Abstract
Background
Genetic studies of cardiomyopathy and heart failure have limited throughput in mammalian models. Adult zebrafish have been recently pursued as a vertebrate model with higher throughput, but genetic conservation must be tested.
Methods and Results
We conducted transcriptome analysis of zebrafish heart and searched for fish homologues of 51 known human dilated cardiomyopathy (DCM)-associated genes. We also identified genes with high cardiac expression and genes with differential expression between embryonic and adult stages. Among tested genes, 30 had a single zebrafish orthologue, 14 had 2 homologues, and 5 had 3 or more homologues. By analyzing the expression data on the basis of cardiac abundance and enrichment hypotheses, we identified a single zebrafish gene for 14 of 19 multiple-homologue genes and 2 zebrafish homologues of high priority for ACTC1. Of note, our data suggested vmhc and vmhcl as functional zebrafish orthologues for human MYH6 and MYH7, respectively, which are established molecular markers for cardiac remodeling.
Conclusions
Most known genes for human DCM have a corresponding zebrafish orthologue, which supports the use of zebrafish as a conserved vertebrate model. Definition of the cardiac transcriptome and fetal gene program will facilitate systems biology studies of DCM in zebrafish.
Keywords: dilated cardiomyopathy, transcriptome, fetal gene program, zebrafish
Introduction
The identification of DMD (Duchenne muscular dystrophy) and ACTC1 (Actin, alpha cardiac muscle 1) as the first causative genes for X-linked and autosomal-dominant dilated cardiomyopathy (DCM), respectively, opened the door for genetic studies of idiopathic heart failure.1,2 Although mutations in dozens of genes have been linked to DCM,3 current genetic testing panels only have a 37% yield.4 Therefore, the discovery of novel DCM genes and/or modifier genes for cardiomyopathy remains a major challenge. The rapid advance of genomic technologies, such as genomewide linkage analysis, genomewide association studies, whole-exome sequencing, and RNA sequencing (RNA-seq), have accelerated the discovery process. Several candidate genes typically result from such studies, which necessitates further experimental validation and mechanistic studies. Mouse is the most prevalent animal model. However, the high cost of mouse and other mammalian models imposes substantial restrictions. More affordable alternative vertebrate animal models with higher throughput are desirable.
Zebrafish (Danio rerio) is a nonmammalian vertebrate model widely used to study developmental genetics and functional genomics. Genetic resources and tools with higher throughput create novel research opportunities for cardiomyopathy, including 1) the capacity to conduct large-scale mutagenesis screening using as mutagens N-ethyl-N-nitrosourea,5 virus,6 or transposons7; 2) the complete sequence of the zebrafish genome; 3) the development of TILLING technology (targeting induced local lesions in genomes), which has been used to generate mutations for about half the genome (http://www.sanger.ac.uk/Projects/D_rerio/zmp/)8; 4) morpholino technology, which can be used to quickly knock down genes during embryogenesis9; and 5) genome editing technology using TALENs (transcription activator-like effector nucleases) and/or CRISPRs (clustered regularly interspaced short palindromic repeats), which can be used to generate targeted gene knockouts, conditional gene knockouts, large-fragment genome deletions, or gene knockins.10
The positional cloning of titin and tnnt2 as causative genes for pickwick and silent heart, 2 embryonic lethal mutants identified from an N-ethyl-N-nitrosourea–based mutagenesis screen, initiated efforts to apply zebrafish as a model for studying human cardiomyopathy.11,12 Additional studies in zebrafish embryos have been conducted to annotate functions of known cardiomyopathy genes, such as actn2, myh, myl, and lamn,13–15 to discover new cardiomyopathy genes such as nexilin, lama4, and ilk,16–18 to mimic the cardiac remodeling process,19 and to screen compounds of potential therapeutic value.20 However, fish embryos cannot be used to recapitulate the full spectrum of human cardiomyopathy, a disease progressing from an initial compensated phase to a later decompensated stage. Accordingly, adult zebrafish models for cardiomyopathy have been recently pursued. Cardiomyopathy-like phenotypes have been reported in adult fish stressed by either chronic anemia and/or doxorubicin.21,22 However, there are still concerns about the conservation of zebrafish cardiomyopathy models, and gene-based adult models have yet to be generated.
Toward the goal of establishing adult zebrafish as a vertebrate model for large-scale genetic studies of cardiomyopathy, we conducted transcriptome studies of zebrafish heart and assessed the conservation of the zebrafish model by seeking zebrafish orthologues of human DCM-associated genes.3,23 In mammals, many fetal genes, which are quiescent in the adult stages but can be reactivated during heart failure, have been used as molecular markers for the pathogenesis of cardiomyopathy.24 Therefore, we also defined the cardiac transcriptome in both larva and adult zebrafish and conducted differential expression analysis between larva and adult heart.
Materials and Methods
Fish Husbandry
Wild-type WIK fish were used for this study. The study was approved by the Mayo Clinic Institutional Animal Care and Use Committee.
RNA Preparation
Fifty hearts from zebrafish larvae 4 days post fertilization (dpf) were dissected and pooled. This stage was selected because of the maturity of heart structures and the accessibility of the hearts for surgical removal. Five hearts consisting of both atrium and ventricle were dissected and pooled from 6-month-old WIK fish. Muscle samples consisting of both fast-twitch and slow-twitch fibers were dissected from an adult fish. Tissues were homogenized using a mortar and pestle (Fisher Scientific), and total RNA was extracted using TRIzol (Sigma) according to the manufacturer’s instructions. RNA quality was assessed using a 2100 Bioanalyzer Instrument (Agilent Technologies) in the Mayo DNA Sequencing Core Facility.
Preparation of Libraries
RNA libraries were prepared according to the manufacturer’s instructions for the TruSeq RNA Sample Prep Kit v2 (Illumina). The liquid-handling EpMotion 5075 robot (Eppendorf) was used for TruSeq library construction. All AMPure bead (Beckman Coulter) clean up, mRNA isolation, end repair, and A-tailing reactions were completed on the 5075 robot. Reverse transcription and adaptor ligation steps were performed manually. The adapter-modified DNA fragments were then enriched by 12 cycles of polymerase chain reaction (PCR) using primers included in the Illumina Sample Prep Kit. Concentration and size distribution of the libraries were determined on an Agilent Bioanalyzer DNA 1000 chip. Sample concentration was confirmed using Qubit fluorometry (Invitrogen). Libraries were loaded onto paired-end flow cells at concentrations of 8 to 10 pM to generate cluster densities of 700,000/mm2 following Illumina’s standard protocol using the Illumina cBot and cBot Paired-End Cluster Kit version 3. The flow cells were sequenced as 51×2 paired-end reads on an Illumina HiSeq 2000 using TruSeq SBS Sequencing Kit version 3 and HCS v2.0.12 data collection software. Base-calling was performed using Illumina’s RTA version 1.17.21.3.
Mapping of Sequence Reads
The generated FASTQ sequence reads from an Illumina HiSeq instrument were aligned to the latest available zebrafish genome assembly (Zv9) by TopHat.25 At most, 2 mismatches were allowed for the first 32 bases in each alignment. Reads with more than 2 mismatches or that mapped to multiple genomic locations (an alignment score <4) were deleted. In all, 32,677 genes were annotated in Ensembl Zv9. The raw read counts for genes were generated by HTSeq (http://www-huber.embl.de/users/anders/HTSeq/doc/overview.html) for further downstream analyses.
Differential Gene Expression Analysis
For analysis of differential gene expression, we eliminated genes without any reads across all samples. Statistical testing was done using the R package DESeq.26 Because the primary goal of our analysis was to explore the underlying cause of differentially expressed genes between different tissues, an adjusted P value/false discovery rate (to adjust for multiple testing using the Benjamin-Hochberg method) cutoff of 0.01 was used to select significantly changed genes. In addition, we used the following 2 criteria to identify genes differentially expressed in embryo and adult: 1) the number of reads per kilobase per million reads (RPKM) for the gene was ≥0.3 in either the embryonic transcriptome or the adult transcriptome; and 2) the change in the expression level was at least 2-fold.
Functional Annotation Bioinformatics Microarray Analysis (DAVID) (http://david.abcc.ncifcrf.gov/home.jsp) was used to assign genes into pathway categories.27
Quantitative Reverse Transcriptase PCR
The Superscript III First-Strand Synthesis System (Invitrogen) was used to generate cDNA from 500 ng RNA. Quantitative reverse transcriptase PCR (qPCR) was carried out using a Roche LightCycler 480 QPCR apparatus in 96-well QPCR plates (Roche Diagnostics Corp). The expression of the genes was normalized using the expression level of gapdh or actb2 by −ΔΔCt (cycle threshold) values. Nine RNA samples (3 each for embryo heart, adult heart, and adult muscle) were analyzed in triplicate by qPCR. The primers are listed in Supplemental Table 1.
Data Analysis
We used reads per kilobase of transcript per million mapped reads (RPKM) as the normalization method to calculate gene expression in each library of each tissue. In the differential gene expression analysis, the R package DESeq was used to normalize the gene raw read counts by considering both library size and expression distribution. To overcome the overdispersion problem in RNA-seq data, a modified negative binomial distribution model was used.
Results
Definition of Cardiac Transcriptome in a Zebrafish Heart
Using the standard paired-end RNA-seq protocol, we obtained more than 74, 114, and 130 million reads for embryonic hearts, adult hearts, and adult muscles, respectively (Supplemental Table 2). More than 75% of reads could be mapped to the Zv9 zebrafish genome. The reads were highly consistent within the 3 biological repeats for each experimental condition, as indicated by the correlation analysis (Supplemental Figure 1).
Two previous transcriptome studies used RPKM cutoffs of 3 or greater28 or 0.329 to delineate expression levels in the heart. Using the cutoff of 3 RPKM, 5,345 genes, or 16% of the 32,677 genes in the zebrafish genome, met the criteria in an adult zebrafish heart, and 6,169 genes, or 19% of the genome, met the criteria in the embryonic zebrafish heart. Using the cutoff of 0.3 RPKM, 14,797 genes or 45% of the genome and 15,217 genes or 47% of the genome are expressed in an adult heart and an embryonic heart, respectively. Previously, it has been shown that the 200 most abundant mRNAs in mouse heart, which account for less than 1% of all 25,000 mouse gene transcripts, make up approximately 65% of the total cardiac mRNA pool.30 In zebrafish, the 200 most abundant genes made up about 66% and 61% of the total mRNA pool in the embryonic heart and the adult heart, respectively.
Using differential gene expression analysis, we identified 2,795 upregulated genes that exhibit high expression in the embryonic heart but low expression in the adult heart and 3,175 downregulated genes with low expression in the embryonic heart but high expression in the adult heart. Among the upregulated genes, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis identified several related to cell cycle and DNA replication (Supplemental Table 3) (P<.05). The downregulated pathways (P<.05) included those involved in carbohydrate metabolism (Supplemental Table 3). We also discovered decreased expression of genes in the calcium signaling pathway at 4 dpf, which were activated in adult heart (Supplemental Table 4). Some of these genes were orthologues of known “fetal genes” involved in calcium handling,31 including atp2a2a (orthologue of SERCA2A, −3.57-fold change), slc8a1a (orthologue of NCX1, −2.46-fold change), and slc8a2a (orthologue of NCX2, −5.62-fold change).
In mammals, the expression of some sarcomeric genes switches from fetal to adult isoforms after birth. Similarly, in zebrafish we detected 25 genes with high expression in the larval stage that were downregulated at the adult stage (Supplemental Table 5A) and 17 genes with low expression during the embryonic stage that were upregulated at the adult stage (Supplemental Table 5B). qPCR analysis of 11 genes in each group (Supplemental Figure 2A) showed high correlation with the fold changes.
Identification of Zebrafish Homologues of 51 DCM-Associated Genes
Previous human genetic studies suggested 51 genes as causing or conferring susceptibility to inheritable DCM.3,23,32 Our survey of the orthology data in the zfin Data report (http://zfin.org/downloads/human_orthos.txt) and Ensembl (http://www.ensembl.org/index.html) identified zebrafish homologues for 46 of these genes. For the 5 DCM genes that did not have a clear homologue, we searched either the literature or the NCBI database for further information. Results were found for 3 of these genes. For the human gene SCN5A, scn5laa and scn5lab have been suggested as orthologues in zebrafish33; these are currently named scn12aa and scn12ab, respectively, in Ensembl. Ensembl suggests that dsc2l may be the zebrafish orthologue of Desmocollin 2.34 Although 3 DSC genes exist in humans that exhibit tissue-specific expression patterns (DSC1, DSC2, and DSC3), there is only 1 dsc2l gene in zebrafish. Although no zebrafish orthologue of phospholamban was suggested from either zfin or Ensembl, a phospholambanlike gene (NM_001201561.1) was annotated in the NIH database (http://www.ncbi.nlm.nih.gov/nuccore) which exhibits 50% protein identity to the human counterpart. Insufficient information was found for the other 2 genes. There are several candidate zebrafish homologues for FOXD4, but the closest homologue is foxd5. Because the gene is indicated as a potential orthologue with a low confidence score in Ensembl, we consider the zebrafish orthologue of FOXD4 to remain unclear. Finally, we found no zebrafish orthologue for cardiotrophin 1, which might encode a cytokine that only exists in mammals.
Of the 49 total zebrafish homologues for human DCM genes that we identified, 30 had a single orthologue in zebrafish. Identities of the proteins encoded by these orthologues ranged from 10.14% to 86.73% (mean, 59%) (Table 1). The other 19 DCM genes had multiple zebrafish homologues (Supplemental Table 6): 14 with 2 homologues and 5 with 3 or more homologues.
Table 1.
Human DCM-Associated Genes (n=30) With 1 Zebrafish Orthologue
| mRNA Expression, RPKM | |||||||
|---|---|---|---|---|---|---|---|
| Human DCM-Associated Gene | Zebrafish Orthologue |
Ensembl Gene ID | Identity | Embryo Heart |
Adult Heart |
Adult Muscle |
|
| ABCC9 | The subunit SUR2A of cardiac ATP-sensitive potassium channel | abcc9 | ENSDARG00000015985 | 81.67% | 3.37 | 24.61 | 4.27 |
| BAG3 | BCL2-associated athanogene 3 | bag3 | ENSDARG00000039486 | 32.52% | 3.06 | 37.17 | 9.09 |
| CAV3 | Caveolin 3 | cav3 | ENSDARG00000024141 | 73.51% | 8.50 | 11.90 | 20.82 |
| CSRP3 | Muscle LIM protein | csrp3 | ENSDARG00000069975 | 71.65% | 82.04 | 125.24 | 0.30 |
| DMD | Dystrophin | dmd | ENSDARG00000008487 | 81.67% | 0.37 | 0.24 | 7.80 |
| DNAJC19 | DnaJ(Hsp40) homolog, subfamily C, member 19 | dnajc19 | ENSDARG00000044420 | 77.59% | 8.05 | 5.92 | 12.07 |
| DSC2 | Desmocollin 2 | dscl* | ENSDARG00000039677 | 30.00% | 3.61 | 5.87 | 4.10 |
| EMD | Emerin | emd | ENSDARG00000095774 | 20.87% | 4.37 | 2.18 | 1.11 |
| EYA4 | Eyes absent homolog 4 | eya4 | ENSDARG00000012397 | 76.06% | 1.49 | 2.16 | 12.05 |
| FKTN | Fukutin | fktn | ENSDARG00000059437 | 64.44% | 1.84 | 0.98 | 0.55 |
| GATAD1 | GATA zinc finger domain containing 1 | gatad1 | ENSDARG00000027612 | 73.23% | 2.50 | 1.18 | 0.57 |
| ILK | Integrin-linked kinase | ilk | ENSDARG00000056964 | 86.73% | 12.24 | 10.03 | 4.48 |
| LAMA4 | Laminin a-4 | lama4 | ENSDARG00000020785 | 43.23% | 13.40 | 7.77 | 6.71 |
| LMNA | Lamin A/C | lmna | ENSDARG00000013415 | 64.91% | 1.91 | 2.31 | 4.27 |
| LAMP2 | Lysosome-associated membrane protein 2 | lamp2 | ENSDARG00000014914 | 25.25% | 10.10 | 26.71 | 6.49 |
| MYBPC3 | Myosin-binding protein C | mybpc3 | ENSDARG00000011615 | 62.72% | 198.23 | 464.24 | 4.47 |
| MYPN | Myopalladin | mypn | ENSDARG00000076485 | 43.31% | 0.22 | 3.24 | 8.77 |
| NEBL | Nebulette | nebl | ENSDARG00000021200 | 10.14% | 0.00 | 0.03 | 0.02 |
| NEXN | Nexilin | nexn | ENSDARG00000057317 | 57.04% | 47.98 | 81.55 | 23.33 |
| PKP2 | Plakophilin-2 | pkp2 | ENSDARG00000023026 | 54.26% | 1.11 | 3.06 | 1.07 |
| PLN | Phospholamban | cardiac phospholambanlike | ENSDARG00000069404 | 50.00% | 96.91 | 290.34 | 1.91 |
| PSEN1 | Presenilin 1 | psen1 | ENSDARG00000004870 | 53.70% | 6.33 | 2.63 | 1.13 |
| PSEN2 | Presenilin 2 | psen2 | ENSDARG00000015540 | 71.00% | 2.91 | 1.74 | 2.50 |
| RBM20 | RNA binding protein 20 | rbm20 | ENSDARG00000087769 | 40.00% | 2.62 | 12.08 | 6.99 |
| SDHA | Succinate dehydrogenase complex, subunit A, Flavoprotein | sdha | ENSDARG00000016721 | 80.00% | 44.39 | 91.56 | 44.49 |
| SGCD | delta-Sarcoglycan | sgcd | ENSDARG00000009789 | 72.00% | 4.46 | 2.95 | 6.86 |
| TAZ | Tafazzin | taz | ENSDARG00000041421 | 60.27% | 2.02 | 3.27 | 0.92 |
| TCAP | Titin-cap or telethonin | tcap | ENSDARG00000007344 | 40.12% | 0.35 | 141.31 | 161.93 |
| TPM1 | α-Tropomyosin | tpm1 | ENSDARG00000087402 | 79.00% | 16.84 | 1.64 | 78.93 |
| VCL | Metavinculin | vcl | ENSDARG00000044968 | 84.00% | 2.98 | 6.75 | 7.90 |
Abbreviations: DCM, dilated cardiomyopathy; RPKM, reads per kilobase per million reads.
All human and zebrafish genes were 1-to-1 orthologues except DSC2, in which many human genes had 1 zebrafish orthologue.
Assessment of Cardiac Abundance of the 30 DCM Genes With a Single Orthologue
It is posited that genes with higher abundance in a particular organ must have important roles in this organ. This abundance hypothesis motivated efforts to generate a cardiac expressed sequence tag collection,35 to define genes with higher expression levels in the heart via RNA-seq analysis,28 and to enrich cardiac mutant lines via an expression-based strategy.36 Given that DCM-associated genes have been shown to have pivotal roles in cardiac diseases, we set out to test the abundance hypothesis by assessing the expression level of the 30 genes with a single zebrafish orthologue.
When RPKM ≥0.3 was used as the cutoff, 28 orthologues (93%) met the criteria in both adult and embryonic heart; dmd and nebl did not meet the criteria (Table 1). We then compared the expression level of the 30 DCM orthologues with those of the whole genome. The average RPKM value of the orthologues for the 30 DCM genes was significantly higher than that of the whole genome (mean, 1.0×100.77 vs 1.0×10−0.33; P<.001) in adult heart (Supplemental Figure 3A). Similarly, the average expression level of the orthologues for the 30 genes was significantly higher in embryonic heart than in the whole genome (mean, 1.0×100.56 vs 1.0×10−0.18) (Supplemental Figure 3B).
When RPKM ≥3 was used as the cutoff, 19 of 30 genes (57%) were considered highly expressed in adult heart, and 17 of 30 genes (63%) were highly expressed in embryo heart. Together, our expressional analysis of 30 DCM genes supports the abundance hypothesis. Moreover, our data support that RPKM ≥0.3 is a reasonable cutoff to define the zebrafish cardiac transcriptome.
Prioritization of Zebrafish Homologues for 19 DCM-Associated Genes With Multiple Homologues
To facilitate future genetic studies of the 19 DCM-associated genes with multiple homologues, we attempted to prioritize the corresponding 59 zebrafish homologues according to their cardiac expression profile. We applied the abundance hypothesis for this prioritization process—among multiple homologues of the same DCM-causative gene, the homologue with higher abundance in the heart is given higher priority. Moreover, we applied a cardiac enrichment hypothesis in our prioritization process—among multiple homologues of the same DCM gene, the homologue exhibiting higher expression in the heart than in the muscle was given higher priority.
By applying these 2 criteria to 28 homologues for the 14 DCM genes with 2 homologues, we could prioritize 1 homologue over the other for 10 DCM genes (Table 2) if the combined score for these candidate genes was determined to be more than 5, which was our arbitrary cutoff value. However, we could not prioritize homologues for the other 4 DCM genes, ANKRD1, LDB3, TMPO, and TTN. In contrast to cardiac expression of ANKRD1 in human, the expression of both ankrd1a and ankrd1d was low in zebrafish heart (RPKM<0.3). For LDB3 and TTN, both ldb3a/b and ttna/b were enriched in the muscle but not in the heart. For TMPO, both tpmoa and tpmob had comparable cardiac and skeletal expression levels. Thus, more experiments are needed to determine if their functions are redundant in DCM pathogenesis.
Table 2.
Cardiac Abundance and Cardiac Enrichment for Zebrafish Homologues of 19 Human DCM-Associated Genes
| Zebrafish Orthologue | Abundance, % | Score* | Cardiac Enrichment |
Score† |
|---|---|---|---|---|
| ACTC1 | ||||
| acta1a | 1.32 | 128.55 | √√√√ | |
| acta1b | 20.22 | √ | 248.67 | √√√√ |
| hm:zewp0073 | 3.77 | 157.02 | √√√√ | |
| actc1a | 74.58 | √√√ | 178.41 | √√√√ |
| actc1b | 0.12 | 0.00 | ||
| ACTN2 | ||||
| actn2 | 99.66 | √√√√ | 258.31 | √√√√ |
| CABZ01111872.1 | 0.34 | 14.23 | √√√ | |
| ANKRD1 | ||||
| ankrd1a | 57.29 | √√ | 0.08 | |
| ankrd1b | 42.71 | √√ | 3.82 | √√ |
| CRYAB | ||||
| cryaba | 0.00 | NA | ||
| cryabb | 100.00 | √√√√√ | 1.02 | |
| DES | ||||
| desma | 99.96 | √√√√ | 2.68 | √√ |
| desmb | 0.04 | 3.47 | √√ | |
| DSP | ||||
| dspa | 35.55 | √ | 0.63 | |
| dspb | 64.45 | √√√ | 2.88 | √√ |
| FHL2 | ||||
| fhl2a | 72.18 | √√√ | 7.99 | √√ |
| fhl2b | 27.82 | √ | 9.28 | √√ |
| LDB3 | ||||
| ldb3a | 22.08 | √ | 0.38 | |
| ldb3b | 77.92 | √√√ | 0.37 | |
| MYH6 and MYH7 | ||||
| myh6 | 7.73 | 1082.61 | √√√√√ | |
| vmhc | 1.10 | 22.40 | √√√ | |
| vmhcl | 87.87 | √√√√ | 1784.02 | √√√√√ |
| CR450736.2 | 0.00 | 0.00 | ||
| CU633479.5 | 0.00 | 0.02 | ||
| CU633479.6 | 0.00 | 0.00 | ||
| si:ch211-24n20.3 | 0.00 | 0.00 | ||
| myh7ba | 3.00 | 10.62 | √√√ | |
| myh7bb | 0.30 | 8.73 | √√ | |
| PDLIM3 | ||||
| pdlim3a | 17.32 | 13.16 | √√√ | |
| pdlim3b | 82.68 | √√√√ | 6.19 | √√ |
| SCN5A | ||||
| scn12aa | 0.08 | 1.10 | ||
| scn12ab | 99.92 | √√√√ | 111.39 | √√√√ |
| SYNE1 | ||||
| syne1a | 69.95 | √√√ | 3.38 | √√ |
| syne1b | 30.05 | √ | 1.19 | |
| SYNE2 | ||||
| syne2a | 0.78 | 0.02 | ||
| syne2b | 99.22 | √√√√ | 2.28 | √√ |
| TMPO | ||||
| tmpoa | 55.88 | √√ | 1.13 | |
| tmpob | 44.12 | √√ | 0.57 | |
| TNNC1 | ||||
| tnnc1a | 89.20 | √√√√ | 1639.25 | √√√√√ |
| tnnc1b | 10.80 | 1.53 | ||
| TNNI3 | ||||
| tnni1a | 0.05 | 0.55 | ||
| tnni1al | 0.01 | 0.01 | ||
| tnni1b | 68.57 | √√√ | 152.79 | √√√√ |
| tnni1c | 0.30 | 0.03 | ||
| tnni1d | 0.23 | 0.20 | ||
| tnni2a.1 | 0.03 | 0.10 | ||
| tnni2a.2 | 1.20 | 0.23 | ||
| tnni2a.3 | 0.18 | 0.00 | ||
| tnni2a.4 | 0.01 | 0.00 | ||
| tnni2b.1 | 0.00 | 0.01 | ||
| tnni2b.2 | 0.15 | 0.00 | ||
| zgc:112242 | 2.99 | 1.59 | ||
| zgc:101560 | 26.26 | √ | 60.47 | √√√ |
| si:dkey-206m15.8 | 0.01 | 0.01 | ||
| TNNT2 | ||||
| tnnt2a | 99.92 | √√√√ | 1427.04 | √√√√√ |
| tnnt2b | 0.06 | 0.30 | ||
| tnnt2c | 0.02 | 1.08 | ||
| TTN | ||||
| ttna | 57.86 | √√ | 0.26 | |
| ttnb | 42.14 | √√ | 0.17 |
Abbreviations: DCM, dilated cardiomyopathy; NA, not applicable.
The abundance is defined as the percentage of expression level for each homologue among all homologues for the same DCM-causative gene (ie, RPKM of 1 homologue/[sum of RPKM of all homologues for the same DCM-causative gene]). The percentage of a homologue more than 20%, 40%, 60%, 80%, or 100% is assigned an abundance priority score of 1 (√) through 5 (√√√√√), respectively.
The score for cardiac enrichment is calculated by the ratio of RPKM in heart to that in muscle. A ratio more than 2-, 10-, 100-, or 1,000-fold is assigned an enrichment priority score of 2 (√√) through 5 (√√√√√), respectively.
We then applied the 2 criteria to analyze 31 homologues for the 5 DCM genes with 3 or more homologues (Table 2). Among the 3 homologues of TNNT2, only tnnt2a scored much higher than the others and thus is recommended. Compared with 3 TNNI homologues in mammals, including TnI-fast, TnI-slow, and TnI-cardiac,37 there are 14 troponin I homologues in zebrafish, including 2 tandem duplications of tnni2a.1-4 and tnni2b1-2. None of them is assigned as the zebrafish TNNI3 orthologue in the zfin database, whereas some of them are recommended by Ensembl with low confidence. According to our RNA-seq data, tnni1b was the major cardiac isoform with both high abundance and cardiac-enriched expression and thus is recommended. Our data also suggested that tnni2a.3 and tnni2b.2 were the 2 major skeletal muscle isoforms. No ACTC1 orthologue is reported in the zfin database, and only acta1b is listed as an orthologue in Ensembl. Our RNA-seq data indicated that actc1a was the major actin isoform and acta1b was the minor isoform in both embryonic and adult hearts. In contrast, actc1b is a skeletal muscle-enriched isoform that accounts for 99.79% of total actin at the mRNA level in adult muscle. Therefore, both actc1a and acta1b should be considered when studying ACTC1-based DCM in zebrafish. qPCR of 10 genes in each condition showed high correlation of the fold changes between the 2 methods (Supplemental Figure 2B) and confirmed the relative expression of TTNI3 and ACTC1 homologues by semiquantitative reverse transcriptase PCR (Supplemental Figure 4).
Prioritization of Zebrafish Myosin Heavy Chain Homologues
Zebrafish have 9 MYH homologues. Seven homologues share more than 80% protein identity with MYH6 and MYH7, but myh7ba and myh7bb share 67% and 66% identities, respectively, with MYH7 (Supplemental Table 6). In a 4 dpf embryonic heart, myh6 (also termed amhc, atrial myosin heavy chain), vmhc (ventricle myosin heavy chain), and vmhcl (ventricle myosin heavy chain like) represent the 3 most abundant homologues (12.13%, 31.36%, and 53.36%, respectively), whereas in an adult heart, vmhcl contributes to 88% of the total myh transcripts. According to the cardiac enrichment hypothesis, myh6 and vmhcl are highly enriched in heart compared with muscle (Table 2, Figure 1A and B).
Figure 1.
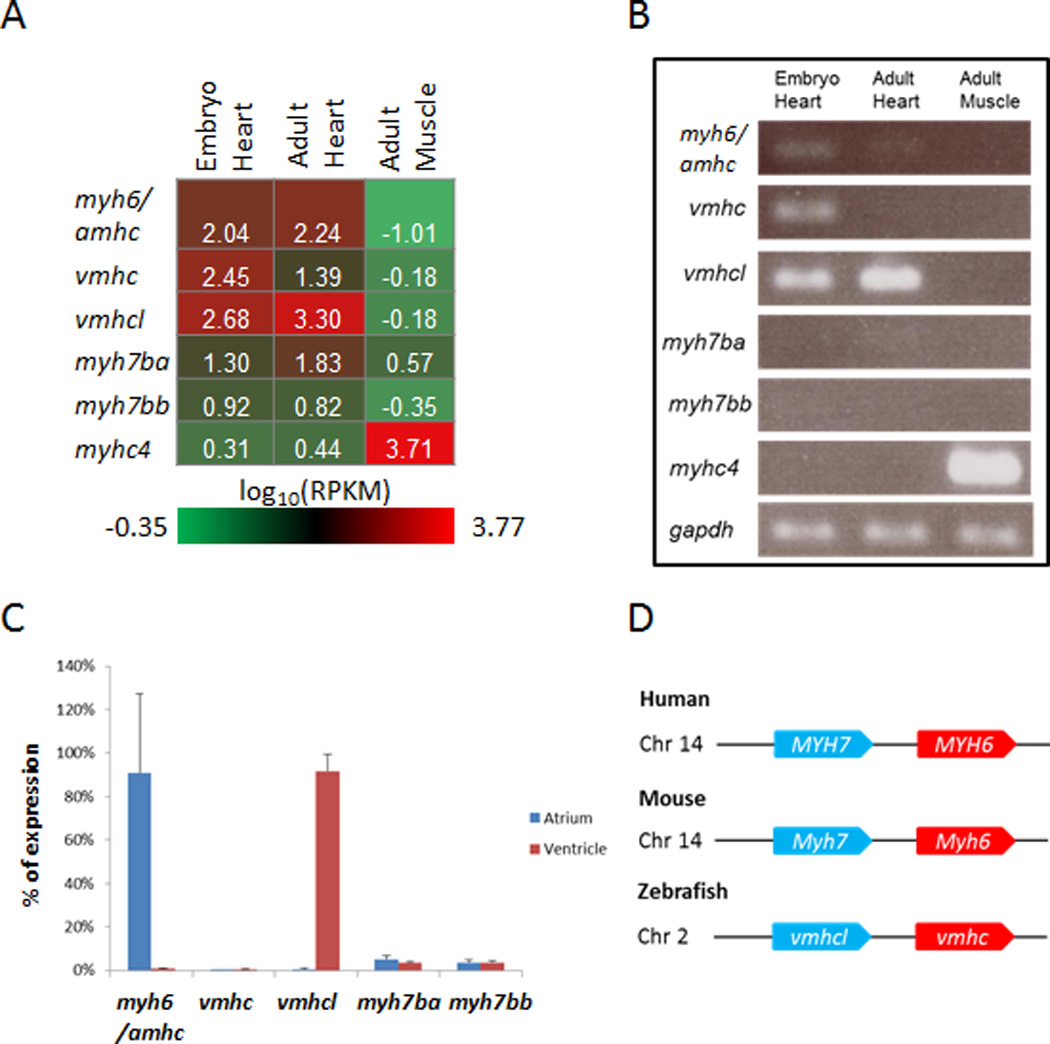
Expressional Analysis of MYH Homologues in Zebrafish. A, Heat map of the average expression in log10(reads per kilobase per million reads [RPKM]) for myh6/amhc, vmhc, vmhcl, myh7ba, myh7bb, and myhc4 (muscle-specific myh) in embryo heart, adult heart, and adult muscle. B, Validation of the RNA sequencing data in (A) showing expression levels using semiquantitative reverse transcriptase polymerase chain reaction (semi-qPCR). gapdh was used as control. C, Expression of myh6/amhc, vmhc, vmhcl, myh7ba, and myh7bb in adult atrium and ventricle as a percentage of total expression level from qPCR, normalized to the expression of gapdh. D, Schematics of genomic region for MYH6/MYH7 homologues in human, mice, and zebrafish. Chr indicates chromosome.
Because previous studies indicated that myh6/amhc expresses specifically in the atrium, while vmhc only expresses in the ventricle,15 we checked chamber specificity for these promising myh homologues (Figure 1C). Consistent with its embryonic expression, myh6/amhc retained its atrium-restricted expression pattern and contributed to more than 90% of the myosin transcripts in atrium. vmhcl expressed specifically in the ventricle and contributed to more than 90% of the myosin transcripts in the ventricle. In contrast to its abundant expression in the embryonic ventricle, vmhc was almost undetectable (less than 0.5%) in either atrium or ventricle of the adult. The expression of myh7ba and myh7bb were also quite low (less than 5%) in both chambers.
In humans, both MYH7 (α-MHC) and MYH6 (β-MHC) are expressed in myocardium and cause cardiomyopathy when mutated.38,39 These genes are in tandem on chromosome 14, with MYH6 located 5.3 kb downstream of MYH7 (Figure 1D), and their expression is developmentally regulated. MYH6 is mainly expressed in embryonic heart, whereas MYH7 becomes the predominant adult isoform.40 Similarly, mouse Myh7 and Myh6 are located in tandem on chromosome 14, with myh6 located 5.3 kb downstream of myh7. Different from human, Myh7 is mainly expressed in the embryonic rodent heart, whereas Myh6 becomes the predominant adult isoform.41
In zebrafish, vmhcl and vmhc are located in tandem on chromosome 2, with vmhc approximately 4.4 kb downstream of vmhcl. Similar to that in human, vmhc mainly expresses in embryo, whereas vmhcl becomes the predominant adult isoform. To determine whether vmhcl and vmhc respond to cardiac stresses, we assessed their expression in doxorubicin-induced cardiotoxicity.21 vhmc expression was significantly activated and peaked at 3 dpi, and vmhcl expression was decreased. The ratio of vmhc/vmhcl was increased by 25-fold compared with that in an uninjected fish heart (Figure 2A). Consistent with fetal gene responses in mammals, we also detected gene activation for nppa and nppb, 2 established molecular markers for cardiac remodeling (Figure 2B), and downregulation of genes in calcium-handling pathways, including pln, ryr2b, atp2a2, and slc8a1a, after doxorubicin treatment (Figure 2C). Interestingly, nppa expression recovered to basal levels at 2 weeks post injection, whereas nppb remained activated at least until 3 weeks post injection.
Figure 2.
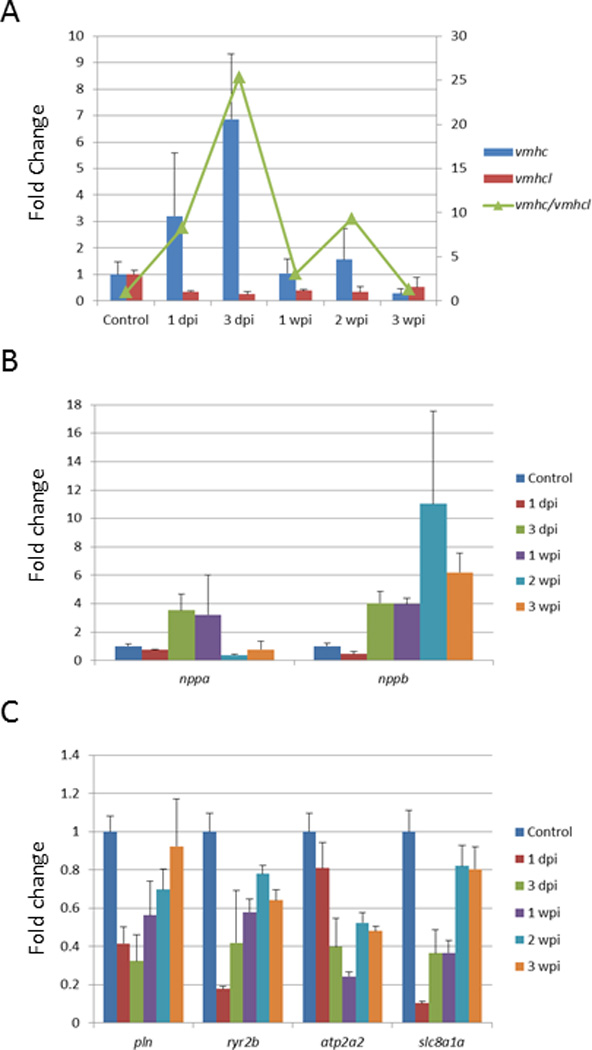
Transcripts of vmhc and vmhcl Respond to Doxorubicin. Adult zebrafish were injected with doxorubicin (20 mg/g), and gene expression in ventricle was determined using qPCR at 1 day, 3 days, 1 week, 2 weeks, and 3 weeks after injection. The expression level was normalized to that of actb2. Shown are expression levels of vmhc and vmhcl (A), nppa and nppb (B), and pln, ryr2b, atp2a2, and slc8a1a (C). In A, the ratio of vmhc to vmhcl was increased compared with that in an uninjected fish heart (green line), right scale. dpi indicates days post injection; wpi, weeks post injection. N=3 in each experimental group.
Discussion
Transcriptome Analysis in a Zebrafish Heart
In this study, we defined the transcriptome of zebrafish heart. Analysis of the expression of zebrafish orthologues for 30 DCM-causative genes showed that more than 60% of the genes are in the top 16% for expression level, and 93% are in the top 45% for expression level. These data provide quantitative support to the abundance hypothesis; that is, genes with higher abundance in a particular organ have more important roles in that organ. Accordingly, the following strategy could be used to improve the efficiency of large-scale genetic studies of DCM. One could initially focus on ≈5,000 genes (≈16% of the genome, identified using RPKM ≥3) as a pilot study, which is predicted to uncover about half of DCM-related genes. If successful, one could extend the study to the additional ≈10,000 genes (≈45% of the genome, identified using RPKM ≥0.3) in an effort to uncover most of the remaining DCM-related genes.
By comparing the cardiac transcriptome of a 4 dpf larval fish and an adult fish, we defined the fetal gene program in a zebrafish heart. Among the approximately 5,000 genes we identified with differential expression, many upregulated genes and their related pathways reflect high cell proliferation in the larval stage. Our pathway analysis also uncovered activated genes in the Hedgehog and the TGF-beta signaling pathway (Supplemental Tables 7 and 8), which have been implicated in cardiogenesis.42 Several genes related to calcium handling and muscle contraction were identified, and their mammalian orthologues have been used as molecular markers for cardiac remodeling. Interestingly, we noted downregulated genes in carbohydrate metabolism in the larval stage, which differs from mammals.43 This discrepancy probably reflects the difference between a zebrafish heart that develops ex utero and consumes the yolk composed of lipid and triacylglycerol and a mammalian heart that develops in utero and mainly consumes glucose delivered via the circulating blood.
Transcriptome Analysis Facilitates the Prioritization of DCM-Associated Genes in Zebrafish
Of the 51 human DCM-associated genes, 19 had more than 1 homologue in zebrafish. Because of a genome duplication event in teleost fish,44 approximately 15% (3,105/20,479) of human genes have more than 1 orthologue in zebrafish. We applied the cardiac abundance hypothesis to assess the expression of different homologues for each DCM-associated gene, which appears to be highly effective. The expression of actn2, cryabb, desma, scn12ab, synbe2b, and tnnt2 contributes to more than 99% of the transcripts within the gene family, which strongly suggests their predominant roles in the heart. We also used the cardiac enrichment hypothesis to prioritize homologues, which partially reflects the subfunctionalization of the duplicated genes. Among the 59 fish homologues, 22 are cardiac-enriched genes and 20 are muscle-enriched genes; many of them exhibit more than 100-fold greater expression levels (Supplemental Table 9). By combining the 2 self-evident hypotheses, our scoring system allows us to effectively prioritize homologues for DCM genes, based on a combined score higher than 5. Although ultimate proof requires evidence from genetic manipulation of each homologue to model DCM pathogenesis, we advise starting with the recommended homologues.
Our systematic analysis of DCM-associated genes underscores the current status of the zfin database and Ensembl. Additional new homologues for the 51 DCM genes may be identified in the future. Some genes may be improperly annotated in the current genome. For example, actc1b should be renamed to reflect its identity as a skeletal muscle actin, not a cardiac actin, whereas acta1a and acta1b should be classified as cardiac muscle actins, not skeletal muscle actins. Combining the evidence from chromosome localization and cardiac expression profiles during development and cardiac stress, our data suggest vmhcl and vmhc as zebrafish functional orthologues for MYH7 and MYH6, respectively. More experimental evidence is required, however, before they are annotated myh7 and myh6, respectively, since no synteny was found between zebrafish and human (data not shown). Also, amhc, not the currently used myh6 in zfin, is a more proper name for this atrium-specific homologue of MYH.
Limitations
We did not observe developmental expression changes between nppa and nppb (1.84- and 1.44-fold changes between larval and adult stage, respectively), despite both genes being molecular markers for cardiomyopathy20–22 and in DOX-induced cardiotoxicity (Figure 2B). This observation could be explained by dynamic expression of nppa and nppb during cardiogenesis, which peaks at day 2 and then decreases.20 In the future, transcriptomes should be analyzed at more time points, especially during cardiogenesis. In addition, total RNA was extracted from whole hearts, which contain heterogeneous cell populations. It is plausible that genes with either cell type–specific or tissue-restrictive expression patterns are underrepresented. To address this concern, specific cell types could be isolated by fluorescence-activated cell sorting, which can then be subjected to RNA-seq analysis. Alternatively, single-cell–based RNA-seq technology can be explored.45
Zebrafish is a Conserved Model for Studying DCM
Recently, it has been shown that 71.4% (14,623/20,479) of human protein-coding genes have at least 1 related zebrafish homologue and that 82% of human disease-associated genes have a zebrafish counterpart.46 Here, we demonstrated that 96% of DCM-associated genes have corresponding homologues in zebrafish, supporting our central hypothesis that zebrafish is a conserved nonmammalian model suitable for studying cardiomyopathies. In fact, as a teleost fish, zebrafish is among the lowest-level vertebrate models with a chambered heart structure and conserved cellular layers including epicardium, myocardium, and endocardium.47
Our systematic identification and prioritization of fish homologues of DCM-associated genes will facilitate genetic studies in zebrafish, including the generation of gene-based DCM models. Besides mutants that have already been generated by TILLING, mutants for the remaining genes can be easily generated by using TALEN and/or CRISPR genome editing technology.10 It is anticipated that the efficient zebrafish model will contribute to the validation of genetic variants discovered in human patients, the elucidation of pathological pathways, and the development of novel therapeutics for DCM.
Supplementary Material
Acknowledgments
We thank Christopher Kolbert, Bruce Eckloff, and Vernadette Simon at the Mayo Medical Genome Facility Gene Expression Core for conducting RNA-seq experiments, and Beninio Gore and Kashia Stragey for zebrafish maintenance.
Funding Sources: This work was supported in part by funds from the NIH (HL81753 and HL107304 to X.X., P30 CA 134274-04 to Y.Z.) and the Mayo Foundation (to X.X.).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Muntoni F, Cau M, Ganau A, Congiu R, Arvedi G, Mateddu A, et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N Engl J Med. 1993;329:921–925. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 2.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 3.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013;123:19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugh TJ, Kelly MA, Gowrisankar S, Hynes E, Seidman MA, Baxter SM, et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med. 2014;16:601–608. doi: 10.1038/gim.2013.204. [DOI] [PubMed] [Google Scholar]
- 5.Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark KJ, Balciunas D, Pogoda HM, Ding Y, Westcot SE, Bedell VM, et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat Methods. 2011;8:506–515. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delvecchio C, Tiefenbach J, Krause HM. The zebrafish: a powerful platform for in vivo, HTS drug discovery. Assay Drug Dev Technol. 2011;9:354–361. doi: 10.1089/adt.2010.0346. [DOI] [PubMed] [Google Scholar]
- 9.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 10.Campbell JM, Hartjes KA, Nelson TJ, Xu X, Ekker SC. New and TALENted genome engineering toolbox. Circ Res. 2013;113:571–587. doi: 10.1161/CIRCRESAHA.113.301765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, et al. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet. 2002;30:205–209. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- 12.Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Xu X. α-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis. FASEB J. 2012;26:4230–4242. doi: 10.1096/fj.12-207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Huang W, Dahme T, Rottbauer W, Ackerman MJ, Xu X. Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovasc Res. 2008;79:97–108. doi: 10.1093/cvr/cvn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- 16.Hassel D, Dahme T, Erdmann J, Meder B, Huge A, Stoll M, et al. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med. 2009;15:1281–1288. doi: 10.1038/nm.2037. [DOI] [PubMed] [Google Scholar]
- 17.Knoll R, Postel R, Wang J, Kratzner R, Hennecke G, Vacaru AM, et al. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- 18.Musso G, Tasan M, Mosimann C, Beaver JE, Plovie E, Carr LA, et al. Novel cardiovascular gene functions revealed via systematic phenotype prediction in zebrafish. Development. 2014;141:224–235. doi: 10.1242/dev.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Hartjes KA, Nelson TJ, Xu X. Cessation of contraction induces cardiomyocyte remodeling during zebrafish cardiogenesis. Am J Physiol Heart Circ Physiol. 2014;306:H382–H395. doi: 10.1152/ajpheart.00721.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker JR, Robinson TY, Sachidanandan C, Kelly AE, Coy S, Peterson RT, et al. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc Res. 2012;93:463–470. doi: 10.1093/cvr/cvr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, et al. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res. 2011;109:658–669. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Hoage T, Bai P, Ding Y, Chen Z, Zhang R, et al. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One. 2009;4:e6596. doi: 10.1371/journal.pone.0006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teekakirikul P, Kelly MA, Rehm HL, Lakdawala NK, Funke BH. Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. J Mol Diagn. 2013;15:158–170. doi: 10.1016/j.jmoldx.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham WT, Gilbert EM, Lowes BD, Minobe WA, Larrabee P, Roden RL, et al. Coordinate changes in Myosin heavy chain isoform gene expression are selectively associated with alterations in dilated cardiomyopathy phenotype. Mol Med. 2002;8:750–760. [PMC free article] [PubMed] [Google Scholar]
- 26.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang da W, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, et al. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Gao C, Peng G, Greer C, Ren S, Wang Y, et al. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ Res. 2011;109:1332–1341. doi: 10.1161/CIRCRESAHA.111.249433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramskold D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol. 2009;5:e1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., 2nd Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res. 2010;106:1459–1467. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 32.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 33.Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, et al. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res. 2010;106:1342–1350. doi: 10.1161/CIRCRESAHA.109.213132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goonesinghe A, Luan XM, Hurlstone A, Garrod D. Desmosomal cadherins in zebrafish epiboly and gastrulation. BMC Dev Biol. 2012;12:1. doi: 10.1186/1471-213X-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ton C, Hwang DM, Dempsey AA, Tang HC, Yoon J, Lim M, et al. Identification, characterization, and mapping of expressed sequence tags from an embryonic zebrafish heart cDNA library. Genome Res. 2000;10:1915–1927. doi: 10.1101/gr.10.12.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Y, Liu W, Deng Y, Jomok B, Yang J, Huang W, et al. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circ Res. 2013;112:606–617. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy AM, Jones L, 2nd, Sims HF, Strauss AW. Molecular cloning of rat cardiac troponin I and analysis of troponin I isoform expression in developing rat heart. Biochemistry. 1991;30:707–712. doi: 10.1021/bi00217a018. [DOI] [PubMed] [Google Scholar]
- 38.Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, et al. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112:54–59. doi: 10.1161/CIRCULATIONAHA.104.507699. [DOI] [PubMed] [Google Scholar]
- 39.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 40.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–2324. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- 42.Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–2396. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- 43.Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- 44.Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, et al. Vertebrate genome evolution and the zebrafish gene map. Nat Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- 45.Ramskold D, Luo S, Wang YC, Li R, Deng Q, Faridani OR, et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol. 2012;30:777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. Anat Rec. 2001;264:1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


