subjects. Considering the similarities in muscle mechanical moment arms, muscle strength, and skeletal dimensions between the two limbs of the same subject, such a comparison may allow a more straightforward assessment of motor unit firing behavior than would be possible between muscles drawn from different subjects (potentially having a larger difference than two muscles from the same subject) [4]. In addition, due to the elusiveness in interpreting the significance of the maximum force measurements in paretic limb (which are limited by incapability of full voluntary motoneuron pool activation) [4], the comparison between paretic and contralateral muscles was made at the same absolute force levels (i.e. using a matched force protocol) rather than at the same fractions of each muscle’s MVC force.
It was observed that after decomposition, lower number of motor units was extracted from paretic muscle than from contralateral muscle. The relatively low decomposition yield for paretic muscles could be due to pathological changes after stroke, which might influence the decomposition performance. For example, paretic muscle atrophy may reduce the total number of valid EMG channels. Firing rate reduction and possible loss of large motor units [51] in paretic muscles may increase the extent of superposition of a large number of relatively small motor units. These factors may influence the decomposition performance.
Since not all the active motor units were able to be extracted from the surface EMG, the number of extracted or analyzed motor units did not necessarily represent motor unit recruitment at a specific force level. It has been shown in various previous studies [29] [31] [32] [64] [67] that the unidentified motor unit action potential trains after the CKC decomposition come from small and/or distant motor units, constituting the physiological noise which is always present in surface EMG, regardless the decomposition technique used. In fact, physiological noise is also present in intramuscular EMG, but there it is suppressed by high selectivity of the detection electrodes. This means that both surface and intramuscular EMG decompositions are limited in representativeness. However, while being an important issue that is common to all the decomposition techniques (surface or intramuscular), the representativeness of an identified motor unit pool should not be mixed with the accuracy of CKC-based EMG decomposition. The latter has been extensively verified in previous studies [29] [31] [32]. Moreover, in [67], the automatic measure of CKC-based decomposition accuracy has been analytically derived and experimentally validated. This measure is fully automatic and can be applied to every identified motor unit. It has been extensively used in our study in order to select only highly accurately identified motor units. All the other motor units were discarded and were not considered in our analysis. Therefore, we believe that the presented results are highly accurate. The issue of representativeness can not be fully addressed in this study as it reaches beyond the current state of the art in the EMG decomposition and remains an important research topic to be addressed in the future. However, relatively large number of identified motor units with significant changes in the firing patterns has been observed, demonstrating the changes in a significant portion (at least) of the active motor unit pool.
This study focused on the motor unit firing rates during the constant force levels, while alterations in motor unit recruitment range were not examined. In addition, the mean firing rates of all the detected motor units were calculated at each specific force level, without tracking the same motor units across different force levels, while the latter is more useful in examining motor unit firing rate modulation. A different force protocol with step contractions in a single trial (rather than separate trials as used in the current study) might be more appropriate for same motor unit tracking across different muscle contraction levels. The experimental protocol can be further improved by determining target forces based on each subject’s paretic muscle MVC (e.g., 10%, 20%, 30%…80%, 90% MVC) and then presenting the same absolute force target for the contralateral muscle. This way the full range of the paretic MVC for each subject can be examined, while in this study the maximum target force of 10 N represented a nearly paretic muscle MVC for stroke subjects with severe weakness but only approximately 30% paretic muscle MVC for stroke subjects with mild weakness.
Finally, it is acknowledged that factors other than motor unit control disorganization can also be associated with the observed motor unit firing rate changes. For example, selective loss of motor units after a stroke might alter motor unit types or specific muscle fiber contraction dynamics - those are factors closely related to motor unit firing rates. Therefore, a comprehensive examination using a range of techniques (e.g., motor unit number estimation, quantitative motor unit action potential analysis, motor unit twitch characterization, etc.) would be necessary to more precisely quantify post-stroke motor unit control property changes.
V. Conclusion
High density surface EMG techniques were used to examine motor unit firing behaviors post-stroke. The tested stroke subjects showed decreased motor unit firing rate in the paretic FDI muscle compared with the contralateral FDI muscle across all the examined force levels (from 2 N to 10 N with a 2 N increment step). A strong linear relation between the global mean motor unit firing rate and muscle contraction level was observed for both paretic and contralateral FDI muscles, whereas the former demonstrated significantly reduced slope or increment rate of motor unit discharge compared with the latter. The CoV of the motor unit firing rates (averaged from all the motor units at different force levels) for the paretic muscle was significantly higher than for the contralateral muscle. In contrast to previous studies that relied on intramuscular EMG decomposition or interference surface EMG analysis, this study provides direct evidence of motor unit firing behavior alterations post-stroke from surface EMG.
Acknowledgments
This study was supported in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grant R24HD050821 and Grant R01NS080839, and in part by the National Natural Science Foundation of China under Grant 81271658. A. Holobar was supported by Slovenian Research Agency (programme P2-0041 (B) Computer Systems, Methodologies and Intelligent Services). M. Gazzoni and R. Merletti were supported by Compagnia di San Paolo and Fondazione CRT in Torino, Italy.
Biographies

Xiaoyan Li received her B.S. degree in Electrical Engineering from Anhui University, and M.S. degree in Biomedical Engineering from University of Science and Technology of China, both in Hefei, China. She obtained her second M.S. degree in Computer Sciences from Loyola University Chicago in 2002, and her Ph.D. degree in Bioengineering from the University of Illinois at Chicago (UIC) in 2008.
Later she was a Postdoctoral Research Fellow in Institute for Neural Computation of the University of California at San Diego (UCSD), CA, and in Department of Physical Medicine and Rehabilitation of Northwestern University, Chicago, IL, respectively, and a Research Associate at the Sensory Motor Performance Program of the Rehabilitation Institute of Chicago, IL. She became a Research Assistant Professor in Physical Medicine and Rehabilitation of Northwestern University in 2013. She is currently an Assistant Professor in Department of Physical Medicine and Rehabilitation of the University of Texas Health Science Center at Houston. Her research interests focus on motor control, neurological disorders and rehabilitation.

Aleš Holobar (M’98) received PhD degree in Computer Science from the Faculty of Electrical Engineering and Computer Science (FEECS), University of Maribor (UM), Slovenia, in 2004. From 2005 to 2008, he was with Laboratory of Engineering of Neuromuscular System and Motor Rehabilitation at Politechnico di Torino, Italy. From 2009, he is associate professor at FEECS, University of Maribor. His main research interests include statistical signal processing, compound signal analysis, identification of multidimensional systems and biomedical imaging with current activities focused on surface electromyography, electroencephalography and biomedical signal processing.

Marco Gazzoni graduated in Computer Science Engineering in 1998 at the Politecnico di Torino. In 2005 he obtained the PhD degree in Biomedical Engineering from the Politecnico di Torino.
From 1998 he collaborates with the Laboratory for Engineering of the Neuromuscular System (LISiN), Politecnico di Torino, Italy. His chief expertise concerns the development of surface EMG techniques (detection and processing) for the non-invasive investigation of the neuromuscular system with applications in basic physiology, ergonomics, rehabilitation, sport science. His main interest is in the development and application of these techniques in the areas of physical rehabilitation, assistive technologies, and prosthetic control.
He is author/coauthor of 30 peer-reviewed papers in international journals, and about 80 abstracts for national and international congresses. He has one international patent application concerning textile electrodes for the detection of electrophysiological signals. From 2007 he is contract professor for the course "Bioengineering of Exercise and Sport" at Politecnico di Torino. From 2013/2014 he is contract professor for the course "Rehabilitation Engineering" at Politecnico di Torino.

Roberto Merletti received the graduate degree in electronics engineering from Politecnico di Torino, Torino, Italy, and the M.S. and Ph.D. degrees in biomedical engineering from The Ohio State University, Columbus, USA. From 1989 to 1994, he was an Associate Professor with the Department of Biomedical Engineering, Boston University, Boston, MA, USA, and a Research Associate at the Neuromuscular Research Center of the same university.
He is now Full Professor of Biomedical Engineering at Politecnico di Torino where he established, in 1996, the Laboratory for Engineering of the Neuromuscular System (LISiN) of which he is currently Director. He has been involved in six EU projects and two ESA projects in the field of engineering of the neuromuscular system. He is a member of the editorial board of four major biomedical engineering journals and published over 200 papers in international peer reviewed journals in the fields of electrical stimulation and non-invasive electromyography.

William Zev Rymer is currently researching regulation of movement in normal and neurologically disordered human subjects including sources of altered motoneuronal behavior in hemispheric stroke survivors, using electro-physiological, pharmacological, and biomechanical techniques.
Dr. Rymer serves as Director of the Sensory Motor Performance Program, a position he has held since 1987, and Director of Research Planning at the Rehabilitation Institute of Chicago (RIC). In addition to his research roles at RIC, Dr. Rymer holds appointments as Professor of Physical Medicine and Rehabilitation, Physiology, and Biomedical Engineering at Northwestern University Feinberg School of Medicine.
Dr. Rymer earned his medical degree from Melbourne University and his PhD in Neurophysiology from Monash University, both in Australia. After postdoctoral training at the National Institutes of Health and Johns Hopkins University Medical School, he became an Assistant Professor of Neurosurgery and Physiology at the State University of New York, Syracuse. In 1978, he came to Chicago as an Assistant Professor of Physiology at the Feinberg School of Medicine at Northwestern University, and he remained as a primary faculty member in Physiology until his appointment at the RIC.

Ping Zhou (S’01–M’05–SM’07) received the B.S. degree in electrical engineering and the M.S. degree in biomedical engineering from the University of Science and Technology of China, Hefei, China, in 1995 and 1999, respectively, and the Ph.D. degree in biomedical engineering from Northwestern University, Evanston, IL, USA, in 2004.
From 1999 to 2014, he was progressively a Research Assistant, Research Associate, full time and adjunct research faculty at the Rehabilitation Institute of Chicago, Chicago, IL, USA. He was also an Adjunct Research Assistant and later Associate Professor in Physical Medicine and Rehabilitation of Northwestern University, Chicago, IL, USA, from 2006 to 2014. He currently holds an Adjunct Associate Professor position in Physical Medicine and Rehabilitation at the University of Texas Health Science Center at Houston, TX, USA. He directs the Neuro-Myoelectric Engineering for Rehabilitation laboratory housed in the outpatient clinic at TIRR Memorial Hermann Research Center. He is also an affiliated Professor in the biomedical engineering program of the University of Science and Technology of China. His research interests include biomedical signal (in particular, EMG) processing, motor unit pathophysiology and electrodiagnosis, myoelectric control, and assistive devices for neurorehabilitation.
Contributor Information
Xiaoyan Li, Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center (UTHealth), and TIRR Memorial Hermann Research Center, Houston, Texas, 77030, USA xiaoyan.li@uth.tmc.edu.
Aleš Holobar, Faculty of Electrical Engineering and Computer Science, the University of Maribor, 2000 Maribor, Slovenia ales.holobar@uni-mb.si.
Marco Gazzoni, Laboratory of Engineering of Neuromuscular System and Motor Rehabilitation (LiSIN), Politecnico di Torino, Torino, Italy, Department of Electronics, Politecnico di Torino, Torino, Italy, marco.gazzoni@polito.it.
Roberto Merletti, Laboratory of Engineering of Neuromuscular System and Motor Rehabilitation (LiSIN), Politecnico di Torino, Torino, Italy, Department of Electronics, Politecnico di Torino, Torino, Italy, roberto.merletti@polito.it.
William Z. Rymer, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, and with Departments of Physical Medicine and Rehabilitation, Physiology, and Biomedical Engineering, Northwestern University, Chicago, Illinois, 60611, USA w-rymer@northwestern.edu
Ping Zhou, Department of Physical Medicine and Rehabilitation, UTHealth, and TIRR Memorial Hermann Research Center, Houston, Texas, 77030, USA; Biomedical Engineering Program of the University of Science and Technology of China, Hefei, China dr.ping.zhou@ieee.org; pzhou@ustc.edu.cn; ping.zhou.1@uth.tmc.edu.
REFERENCES
- 1.Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004 Jul;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- 2.Mackay J, Mensah GA. The Atlas of Heart Disease and Stroke. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 3.Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR. Impact of stroke on healthrelated quality of life in the noninstitutionalized population in the United States. Stroke. 2006;37:2567–2572. doi: 10.1161/01.STR.0000240506.34616.10. [DOI] [PubMed] [Google Scholar]
- 4.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995 Oct;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 5.Sommerfeld DK, Gripenstedt U, Welmer AK. Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am J Phys Med Rehabil. 2012 Sep;91:814–820. doi: 10.1097/PHM.0b013e31825f13a3. [DOI] [PubMed] [Google Scholar]
- 6.Segura RP, Sahgal V. Hemiplegic atrophy: electrophysiological and morphological studies. Muscle Nerve. 1981 May-Jun;4:246–248. doi: 10.1002/mus.880040312. [DOI] [PubMed] [Google Scholar]
- 7.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002 Dec;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 8.McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motoneurones of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1973 Apr;36:183–93. doi: 10.1136/jnnp.36.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara Y, Masakado Y, Chino N. The physiological functional loss of single thenar motor units in the stroke patients: when does it occur? Does it progress? Clin Neurophysiol. 2004 Jan;115:97–103. doi: 10.1016/j.clinph.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, Ushijima R. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006 Dec;250:27–32. doi: 10.1016/j.jns.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wang YC, Suresh NL, Rymer WZ, Zhou P. Motor unit number reductions in paretic muscles of stroke survivors. IEEE Trans Inf Technol Biomed. 2011 Jul;15(4):505–512. doi: 10.1109/TITB.2011.2140379. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Liu J, Li S, Wang YC, Zhou P P. Examination of hand muscle activation and motor unit indices derived from surface EMG in chronic stroke. IEEE Trans Biomed Eng. 2014 Jun 25; doi: 10.1109/TBME.2014.2333034. [Epub ahead of print] PMID: 24967982 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond MC, Fitts SS, Kraft GH, Nutter PB, Trotter MJ, Robinson LM. Co-contraction in the hemiparetic forearm: quantitative EMG evaluation. Arch. Phys. Med. Rehabil. 1988 May;69:348–351. [PubMed] [Google Scholar]
- 14.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 15.Tang A, Rymer WZ. Abnormal force--EMG relations in paretic limbs of hemiparetic human subjects. J Neurol Neurosurg Psychiatry. 1981 Aug;44:690–698. doi: 10.1136/jnnp.44.8.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Li X, Rymer WZ. EMG-force relations during isometric contractions of the first dorsal interosseous muscle after stroke. Topics in Stroke Rehabilitation. 2013 Nov-Dec;20:537–544. doi: 10.1310/tsr2006-537. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Suresh A, Zhou P, Rymer WZ. Alterations in the peak amplitude distribution of the surface electromyogram poststroke. IEEE Trans Biomed Eng. 2013 Mar;60:845–852. doi: 10.1109/TBME.2012.2205249. [DOI] [PubMed] [Google Scholar]
- 18.Blaschak MJ, Powers RK, Rymer WZ. Disturbances of motor output in a cat hindlimb muscle after acute dorsal spinal hemisection. Exp Brain Res. 1988;71:377–387. doi: 10.1007/BF00247497. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Shin H, Zhou P, Niu X, Liu J, Rymer WZ. Power spectral analysis of surface electromyography (EMG) at matched contraction levels of the first dorsal interosseous muscle in stroke survivors. Clin Neurophysiol. 2014 May;125:988–994. doi: 10.1016/j.clinph.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P, Suresh NL, Rymer WZ. Model based sensitivity analysis of EMG-force relation with respect to motor unit properties: applications to muscle paresis in stroke. Ann Biomed Eng. 2007 Sep;35:1521–1531. doi: 10.1007/s10439-007-9329-3. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JL, Mayer RF. Physiological alterations of motor units in hemiplegia. J Neurol Sci. 1982;54:401–412. doi: 10.1016/0022-510x(82)90203-9. [DOI] [PubMed] [Google Scholar]
- 23.Hu XL, Tong KY, Hung LK. Firing properties of motor units during fatigue in subjects after stroke. J Electromyogr Kinesiol. 2006 Oct;16:469–476. doi: 10.1016/j.jelekin.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Chou LW, Palmer J, Binder-Macleod SA, Knight CA. Motor unit rate coding is severely impaired during forceful and fast muscular contractions in individuals post stroke. J Neurophysiol. 2013 Jun;109:2947–2954. doi: 10.1152/jn.00615.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Holobar A, Gazzoni M, Botter A, Merletti R, Rymer WZ, Zhou P. Post stroke motor unit firing rate alteration examined by high density surface electromyography decomposition. Annual Conference of Society for Neuroscience; San Diego, USA. Nov, 2013. [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient - 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 27.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Vanspall B, Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993 Jan;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 28.Li ZM, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL. Multi-directional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003 Dec;18:908–915. doi: 10.1016/s0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 29.Holobar A, Farina D, Gazzoni M, Merletti R, Zazula D. Estimating motor unit discharge patterns from high-density surface electromyogram. Clin Neurophysiol. 2009 Mar;120:551–562. doi: 10.1016/j.clinph.2008.10.160. [DOI] [PubMed] [Google Scholar]
- 30.Holobar A, Zazula D. Multichannel blind source separation using convolution kernel compensation. IEEE Transactions on Signal Processing. 2007;55:4487–4496. [Google Scholar]
- 31.Marateb HR, McGill KC, Holobar A, Lateva ZC, Mansourian M, Merletti R. Accuracy assessment of CKC high-density surface EMG decomposition in biceps femoris muscle. J Neural Eng. 2011 Dec;8:066002. doi: 10.1088/1741-2560/8/6/066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng. 2010 Jun;18:221–229. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]
- 33.Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev. 2008;45:261–272. doi: 10.1682/jrrd.2007.02.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon T, Brushart TM, Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol Res. 2008 Dec;30:1012–1022. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- 35.McComas AJ, Sica RE, Upton AR, Aguilera N, Currie S. Motoneurone dysfunction in patients with hemiplegic atrophy. Nat New Biol. 1971 Sep;233:21–23. doi: 10.1038/newbio233021a0. [DOI] [PubMed] [Google Scholar]
- 36.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004 Mar;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005 Dec;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 38.Carp JS, Powers RK, Rymer WZ. Alterations in motoneuron properties induced by acute dorsal spinal hemisection in the decerebrate cat. Exp Brain Res. 1991;83:539–548. doi: 10.1007/BF00229832. [DOI] [PubMed] [Google Scholar]
- 39.Powers RK, Rymer WZ. Effects of acute dorsal spinal hemisection on motoneuron discharge in the medial gastrocnemius of the decerebrate cat. J Neurophysiol. 1988 May;59:1540–1556. doi: 10.1152/jn.1988.59.5.1540. [DOI] [PubMed] [Google Scholar]
- 40.Suresh AK, Hu X, Powers RK, Heckman CJ, Suresh NL, Rymer WZ. Changes in motoneuron afterhyperpolarization duration in stroke survivors. J Neurophysiol. 2014 Sep;112:1447–1456. doi: 10.1152/jn.01091.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanova TD, Knorr S, Macdonell CW, Pollock CL, Garland SJ. Motoneurone afterhyperpolarisation time-course following stroke. Clin Neurophysiol. 2014 Mar;125:544–551. doi: 10.1016/j.clinph.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004 Dec;59:1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- 43.Knight CA, Kamen G. Relationships between voluntary activation and motor unit firing rate during maximal voluntary contractions in young and older adults. Eur J Appl Physiol. 2008 Aug;103:625–630. doi: 10.1007/s00421-008-0757-z. [DOI] [PubMed] [Google Scholar]
- 44.van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998 Nov;513:295–305. doi: 10.1111/j.1469-7793.1998.295by.x. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dattola R, Girlanda P, Vita G, Santoro M, Roberto ML, Toscano A, Venuto C, Baradello A, Messina C. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol. 1993;33:109–114. doi: 10.1159/000116915. [DOI] [PubMed] [Google Scholar]
- 46.Spaans F, Wilts G. Denervation due to lesions of the central nervous system. An EMG study in cases of cerebral contusion and cerebrovascular accidents. J Neurol Sci. 1982 Dec;57:291–305. doi: 10.1016/0022-510x(82)90036-3. [DOI] [PubMed] [Google Scholar]
- 47.Martinez A. Cruz, del Campo F, Mingo MR, Perez Conde MC. Altered motor unit architecture in hemiparetic patients. A single fibre EMG study. J Neurol Neurosurg Psychiatry. 1982 Aug;45:756–757. doi: 10.1136/jnnp.45.8.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown WF, Snow R. Denervation in hemiplegic muscles. Stroke. 1990 Dec;21:1700–4. doi: 10.1161/01.str.21.12.1700. [DOI] [PubMed] [Google Scholar]
- 49.Chang CW. Evident trans-synaptic degeneration of motor neurons after stroke: a study of neuromuscular jitter by axonal microstimulation. Electroencephalogr Clin Neurophysiol. 1998 Jun;109:199–202. doi: 10.1016/s0924-980x(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 50.Lukacs M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin Neurophysiol. 2005 Jul;116:1566–1570. doi: 10.1016/j.clinph.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Lukacs M, Vecsei L, Beniczky S. Large motor units are selectively affected following a stroke. Clin Neurophysiol. 2008 Nov;119:2555–2558. doi: 10.1016/j.clinph.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Lukacs M, Vecsei L, Beniczky S. Changes in muscle fiber density following a stroke. Clin Neurophysiol. 2009 Aug;120:1539–1542. doi: 10.1016/j.clinph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Stashuk D. EMG signal decomposition: how can it be accomplished and used. J Electromyogr Kinesiol. 2001 Jun;11:151–173. doi: 10.1016/s1050-6411(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 54.McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods. 2005 Dec;149:121–133. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Nawab SH, Wotiz RP, DeLuca CJ. Decomposition of indwelling EMG signals. J Appl Physiol. 2008 Aug;105:700–710. doi: 10.1152/japplphysiol.00170.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merletti R, Aventaggiato M, Botter A, Holobar A, Marateb H, Vieira TM. Advances in surface EMG: recent progress in detection and processing techniques. Crit Rev Biomed Eng. 2010;38:305–345. doi: 10.1615/critrevbiomedeng.v38.i4.10. [DOI] [PubMed] [Google Scholar]
- 57.Zwarts MJ, Lapatki BG, Kleine BU, Stegeman DF. Surface EMG: how far can you go? Suppl Clin Neurophysiol. 2004;57:111–119. doi: 10.1016/s1567-424x(09)70349-6. [DOI] [PubMed] [Google Scholar]
- 58.Rau G, Schulte E, Disselhorst-Klug C. From cell to movement: to what answers does EMG really contribute. J. Electromyogr. Kinesiol. 2004;14:611–617. doi: 10.1016/j.jelekin.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Kleine BU, van Dijk JP, Lapatki BG, Zwarts MJ, Stegeman DF. Using two-dimensional spatial information in decomposition of surface EMG signals. J Electromyogr Kinesiol. 2007 Oct;17:535–548. doi: 10.1016/j.jelekin.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Nawab SH, Chang SS, DeLuca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol. 2010 Oct;121:1602–1615. doi: 10.1016/j.clinph.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeLuca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol. 2010 Aug;104:1034–1046. doi: 10.1152/jn.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farina D, Enoka RM. "Surface EMG decomposition requires an appropriate validation. J Neurophysiol. 2011 Feb;105:981–982. doi: 10.1152/jn.00855.2010. [DOI] [PubMed] [Google Scholar]
- 63.DeLuca CJ, Nawab SH. Reply to Farina and Enoka: The reconstruct-and-test approach is the most appropriate validation for surface EMG signal decomposition to date. J Neurophysiol. 2011 Feb;105:983–984. doi: 10.1152/jn.00855.2010. [DOI] [PubMed] [Google Scholar]
- 64.Holobar A, Glaser V, Gallego JA, Dideriksen JL, Farina D. Non-invasive characterization of motor unit behaviour in pathological tremor. J Neural Eng. 2012;9:056011. doi: 10.1088/1741-2560/9/5/056011. [DOI] [PubMed] [Google Scholar]
- 65.Slack D, Nelson L, Patterson D, Burns S, Hakimi K, Robinson L. The feasibility of hypnotic analgesia in ameliorating pain and anxiety among adults undergoing needle electromyography. Am J Phys Med Rehabil. 2009 Jan;88:21–29. doi: 10.1097/PHM.0b013e31818e00bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kallenberg LA, Hermens HJ. Motor unit properties of biceps brachii in chronic stroke patients assessed with high-density surface EMG. Muscle Nerve. 2009 Feb;39:177–185. doi: 10.1002/mus.21090. [DOI] [PubMed] [Google Scholar]
- 67.Holobar A, Minetto MA, Farina D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J Neural Eng. 2014 Feb;11:016008. doi: 10.1088/1741-2560/11/1/016008. [DOI] [PubMed] [Google Scholar]