Abstract
Advances in bacterial DNA sequencing allow for characterization of the human commensal bacterial community (i.e., microbiota) and its corresponding genome (i.e., microbiome). Surveys of healthy adults reveal that each unique body habitat (e.g., gut, skin, oral cavity, vagina) is characterized by a signature composite of bacteria. Aging is accompanied by a myriad of clinical issues, including a basal pro-inflammatory state (i.e.,inflamm-aging), that directly interface with the microbiotaof older adults and enhance susceptibility to disease. Studies in older adults demonstrate that the gut microbiotacorrelates with diet, location of residence (e.g., community dwelling, long term care settings), and basal level of inflammation.Links exist between the microbiotaand a variety of clinical issues plaguing older adults including physical frailty, Clostridium difficile colitis, vulvovaginal atrophy, colorectal carcinoma and atherosclerotic disease. Manipulation of the microbiota andmicrobiome of older adults holds promise as an innovative strategy to affect comorbidities associated with aging.
General Aspects Of Thehuman Microbiota And Microbiome
Leeuwenhoek's discovery of“wee beasties” from mouth scrapings was the first evidence that humans are co-inhabited by microbes. Since 1890, when Koch isolated the anthrax bacteria(1), culture methods have improved. However, most microbes (e.g., bacterial microorganisms) cannot be cultured using conventional methods(2).In 1977, Woese and Fox proposed that the 16S ribosomal RNA subunitcan classify bacteria, including our commensal microbial flora(3).This small RNA subunit isevolutionarilyconserved among prokaryotes, but it contains nine hypervariable regions (V1-V9) useful for phylogenetic analysis. Ongoing advances in next generation sequencing of 16S ribosomal RNA genes, and whole genome shot gun sequencing, allow for large-scale analysis and characterization of the human bacterial community(i.e., the microbiota); the genome of the microbiotais referred toas the microbiome(4, 5). It is estimated that there are about 100 trillion bacteria associated with humans that outnumberour human cells by a factor of ten(6). Therefore, the primary eukaryotic genome of humans is supplemented by thissecond prokaryotic genome of microbes(7). This represents a paradigm shift for medicine in which our relationship with microbes is nowviewed as a complex symbiosis instead of a potential source for clinical infectious disease. The purpose of this review is to highlight the role of the microbiota and microbiome in health and disease with its potential clinical relevance to older adults.
Survey of Microbiota from Human Body Habitats
In 2009,Costello et al. reported the results of a surveyof nine healthy adults of both sexes from multiple body habitats: stool, oral cavity,nostrils,external auditory canal, hair on head, and a variety of skin sites. Using 16S ribosomal RNA gene sequencing, theyfound that across all body habitats, most sequences belonged to four bacterial phyla: Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes. Each body habitat had a uniquecomposition of bacterial microbiota that reflected properties of the local environment. Within each habitat, there was large variation between individuals; however,there was stabilitywhen comparing the baseline with follow-up sampling within the same individual(8).
The Human Microbiome Project Consortium
The Human Microbiome Project consortium is a multidisciplinary international effort to characterize the human microbiota and microbiome(4). In 2012, theconsortium reported a large study that recruited 242 healthy adults,of both gendersbetween the ages of 18 and 40 years,in the United States to further characterize the microbiotaand microbiome. Women underwent sampling at 18 body sites,including the skin, nose, mouth, throat, vagina and feces, and men were sampled at 15 sites(9). Of these subjects, 131 were resampled, with amean of 219 days after the initial sample, to determine the stability of the microbiota and microbiome over time. Bacterial communities were compared using taxonomic profiling of 16S rRNA gene sequences using pyrosequencing, and metagenomic profiling using whole genome shotgun sequencing. The study confirmed previous findings that each body habitat had a distinct microbial communitywith a signature composite of taxa (Table 1). Most metabolic pathways were uniformly distributed across individuals and body habitats, indicating a redundancy in bacterial metabolism. The oral cavity and the stool hadthe most diverse bacterial communities (i.e., the most alpha diversity). Conversely, the vaginal microbial community showed the lowest alpha diversitywith domination by Lactobacillusspecies. The oral cavity had the lowest diversity between subjects (i.e., beta diversity), as opposed to the skin which had the highest beta diversity. The gut microbiota showed an inverse relationship between Bacteroidetes and Firmicutes; subjects dominant in Bacteroideteshad a minority of Firmicutes(9). Overall, bacterial communities were relatively stable comparing the baseline to repeat sampling within the same subject, but the bacterial communitiesshowed large variation between subjects. The stability of the microbiome within an individual suggests a mutually beneficial stable coexistencebetween the microbiota and the human host. The implication is that any disturbance in the microbiota may be predictive of disease.
Table 1.
Predominating Phyla of Specific Human Habitat
| Body Habitat | Predominating Phylaa |
|---|---|
| Mouth | Firmicutes (e.g., Streptococcus) >Proteobacteria (e.g., Haemophilus), Bacteroidetes (e.g., Prevotella) |
| Gut | Bacteroidetes (e.g., Bacteroides), Firmicutes (e.g., Streptococcus) |
| Skin | Actinobacteria (e.g., Propionibacterium) >Firmicutes (e.g., Staphylococcus) |
| Vagina | Firmicutes (e.g., Lactobacillus) |
Predominating Phlya of Specific Body Habitats (adapted from references 8, 9).
Respective genera in dominating phyla are listed in parenthesis.
Lifespan, The Microbiota, And The Immune Response
Newborn children have bacterial communities that reflect vaginal or Cesarean section delivery. Vaginally-delivered newborns have bacterial communities dominated byvaginal flora, such as Lactobacillus species. Conversely, newborns delivered by Cesarean section have microbiota dominant in skin flora, such as Staphylococcusspecies(10). The infant gut microbiota become more diverse over time as diet changes. For example, ingestion of solid food is associated with an increase in gut Bacteroidetes(11). The composition of the gut microbiotain children approximatesthe composition seen in adults by the age of three years (12).
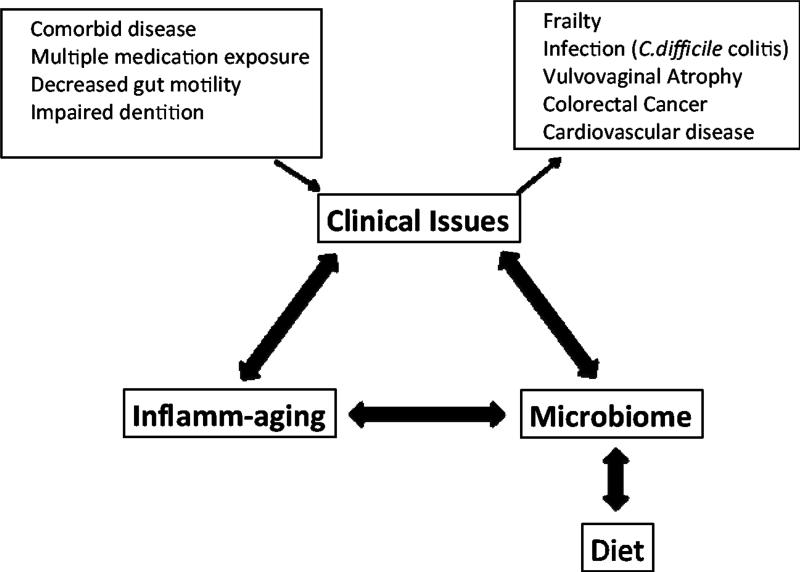
Aging is accompanied by a myriad of clinical issues (Figure 1). Older adults(i.e., over age 65 years) have a high prevalence of co-morbid disease(13) and concomitant exposure to multiple medications, including antibiotics. The aging alimentary tract is subject to a variety of changes:impaired dentition and salivary function, decreased motility with constipation, diverticular disease, and dietary modification(14). Together, these factors may contribute to changes in the microbiota among older adults, and may ultimately enhance susceptibility to infectious diseases. For example Clostridium difficile colitis is thought to arise from a disturbance in the microbiota that is associated with antibiotic use. One can speculate that other infectious diseases (e.g.,urinary tract infection,pneumonia), as well aschronic health conditions, may be associated with changes in the microbiota and the associatedmicrobiome(15, 16).
Figure 1.
Association Between the Microbiome and Clinical Issues Impacting Older Adults.
The term “inflamm-aging” was created to describe the pro-inflammatory environment that exists in older adults(17). Older adults have high concentrations of acute phase reactantsand pro-inflammatory cytokines, andthis inflammation dysregulation is accompanied by impaired immune responses to pathogens and vaccines(18). Inflammation has been implicated in the development of several age-related co-morbid conditions, includingatherosclerosis and neurodegeneration(19, 20). Inflamm-aging may relatetochronic antigenic stimulation from chronic viral infections likeCytomegalovirus (CMV)(21)or fromgut bacterial translocation. Bacterial translocation is supported by high blood levels of lipopolysaccharide (LPS) binding protein and high urinary excretion of microbial by-products among older adults(22, 23).
Accumulating evidence in animal models suggest that the microbiota and microbiome help shape the innate and adaptive immune system(24) with a delicate balance of pro- and anti-inflammatory responses. Toll like receptors (TLRs), pattern recognition receptors that function as sensors of microbial products like LPS, initiate the inflammatory response. TLRs detect commensal bacteria in the gut, and TLR activation by commensals is necessary for protection against gut injury and maintaining homeostasis(25). Additionally, short-chain fatty acids (e.g., butyrate), products of bacterial fermentation by commensals, have anti-inflammatorypropertiesin multiple immune cell types(26). Some Clostridium strains in the gut also show anti-inflammatory properties, by induction of the anti-inflammatory cytokine IL-10 and CD4+ regulatory T cells(27).
There is also accumulating evidence that the microbiome may affect the aging process, as demonstrated in studies of invertebrates such as Caenorhabditis elegans (C. elegans). For example, C.elegans is normally grown in co-culture with bacteria that serve as a source of nutrients and other products that the nematode cannot synthesize itself. Several studies have shown an increase or decrease in worm lifespan depending on the type of coexisting bacteria, and whether specific bacterial products are produced(28).
In conclusion, the above studies suggest that the microbiota may shape the host immune system, contribute to inflamm-aging, lead tochronic health conditions, and modulate the aging process.
The Microbiotaof Older Adults
The ELDERMET consortium was established in 2007 to characterize the intestinal microbiota of older adults. In 2011, the investigators reported the study of 161 adult subjects from Ireland, aged 65 years and older, who underwent fecal sampling; a subset of 26 subjects were re-sampled three months later. Nine younger subjects served as controls. Among older adult subject fecal samples, the phylum Bacteroideteswas dominant at 57% compared with 40% for the phylum Firmicutes. Conversely, the younger control subject fecal samples were dominated by Firmicutes at 51% compared to Bacteroidetes at 41%. Antibiotic exposure was associated with a relative increase in Bacteroidetes and decrease in Firmicutes, Actinobacteria, andProteobacteria. The degree and type of changes in taxa with antibiotic exposure differed between subjects. Overall, the composition of the intestinal microbiota showed much individual variation among older adults(29).
Gut Microbiota, Inflammation and Physical Frailty among Older Adults
In 2012, a follow up study evaluated the intestinal microbiota of 178 older adults of Irish descentaged 64 to 102 years. Thirteen young adults, with a mean age of 36 years, served as a control group. The subjects were stratified by diet and community residence setting to understand the individual variation of intestinal microbiota, and its impact on measures of inflammation and physical frailty. Subjects stratified by residence setting included: 83 whowere community dwellers; 20 who attended an out patient day hospital; 15 who were in short-term (<6 weeks) rehabilitation care; and, 60 who were in long-term residential care(30). UniFracbeta-diversity analysis, a measure of similarity between communities, showed clear separation of the intestinal microbiota between long-term residential subjects andcommunity-dwelling subjects.
Long-term residential subjects were noted to have a higher proportion of the Bacteroidetes phylumin their gut compared to a higher proportion of Firmicutes among the community dwellers. Overall, it took about one year for the intestinal microbiota of long-term residential subjects to deviate from community dwellers.
Analysis of the subjects revealed four diet groups (DG) that were characterized by fat and fiber content (DG 1: low fat/high fiber, DG2: moderate fat/high fiber, DG3: moderate fat/low fiber, DG4: high fat/low fiber). Groups DG1 and 2 included 98% of the day hospital and community dwelling subjects. DG3 and DG4 included 83% of the long-term residential care subjects. The heterogeneity of the microbiota was noted to be most diverse within the DG1 (low fat/high fiber) group and least diverse among the DG3 and DG4 groups. Markers of inflammation were also measured in the subjects. SerumC-reactive protein (CRP), IL-6, IL-8,and TNF-α were found to be higher in long-term residential care subjects and subjects in short term rehabilitation care as compared to community dwellers. Functional status and measures of frailty(e.g., Barthel Index of Activities of Daily Living, Functional Independence Measure, Geriatric Depression Test, Charlson index of co-morbidity, and mini-mental status exams) showed evidence for impaired function and increased frailtyamong the long-term residential care subjects compared to all other groups(30). Overall, site of residence and diet were associated with patterns of intestinal microbiota that also correlated with systemic inflammation and functional impairment (Table 2). Although these associations are without proven cause and effect, one could hypothesize that changes in diet might influence the microbiota and functional capacity of older adults.
Table 2.
Correlation of Microbiota, Diet, Inflammation, and Frailty among Older Adults
| Associations | Long term care (> 6 weeks) | Rehabilitation care (< 6 weeks) | Day Hospital | Community Dwellers |
|---|---|---|---|---|
| Diet Group DG1 low fat/high fiber DG2 moderate fat/high fiber DG3 moderate fat/low fiber DG4 high fat/low fiber |
DG3, DG4 predominate | Variablea | DG1, DG2 predominate | DG1, DG2 predominate |
| Inflammatory Markers (TNF-α, IL-6, IL-8, CRP) | Highest | Intermediateb | Intermediateb | Lowest |
| Functional status/Frailty | Impaired function & frailty predominate | Intermediatec | Intermediatec | Normal function predominates |
| Microbiota Predominating Phyla | Bacteroidetes predominate | Variabled | Variabled | Firmicutes predominate |
Dietary patterns were variable among subjects in rehabilitation care (<6 weeks).
Inflammatory markers for subjects in rehabilitation care (<6 weeks) and day hospital subjects fell in an intermediate range between levels seen in long stay and community dwellers.
Functional status and frailty measures showed a spectrum between community dwellers and long stay subjects. Rehabilitation and day hospital subjects showed variability of function compared to community dwellers that had a predominance of normal function and long stay subjects that had a predominance of impaired function and frailty.
Predominance of either Bacteroidetes andFirmicutes varied among rehabilitation and day hospital subjects, compared to long stay and community dwellers which were more defined with respect to predominance of Phyla.
Contents of table are adapted from reference 30.
Studies onthe island of Sardinia reveal a geographic region that is distinguished by unusually high numbers of centenarians who eat a simple Mediterranean diet(31). The NU-AGE project was designed to determine whether the Mediterranean dietcan affect the process of inflamm-aging, the microbiota, and functional status. This ongoing study includes 1,250 older individuals, aged 65-79 years, from Europe. For one year, half the cohortis designed to ingest the Mediterranean diet and half ingest their normal diet. Investigators will determine whether the Mediterranean diet changes the gut microbiota, inflammatory markers, metabolic signatures, and functional status, compared to a normal diet among older adults (32).
The Role of the Microbiota in Clostridium difficile colitis in Older Adults
Perturbation of the gut microbiota (i.e., dysbiosis) may have implications in many infectious disease processes of older adults, particularly Clostridium difficile colitis.The association of Clostridium difficile infection (CDI) with preceding antibiotic exposure suggests that the intestinal microbiota are altered and predispose the patient to Clostridium difficile colonization, toxin production and disease when exposed to the pathogen(33). Older adults, aged 65 and older, account for the majority of morbidity and mortality associated with CDI (15), and CDI is a common cause of acute diarrhea among nursing home residents. The high burden of CDI among older adults likely relates to their contact with healthcare environments (e.g., hospitals, nursing homes) where they are exposed to both antibiotics and Clostridium difficile spores. Antibiotic treatment of an initial episode of CDI (e.g., with oral metronidazole or vancomycin) results in recurrent disease in up to35% of patients. Manipulation of the fecal microbiota by fecal transplantation from healthy donors is a plausible approach for recurrent disease refractory to repeated antibiotic treatment(34). A recent randomized trial showed that fecal microbiota transplantation (FMT) was effective in the therapy of recurrent CDI. Forty-three patients were randomized to receive either FMT plusoral vancomycin treatment for 4-5 days, oral vancomycin treatment for 14 days alone, or oral vancomycin treatment for 14 days plus bowel lavage on day 4 or 5. Enrolled subjects included older adults with a mean age of > 65 years in all three treatment arms. The primary endpoint was cure without evidence for relapse within 10 weeks of treatment initiation. The infusion of donor feces plus oral vancomycin treatment was found to be superior toboth regimens of vancomycin without donor feces infusion(35). The diversity of the fecal microbiota was low in subjects before fecal infusion, but it approached the diversity of donor microbiota two weeks after fecal infusion.
Recently published results from an animal model showed that a phylogenetically diverse mixture of 6 bacterial strains (selected among the phyla of Firmicutes, Bacteroidetes, and Actinobacteria) cured C. difficile colitis in mice and caused expansion of healthy commensals(36). Data from the human trial and mouse model support the concept that manipulating the intestinal microbiotaby adoptive bacteriotherapyis a plausible strategy to reconstitute the intestinal microbiota and preventre current CDI among older adults.
The Role of the Microbiota in Other Chronic Conditions of Older Adults
The microbiota have been implicated in other physical conditions that affect older adults. Menopause is often accompanied by vulvovaginal atrophy, a syndrome that consists of vaginal dryness, soreness, and dyspareunia. Studies of the vaginal microbiota in postmen opausal women reveal that vulvovaginal atrophy is associated with a low abundance of Lactobacillus (which normally dominates the vaginal flora) and high bacterial diversity(37, 38). Intake of oral lactobacilli may play a role in restoration of vaginal flora as suggested by a small, randomized controlled study in postmen opausal women (39). Restoration of vaginal flora may improve symptoms of vulvovaginal atrophy, but requires further investigation.
Multiple reports suggest that intestinal microbiota play a role in the development of colon cancer, a common disease among older adults(40). Genomic analysis of the microbiota of colon carcinomas and adenomas show an enrichment of Fusobacterium species compared to matched non-cancerous tissue samples(41-43). Patients with colorectal cancer, and colon adenomas, also show enrichment of Fusobacterium species in stool samples(43).In a study comparing stool microbiota of subjects with and without colon cancer, a decrease in butyrate producing bacteria and an increase of opportunistic pathogens were noted in subjects with colon cancer(44). These changes are hypothesized to contribute to the inflammatory environment that may promote tumorigenesis.
The microbiota may also play a role in cardiovascular disease among older adults. The association between periodontal infection and cardiovascular risk is well established in the literature (45). Increased burden of periodontal disease-causing bacteria in the oral cavity, commonly seen among older adults including nursing home residents, is associated with both increased carotid intima media thickness and hypertension (46, 47). Evaluation of the microbiota in the oral cavity and atherosclerotic plaques of subjects with atherosclerosis (compared to healthy controls) shows that the relative abundance of bacterial phyla seen in atherosclerotic plaquescorrelated with the bacterial phyla seen in the oral cavity(48). Production of trimethylamine-N-oxide (TMAO),a pro-atherosclerotic metabolite of phosphatidylcholinein the intestine, has also implicated the gut microbiota in cardiovascular disease. Among a cohort of 4007 subjects with a mean age of 63 years, higher baseline plasma levels of TMAO were predictive of increased cardiovascular events after adjustment of standard risk factors. These data suggest a link between the metabolism of gut microbiota and cardiovascular disease(49), which may have new implications for treating cardiovascular disease in the future.
Overall, the microbiota have been linked to a number of disease processes, both infectious and degenerative, that affect older adults. Current data only describe associations; further investigationis required to assess cause and effect link and whether intervention strategies are effective.
Overall Conclusions
Elie Metchnikoff theorized in the early 20th century that the process of aging was the result ofreactive phagocytes destroying healthy tissueas a response to autotoxins in the intestine. He believed that aging could be delayed by manipulation of the intestinal flora with daily ingestion of yogurt(50). The human immune system is likely influenced by the gut microbiota, and their interaction plausibly contributes to the process of inflamm-aging. Ongoing studies suggest that dietis associated with both the gut microbiota andsystemic inflammation with an impact on functional status of older adults. Future studies are needed to determine a causal relationship.
The complex relationship between humans and the one trillion bacterial microbiota that form our microbiome remains largely unexplored. The implications for medicine are provocative, since it is likely that our complex symbiosis affects every aspect of health. Manipulating the intestinal microbiota and microbiome may be beneficial for maintaining health and treating disease, particularly among older adults. The relationship between the microbiome of other human ecological niches (e.g., oral cavity, skin, vagina) and the development of other clinical diseases that are common among older adults (e.g., pneumonia, urinary tract infection, reactive airways disease, and other malignancies) remain important areas of future study.
ACKNOWLEDGMENTS
This work was supported by grantsT32 AI007517 and K07AG030093 from the National Institutes of Health.
Sponsor's Role: N/A
Footnotes
Author Contributions: Both authors participated in writing the manuscript.
Conflict of Interest: The authors do not have any financial conflicts of interest.
REFERENCES
- 1.Kruif Pd. Microbe Hunters. 1st ed. Cornwall Press; Cornwall, NY: 1926. [Google Scholar]
- 2.Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: A reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc Natl Acad Sci U S A. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosino JF, Highlander S, Luna RA, et al. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luckey TD. Introduction to intestinal microecology. Am J Clin Nutr. 1972;25:1292–1294. doi: 10.1093/ajcn/25.12.1292. [DOI] [PubMed] [Google Scholar]
- 7.Sleator RD. The human superorganism - of microbes and men. Med Hypotheses. 2010;74:214–215. doi: 10.1016/j.mehy.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 8.Costello EK, Lauber CL, Hamady M, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlamangla A, Tinetti M, Guralnik J, et al. Comorbidity in older adults: Nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 14.Biagi E, Candela M, Fairweather-Tait S, et al. Aging of the human metaorganism: The microbial counterpart. Age (Dordr) 2012;34:247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatr Med. 2014;30:79–93. doi: 10.1016/j.cger.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes C. Review: systemic inflammation and Alzheimer's disease. Neuropathol Appl Neurobiol. 2013;39:51–68. doi: 10.1111/j.1365-2990.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills M, Akbar A, Beswick M, et al. Report from the second cytomegalovirus and immunosenescence workshop. Immun Ageing. 2011;8:10. doi: 10.1186/1742-4933-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collino S, Montoliu I, Martin FP, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8:e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehle JR, Jr., Leng X, Kitzman DW, et al. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 28.Heintz C, Mair W. You are what you host: microbiome modulation of the aging process. Cell. 2014;156:408–411. doi: 10.1016/j.cell.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 31.Poulain M, Pes GM, Grasland C, et al. Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Exp Gerontol. 2004;39:1423–1429. doi: 10.1016/j.exger.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Santoro A, Pini E, Scurti M, et al. Combating inflammaging through a Mediterranean whole diet approach: The NU-AGE project's conceptual framework and design. Mech Ageing Dev. 2014;136-137:3–13. doi: 10.1016/j.mad.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Pamer EG. Fecal microbiota transplantation: effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014;7:210–214. doi: 10.1038/mi.2013.117. [DOI] [PubMed] [Google Scholar]
- 34.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 35.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 36.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2013;21:450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hummelen R, Macklaim JM, Bisanz JE, et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS One. 2011;6:e26602. doi: 10.1371/journal.pone.0026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petricevic L, Unger FM, Viernstein H, et al. Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2008;141:54–57. doi: 10.1016/j.ejogrb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Cai G, Qiu Y, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ordovas JM, Mooser V. Metagenomics: The role of the microbiome in cardiovascular diseases. Curr Opin Lipidol. 2006;17:157–161. doi: 10.1097/01.mol.0000217897.75068.ba. [DOI] [PubMed] [Google Scholar]
- 46.Desvarieux M, Demmer RT, Jacobs DR, Jr., et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28:1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intima-media thickness: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackowiak PA. Recycling Metchnikoff: Probiotics, the Intestinal Microbiome and the Quest for Long Life. Front Public Health. 2013;1:52. doi: 10.3389/fpubh.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]