Abstract
Objectives
The importance of detecting human papillomavirus (HPV) in head and neck squamous cell carcinoma (HNSCC) has resulted in a growing expectation for HPV testing of clinical samples. Although testing protocols vary, most pertain to primary tumor biopsies/resections. Testing of fine needle aspirates and core biopsies (FNACB) is advantageous, but it is unclear whether technical and biologic factors adversely affect the fidelity of HPV detection in these samples.
Methods
Data was collected for 85 patients with regionally metastatic HNSCC that had undergone FNACB with HPV analysis as part of clinical care. HPV testing consisted of p16 immunostaining and HPV in-situ hybridization (ISH). The FNACBs were compared with the subsequent biopsies/resections for HPV status.
Results
P16 staining was present in 60 (71%) cases. P16 positivity was predictive of oropharyngeal origin (p<0.001), and correlated with the presence of HPV by ISH (98% correlation). On comparison of the metastases and primary cancers, HPV status was concordant in 58 of 59 cases (98%).
Conclusions
For patients with metastatic HNSCC, p16 staining reliably reflects HPV status of the primary tumor. P16 staining of FNACBs may obviate the need for more invasive sampling of the primary cancer solely for the purpose of HPV testing.
Keywords: aspiration biopsy, fine needle aspiration, HPV, head and neck
Introduction
The human papillomavirus (HPV), particularly type 16, has been established as an important cause of about 25% of head and neck squamous cell carcinomas (HNSCC) overall and up to 80% of those HNSCCs arising in the oropharynx (OPSCC) [1,2]. The identification of HPV in OPSCC identifies a distinct form of HNSCC that is highly responsive to various forms of therapy and is associated with a favorable clinical outcome [3–5]. Accordingly, there has been a move among cancer care organizations towards mandatory HPV testing for all patients with OPSCC. Based on a comprehensive meta-analysis of the medical literature, Cancer Care Ontario recently established guidelines for HPV testing that called for: 1) HPV testing of all OPSCCs; 2) HPV testing of metastatic squamous cell carcinomas to neck lymph nodes of patients with occult primary tumors; and 3) initial determination of HPV status in OPSCCs using p16 immunohistochemical staining (https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=279836). Although these recommendations are reasonable, they do not adequately address some of the more germane issues surrounding HPV testing in the clinical arena.
Patients commonly enter oncologic care based on a neck mass diagnosed as metastatic HNSCC by cytopathologic assessment. For these patients who have not yet had a tissue diagnosis of the carcinoma at its primary site, future guidelines must address practical concerns regarding the nature and sufficiency of HPV testing in fine needle aspirates and core biopsies (FNACBs). Potentially, use of these samples as a substrate for HPV assessment could clarify HPV tumor status and eliminate the requisite excisional tissue biopsy via a more aggressive surgical procedure. While the feasibility of p16 immunostaining in fine needle aspirates and biopsies of cervical lymph node metastases has been confirmed in a limited number of studies [6–8], p16 status of nodal metastases as a proxy for HPV status of the HNSCC at its primary site has not been confirmed in a comparative analysis. Toward this end, we analyzed our clinical experience of p16 immunostaining of FNACBs in patients presenting with nodal metastases in an effort to: 1) confirm the specificity of p16 staining for oropharyngeal origin; 2) correlate p16 immunostaining with actual presence of high risk HPV as determined by HPV DNA; and 3) establish the fidelity of p16 status across regional and primary sites.
Patients and Methods
Cases
Data was collected on all cases where, as part of clinical care at the Johns Hopkins Hospital, p16 staining was performed on fine needle aspirates of metastatic squamous cell carcinomas involving cervical lymph nodes. At our institution, it is now common practice to supplement these fine needle aspirates with core needle biopsies to enhance sample adequacy, and these core biopsies were also used for HPV analysis (Figure 1). The fine needle aspirates were ultrasound guided and performed using a 25-gauge spinal needle, and the core biopsies were performed with a 20 gauge core biopsy device. Medical records including the cytopathology and surgical pathology reports were reviewed to document patient age and gender, tumor diagnosis, the primary site of tumor origin, and results of p16 staining and HPV DNA in situ hybridization (ISH). All pathology slides were reviewed including the cytopathology slides and, when available, the histopathology slides taken of the subsequent tumor resections and biopsies. For those cases with incomplete data sets (e.g. p16 immunohistochemistry and/or HPV DNA ISH not originally performed on primary tumor resection), the blocks were retrieved to complete HPV analysis.
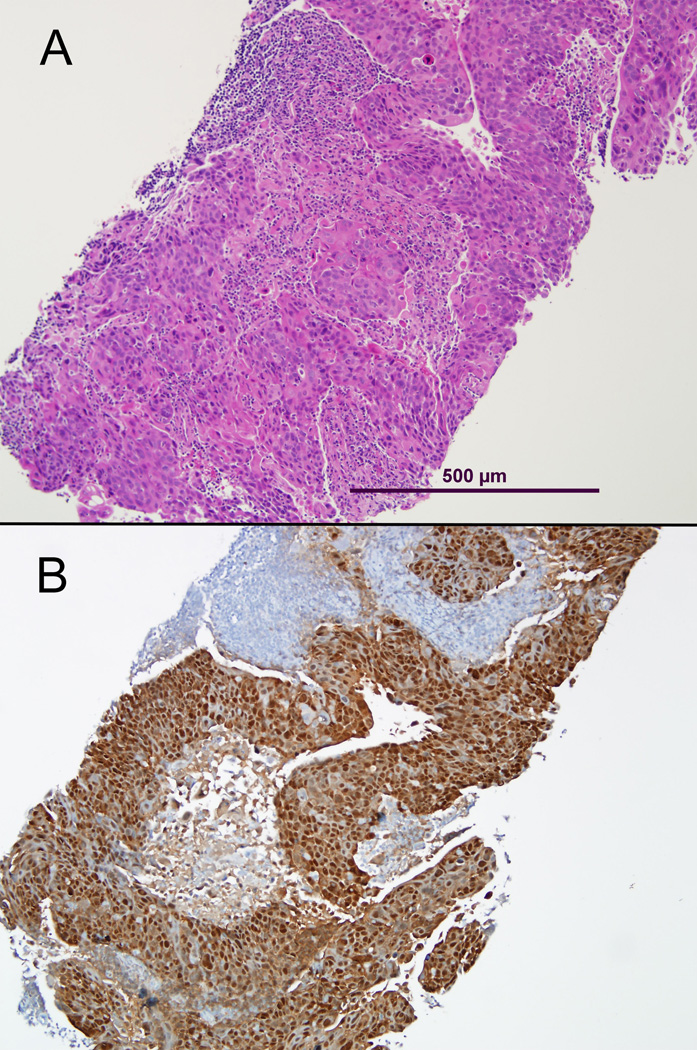
Figure 1.
Core needle biopsy of an oropharyngeal squamous cell carcinoma metastatic to a cervical lymph node. This type of sampling not only preserves the histologic context (A, routine hematoxylin and eosin stain), but it provides ample tissue for ancillary studies such as HPV testing (B, p16 immunohistochemical stain).
P16 immunohistochemistry
Five-micron sections were de-paraffinized. Antigen retrieval was performed using heat-induced epitope retrieval with 10mM citrate buffer. P16 expression was evaluated by use of a mouse monoclonal antibody against p16 (MTM Laboratories, Heidelberg, Germany) visualized using the Ventana XT autostainer using the 1-view secondary detection kit (Ventana). When dealing with p16 staining in tissue sections of HNSCC, the pattern of p16 expression is generally observed to be dichotomous. In other words, p16 staining is either absent (no staining) or present (moderate to strong diffuse nuclear and cytoplasmic staining). In equivocal cases, the presence of nuclear and cytoplasmic staining, even when faint, is strongly associated with the presence of HPV [9]. Accordingly, p16 expression was scored as positive if any cytoplasmic and nuclear staining was observed in the FNACBs, even in the presence of cellular degradation where it was difficult to quantify the percentage of positive tumor cells (Figure 2). For the resected primary carcinomas, p16 expression was scored as positive if nuclear and cytoplasmic staining was present in ≥70% of the tumor specimen. An HPV16-positive oropharyngeal cancer was used as a positive control.
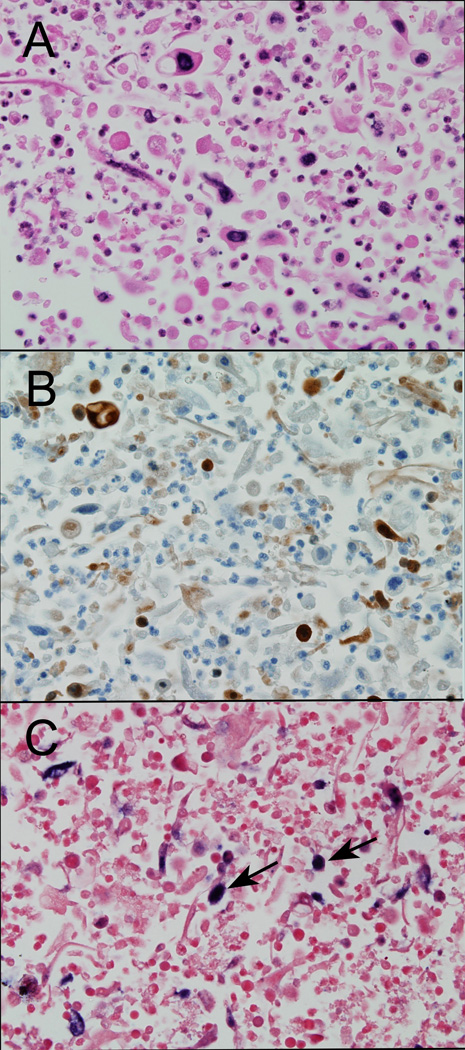
Figure 2.
Fine needle aspirate (cell block) of a metastatic oropharyngeal squamous cell carcinoma evaluated by routine hematoxylin and eosin staining (A), p16 immunostaining (B) and HPV in situ hybridization (C). Despite the presence of cell degradation (right column), the tumor cells retain p16 immunoreactivity (B) and HPV16 hybridization signals (C, arrows).
HPV DNA in situ hybridization
In this study, HPV DNA in situ hybridization (ISH) rather than a PCR-based detection assay was used to confirm HPV status. ISH permits direct microscopic confirmation of the presence and distribution of HPV. In contrast, PCR-based assays are unable to determine if viral DNA arises from the population of cancer cells or the surrounding non-neoplastic tissues; and they do not allow for the recognition of tumor free samples and thus the identification of false negative results.
Five-micron sections from the formalin-fixed paraffin-embedded tumor blocks were evaluated for the presence of HPV DNA by ISH. Two different detection methods were used. A type 16-specific assay was performed using the in situ hybridization-catalyzed signal amplification method for biotinylated probes (DAKO GenPoint, Carpinteria, CA). Briefly, the 5-micron tissue sections underwent deparaffinization, heat-induced target retrieval in citrate buffer, and digestion using Proteinase K (Roche Diagnostics, Indianapolis, IN). Slides were subsequently hybridized with a biotinylated HPV type 16-specific probe (DAKO, Carpintera, CA). Signal amplification was performed by consecutive application of a streptavidin-HRP complex and AQ2 biotinyl tyramide. Visualization of hybridization signals was performed by incubation with the chromogenic substrate diaminobenzidine.
For broader high-risk HPV detection, we also used the Ventana Inform HPV III Family 16 Probe (B) kit (Ventana Medical Systems, Tucson, AZ). For this assay, slides were conditioned using Ventana cell conditioner #2 and ISH-protease 3. Hybridization utilized the HPV III Family 16 probe set that captures high risk HPV genotypes 16, 18, 33, 35, 45, 51, 52, 56, and 66. Signals were detected using the ISH iView Blue Plus Detection Kit, which is an indirect biotin-streptavidin system that detects fluorescein-labeled probes. The kit uses an alkaline phosphatase enzyme and NBT/BCIP substrate chromogen reaction that provides an intense blue, permanent color as well as a red counter stain. All reagents are provided prediluted and ready-to-use on BenchMark Series automated slide stainers (Ventana Medical Systems, Tucson, AZ).
For both detection assays, punctate hybridization signals localized to the tumor cell nuclei defined an HPV-positive tumor (Figure 3). An HPV16-positive oropharyngeal cancer and HPV16-positive SiHa and CaSki cell lines were used as positive controls.
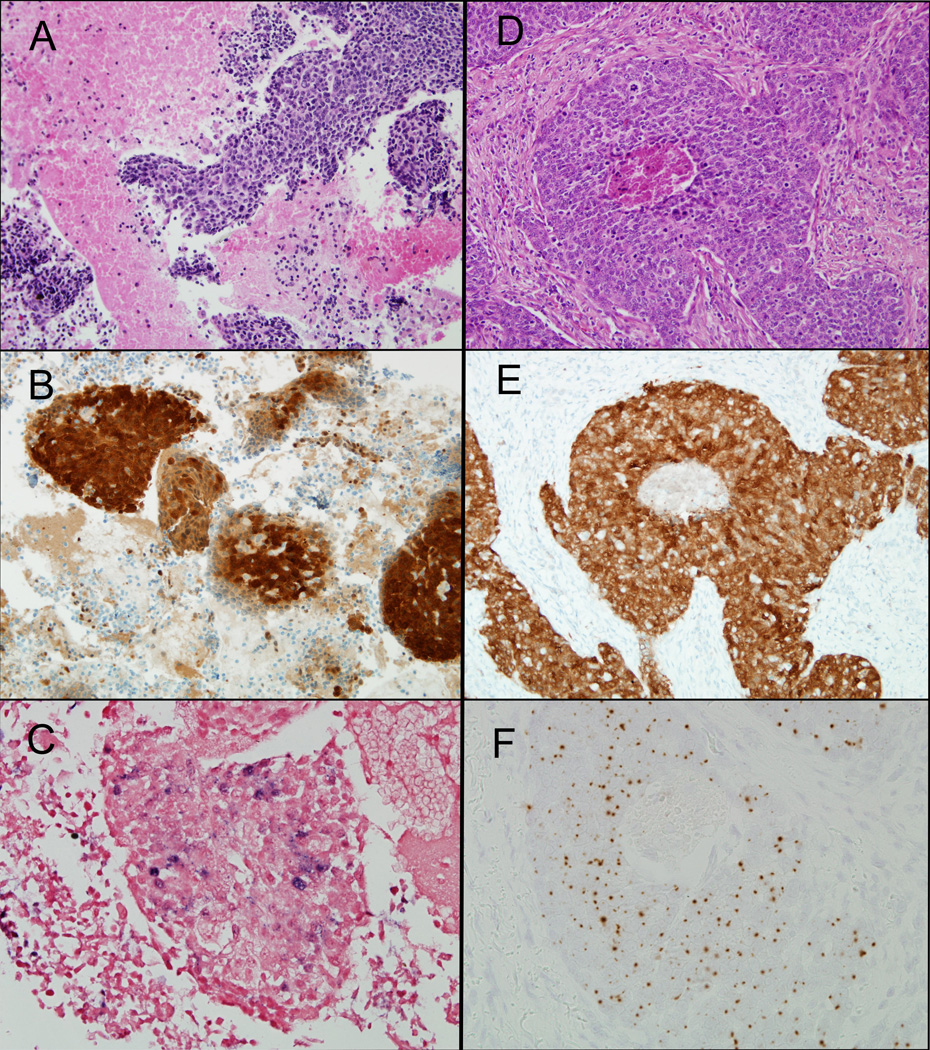
Figure 3.
HPV status is consistently maintained across stages of tumor progression. The fine needle aspirate is shown in the left column, and the resection of the primary oropharyngeal carcinoma is shown in the right column. Both samples show p16 staining (B and E) and the presence of DNA in situ hybridization signals (C, Ventana Inform HPV III Family 16 Probe) (F, DAKO HPV-16 type specific probe).
Results
P16 staining was performed on lymph node aspirates and core biopsies from 85 patients with metastatic HNSCC. Seventy-one (84%) patients were male. Patient ages ranged from 20–89 years (median, 59; mean 58). The primary sites of tumor origin were the oropharynx (tonsil, n = 31; base of tongue, n = 23; soft palate, n = 1), oral cavity (n = 7), larynx (n = 5), facial skin (n = 3), nasopharynx (n = 1) and lung (n = 1). In 13 (15%) cases the primary site was unknown. Of these 85 specimens, 43 (51%) were obtained by fine needle aspiration (cell blocks, n=42; cytospin, n=1), and 42 (49%) were obtained by core biopsy. In 5 cases, cellularity was inadequate for supplemental analysis by DNA in situ hybridization (ISH).
Positive p16 staining was present in 60 (71%) of the FNACBs. A summary of p16 staining as a function of tumor site and HPV status is provided in Figure 4. By site of primary tumor origin, p16 staining was noted in 51 of the 55 (93%) lymph node metastases from the oropharynx, 7 of 13 (54%) metastases from unknown primary sites, and 2 of 17 (13%) metastases from non-oropharyngeal sites. For those patients with metastatic squamous cell carcinomas of known primary site, p16 positivity was strongly predictive of oropharyngeal origin (93% vs. 13%, p< 0.001, Fisher’s exact).
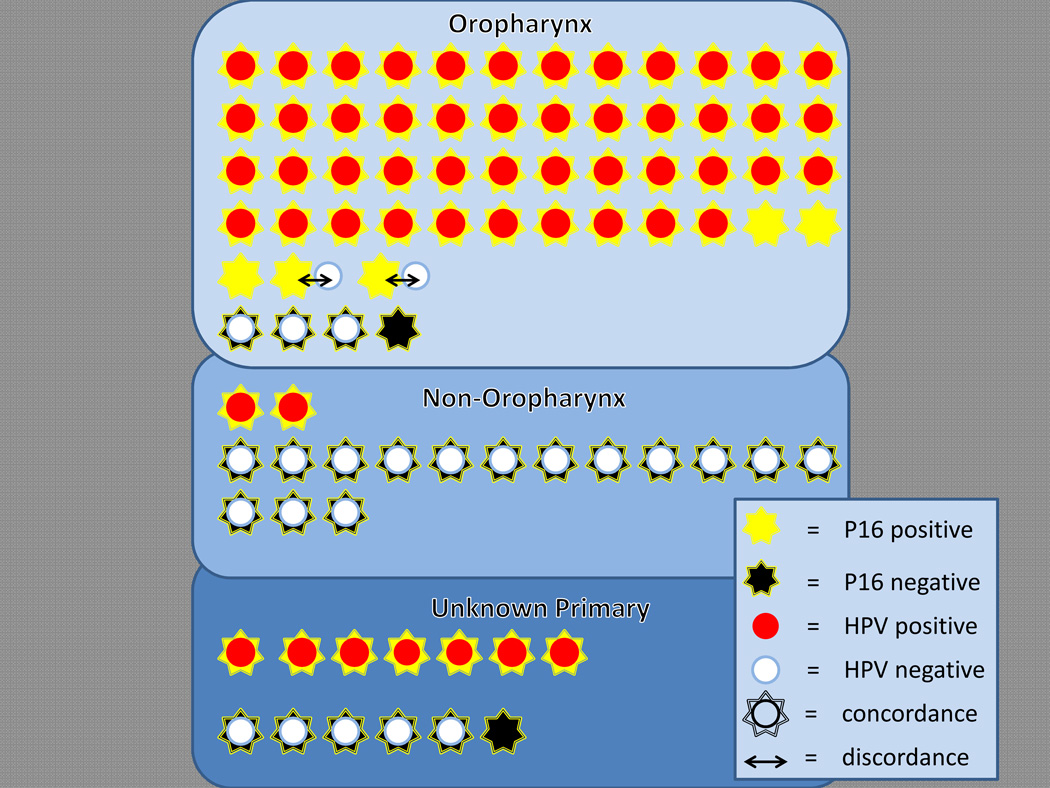
Figure 4.
P16 immunostaining of metastatic HNSCCs showing distribution of staining by tumor origin and as a function of HPV in situ hybridization (ISH). Each star (p16 staining) and corresponding circle (ISH) represent results from individual fine needle aspiration/biopsy samples. Superimposed symbols reflect concordant results, whereas non-overlapping symbols (↔) reflect discordant results. In 5 cases, p16 staining was performed alone without corresponding ISH analysis. P16 positive metastases tend to be from the oropharynx, and they tend to be HPV positive by ISH.
When dealing with FNACBs of metastatic head and neck squamous cell carcinomas, p16 staining helps identify not only the site of tumor origin, but also the presence of HPV [10]. There was a 98% correlation between HPV ISH and p16 staining confirming p16 staining as a reliable surrogate marker for the presence of HPV (Figure 4). HPV DNA ISH was not performed in 5 cases (4 from oropharynx, 1 from unknown primary) due to insufficient cellularity or cellular degradation. In the FNACBs of sufficient cellularity and cellular integrity, HPV ISH signals were detected in 55 (69%) samples; and they were not detected in 25 (31%) samples. There was 98% correlation between HPV ISH and p16 staining. All 55 HPV ISH positive cases were p16 positive, and 23 HPV ISH negative cases demonstrated absence of p16 staining. The 2 discordant cases (p16 positive/HPV ISH negative) were from OPSCCs that were p16 positive/HPV ISH negative (n = 1) and p16 positive/HPV ISH positive (n = 1) based on HPV analysis of the resection specimens.
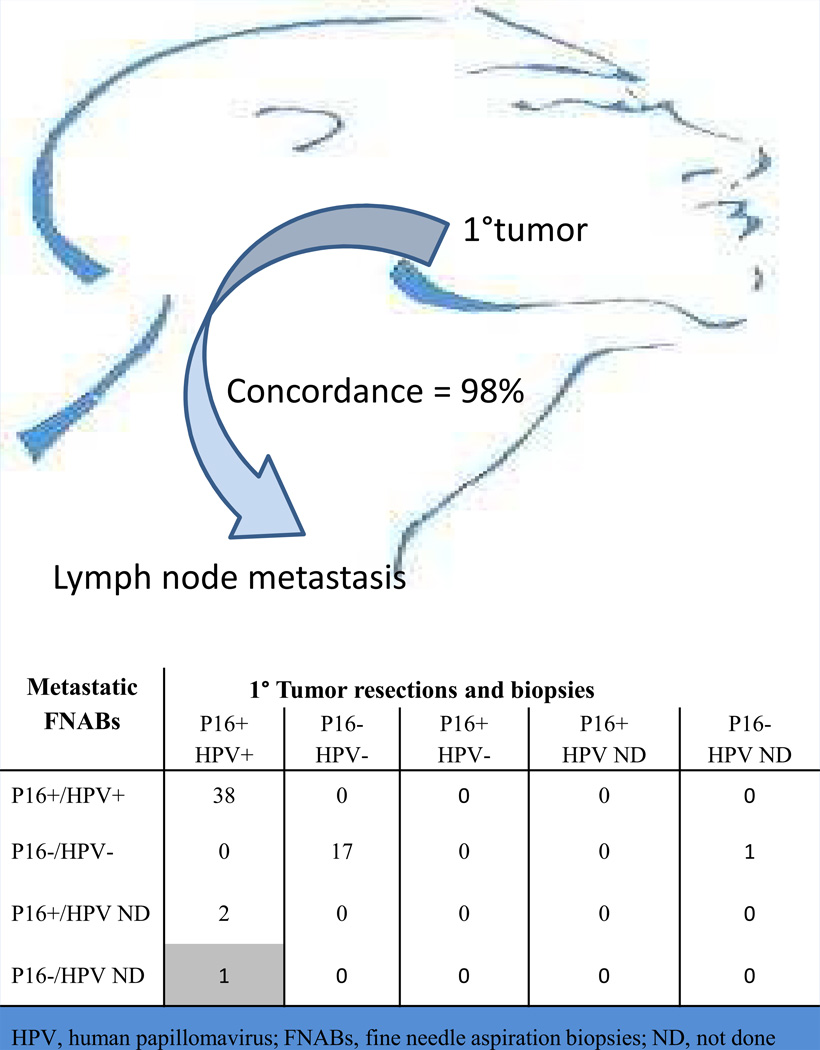
To establish the fidelity of p16 status across primary and metastatic sites, we also evaluated HPV status of the primary tumor resections and biopsies. A comparison of HPV status is summarized in Figure 5. A surgical pathology specimen (i.e. excisional biopsy or resection) was available for 59 (69%) of the paired primary HNSCCs. There was 98% concordance. The single discrepant case was an HPV+/p16+ tonsillar carcinoma where the FNACB was p16 negative. On retrospective review of this discordant case, the material in the fine needle aspiration cell block was suboptimal due to scant cellularity. For HPV positive carcinomas, HPV status was consistently retained through progression to regional nodal spread, and this retention was reliably confirmed in p16 assessment of the FNACBs (Figures 3 and 5).
Figure 5.
Comparison of HPV detection parameters for primary head and neck cancers and their paired metastases in cervical lymph nodes. The primary tumors were sampled by surgical resection or biopsy, and the metastases were sampled by fine needle aspiration or core biopsy (FNACB).
Discussion
Current guidelines have called for routine HPV assessment for those squamous cell carcinomas arising from the oropharynx (OPSCC). Nodal metastases have also been identified as an appropriate substrate for HPV analysis, but only for those patients with clinically occult primary tumors. For patients with nodal metastasis from a known oropharyngeal mass, it is not known whether nodal metastases can reliably substitute for the primary tumor when establishing HPV status. Potentially, a number of biological and technical factors could drive HPV discordance at these two sites including: 1) the timing (early vs. late) and necessity (transient vs. obligatory) of HPV infection during clinical tumor progression, 2) tumor degradation in lymph node metastases, and 3) insufficient tumor sampling afforded by fine needle aspiration biopsy.
Based on clinical HPV testing of tissue samples, over 80% of OPSCCs are HPV positive [2]. We were able to detect p16 staining in the vast majority (93%) of metastatic oropharyngeal squamous cell carcinomas in patients who had undergone FNACBs of involved regional lymph nodes. In effect, technical factors that could potentially influence p16 staining in FNACBs such as cystic degeneration and limited sampling did not reduce the expected high rate of p16 positivity. Conversely, the high rate of p16 staining did not reflect non-specific staining or an inappropriately low threshold for p16 detection. In contrast to metastases from the oropharynx, p16 staining was not as commonly encountered in metastases from non-oropharyngeal sites (93% vs. 13%, p< 0.001). This preferential distribution of p16 staining for carcinomas of the oropharynx supports the use of p16 staining as a staging tool in discerning site of tumor origin for those patients presenting with cervical lymph node metastases [7,8,11].
Immunostaining for p16 protein has recently been regarded as a practical alternative or complementary procedure for HPV testing of oropharyngeal carcinomas, based on a high correlation between HPV detection and p16 overexpression in tissue-based studies [10,12–14]. We found that the use of p16 staining as a surrogate marker of HPV status is also reliable and valid for FNACBs, even in specimens with marked cellular degeneration. In the 80 cases where the FNACBs were evaluated using both methods, all but 2 cases were concordant for an overall concordance rate of 98%. This strong correlation, together with the simplicity, low cost and high sensitivity of p16 immunocytochemistry suggests that p16 staining could function as a standalone test in the evaluation of FNACBs, at least in its role as a prognostic indicator.
HPV has been detected in dysplasia of the tonsillar crypts; and its presence in these early lesions has been taken to support its biologic role as a relevant initiator of tumorigenesis [15]. We found that the HPV status of primary HNSCCs is consistently retained in regional lymph node metastases, supporting the critical role of HPV in maintenance of the malignant phenotype. Indeed, the presence of transcriptionally active HPV across all stages of clinical progression including distant metastases long after treatment of the primary tumor dispels any notion of a “hit and run” mechanism where HPV presence becomes superfluous over time [16–18]. The fidelity of HPV status across temporally and spatially separated lesions is of considerable practical significance. For the squamous cell carcinoma in the lung of a patient with a prior HPV-related OPSCC, HPV detection provides a direct link between the two tumors and provides a useful tool for discriminating between lung metastases and new lung primaries [17,19]. Most patients with HNSCC already have metastatic spread to regional lymph nodes at the time of presentation, about 15% of patients present with a neck mass as the first and only clinical manifestation, and 3% to 9% of the primary tumors elude detection despite a thorough clinical, radiographic, endoscopic and histopathologic evaluation [20]. FNACB represents an initial step in the evaluation of these patients. In light of the high fidelity of HPV status across stages of clinical progression, exploitation of these FNACB samples as a substrate for HPV assessment could facilitate the diagnosis of a HPV-related HNSCC, direct the search for site of origin, predict clinical outcome, and guide therapy all while abrogating the need for tissue acquisition of the primary tumor via a surgical procedure.
The few studies that have addressed HPV testing of cytological samples have primarily tried to adapt tissue-targeted approaches (e.g. p16 immunohistochemistry and HPV ISH) to archived cytological specimens [6–8]. In most instances, HPV testing of cytological specimens is restricted to a small subset of cases in which ample cellular material is available for the construction of cell blocks. In a prior analysis of FNA specimens, only 20% of the clinical samples were sufficiently cellular for construction of cell blocks for HPV testing [7]. At the Johns Hopkins Hospital, the FNA is now routinely augmented by the supplemental core needle biopsy in an effort to enhance sample adequacy for HPV testing of metastatic HNSCCs. The core needle biopsy has been advocated as a complementary method for sampling head and neck lesions in patients undergoing FNAs [21,22]. Like the fine needle aspirate, it is minimally invasive, cost effective and safe. Specifically, the risk of major bleeding and tumor cell displacement (“seeding”) is very low [22]. Although the core biopsy may not significantly improve the overall sensitivity of diagnosing squamous cell carcinoma in lymph node metastasis, it has an immense impact on sample adequacy. In a meta-analysis of core needle biopsies used in the evaluation of head and neck lesions, adequate material for histologic diagnosis and ancillary studies was obtained in 95% of cases [22]. The development of liquid phase assays (e.g. Hybrid Capture 2 [23,24], Cervista® [25,26]) has recently been impelled by a need to circumvent the limiting prerequisite for high cellularity, but until these assays are validated in larger studies, the fine needle aspirate supplemented by the core needle biopsy provides a practical approach for determining HPV status of metastatic HNSCCs. Indeed, the high fidelity of this approach would seem to render the need for additional tissue sampling of the primary tumor superfluous.
Acknowledgments
Funding: This work has been funded by the National Institute of Dental and Craniofacial Research (P50 DE019032).
References
- 1.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Umudum H, Rezanko T, Dag F, Dogruluk T. Human papillomavirus genome detection by in situ hybridization in fine-needle aspirates of metastatic lesions from head and neck squamous cell carcinomas. Cancer. 2005;105:171–177. doi: 10.1002/cncr.21027. [DOI] [PubMed] [Google Scholar]
- 7.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 8.Zhang MQ, El-Mofty SK, Davila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: A tool for identifying the site of an occult head and neck primary. Cancer. 2008;114:118–123. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZW, Weinreb I, Kamel-Reid S, Perez-Ordonez B. Equivocal p16 immunostaining in squamous cell carcinoma of the head and neck: Staining patterns are suggestive of hpv status. Head Neck Pathol. 2012;6:422–429. doi: 10.1007/s12105-012-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JS., Jr P16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–S82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: A highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- 12.Hoffmann M, Ihloff AS, Gorogh T, Weise JB, Fazel A, Krams M, Rittgen W, Schwarz E, Kahn T. P16(ink4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–1602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 13.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W, Gillison ML. Validation of methods for oropharyngeal cancer hpv status determination in us cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Naggar AK, Westra WH. P16 expression as a surrogate marker for hpv-related oropharyngeal carcinoma: A guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 15.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 16.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: An unrecognized pattern of distant spread in patients with hpv-related head and neck cancer. J Neuro-oncol. 2013;112:449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop JA, Ogawa T, Chang X, Illei PB, Gabrielson E, Pai SI, Westra WH. Hpv analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2012;36:142–148. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrad M, Zhao H, Gao G, Wang X, Lewis JS., Jr Transcriptionally-active human papillomavirus is consistently retained in the distant metastases of primary oropharyngeal carcinomas. Head Neck Pathol. 2014;8:157–163. doi: 10.1007/s12105-013-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weichert W, Schewe C, Denkert C, Morawietz L, Dietel M, Petersen I. Molecular hpv typing as a diagnostic tool to discriminate primary from metastatic squamous cell carcinoma of the lung. Am J Surg Pathol. 2009;33:513–520. doi: 10.1097/PAS.0b013e3181938319. [DOI] [PubMed] [Google Scholar]
- 20.de Braud F, al-Sarraf M. Diagnosis and management of squamous cell carcinoma of unknown primary tumor site of the neck. Sem Oncol. 1993;20:273–278. [PubMed] [Google Scholar]
- 21.Witt BL, Schmidt RL. Ultrasound-guided core needle biopsy of salivary gland lesions: A systematic review and meta-analysis. Laryngoscope. 2014;124:695–700. doi: 10.1002/lary.24339. [DOI] [PubMed] [Google Scholar]
- 22.Novoa E, Gurtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: A meta-analysis and systematic review of the literature. Head Neck. 2012;34:1497–1503. doi: 10.1002/hed.21821. [DOI] [PubMed] [Google Scholar]
- 23.Smith DF, Maleki Z, Coughlan D, Gooi Z, Akpeng B, Ogawa T, Bishop JA, Frick KD, Agrawal N, Gourin CG, Ha PK, Koch WM, Richmon JD, Westra WH, Pai SI. Human papillomavirus status of head and neck cancer as determined in cytologic specimens using the hybrid-capture 2 assay. Oral Oncol. 2014;50:600–604. doi: 10.1016/j.oraloncology.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop JA, Maleki Z, Valsamakis A, Ogawa T, Chang X, Pai SI, Westra WH. Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: A convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 2012;120:18–25. doi: 10.1002/cncy.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomides CC, Bibbo M, Wang ZX. Assessment of fine needle aspiration specimen adequacy for high-risk hpv detection and genotyping in oropharyngeal squamous cell carcinoma. Acta Cytol. 2012;56:196–198. doi: 10.1159/000335730. [DOI] [PubMed] [Google Scholar]
- 26.Guo M, Khanna A, Dhillon J, Patel SJ, Feng J, Williams MD, Bell DM, Gong Y, Katz RL, Sturgis EM, Staerkel GA. Cervista hpv assays for fine-needle aspiration specimens are a valid option for human papillomavirus testing in patients with oropharyngeal carcinoma. Cancer Cytopath. 2014;122:96–103. doi: 10.1002/cncy.21375. [DOI] [PubMed] [Google Scholar]