Abstract
Objectivey
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia frequently affecting patients with synucleinopathies but its exact prevalence in multiple system atrophy (MSA) is unclear. Whether questionnaires alone are sufficient to diagnose RBD is also unknown.
Methods
Cross-sectional study of patients with probable MSA from six academic centers in the US and Europe. RBD was ascertained clinically and with polysomnography; and meta-analysis according to PRISMA guidelines for studies published before September 2014 that reported the prevalence of RBD in MSA. A random-effects model was constructed using weighted prevalence proportions. Only articles in English were included. Studies were classified into those that ascertained the presence of RBD in MSA clinically and with polysomnography. Case reports or case series (≤5 patients) were not included.
Results
Forty-two patients completed questionnaires and underwent polysomnography. Of those, 32 (76.1%) had clinically-suspected RBD and 34 (81%) had polysomnography-confirmed RBD. Two patients reported no symptoms of RBD but had polysomnography-confirmed RBD.
The primary search strategy yielded 374 articles of which 12 met the inclusion criteria The summary prevalence of clinically suspected RBD was 73% (95% CI, 62%-84%) in a combined sample of 324 MSA patients. The summary prevalence of polysomnography-confirmed RBD was 88% (95% CI, 79%-94%) in a combined sample of 217 MSA patients.
Interpretation
Polysomnography-confirmed RBD is present in up to 88% of patients with MSA. RBD was present in some patients that reported no symptoms. More than half of MSA patients report symptoms of RBD before the onset of motor deficits.
Keywords: α-synuclein, sleep disorders, parasomnias, polysomnography, parkinsonism
Introduction
Normal sleep is divided into periods with and without rapid eye movements (i.e., REM and non-REM sleep). During REM sleep, normally about 20% of total sleep time, skeletal muscle tone is lost (i.e., atonia) and subjects are immobile except for their eyes. Dreams usually occur during REM sleep.
The inhibition of muscle tone during REM sleep requires the activity of pontomedullary brainstem nuclei [9, 24]. Lesions affecting these neurons result in a peculiar parasomnia named REM sleep behavior disorder (RBD). RBD was first described in 1986 [31] in patients who were acting out their dreams. The behavior included moving their limbs, sleep talking, shouting, or screams. In extreme cases, patients hurt themselves and their spouses. Interestingly, if patients are waken-up by their bed partner they frequently describe a dream that explains their behavior.
RBD is often associated with synucleinopathies including Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA); and in patients with these disorders frequently predates motor and cognitive deficits [23]. In postmortem studies of patients with RBD neurodegeneartion with abnormal deposition of α-synuclein affecting pontomedullary brainstem nuclei is the most frequent finding [3, 7].
Several studies have reported that RBD is frequent in patients with MSA [8, 10, 16, 22, 26, 27, 32, 33, 36, 37, 40, 41] but its exact prevalence and whether RBD affects all patients with MSA is not known. Moreover, whether questionnaires are sufficient to diagnose RBD or polisomnography showing abnormal muscle activation (i.e., lack of atonia) and vocalizations/motor behavior during REM is required for the diagnosis is unclear.
To answer these questions we investigated the prevalence of RBD in patients with MSA prospectively enrolled in a multicenter study of academic medical centers in the US and Europe. We also performed a systematic review and meta-analysis of published studies on the prevalence of RBD in MSA.
Methods
Prevalence study
Cross-sectional study including patients with probable MSA[18] evaluated at the New York University School of Medicine (New York, NY), Mayo Clinic (Rochester, MN), Vanderbilt Medical Center (Nashville, TN), University of Michigan (Ann Arbor, MI), Stanford University School of Medicine (Stanford, CA) and the University Clinic of Navarra (Pamplona, Spain). Patients with both phenotypes (MSA-P and MSA-C) were included [18]. All patients underwent clinical and neurological evaluations by a board-certified neurologist. Institutional review boards at each participating site approved this study and written informed consent was obtained from all participants at enrolment.
RBD was ascertained clinically, using validated questionnaires for RBD [13, 28] and with polysomnography. Polysomnography was performed using dedicated inputs for electroencephalogram (EEG, according to the 10-20 system: Fp1, Fp2, F3, F4, F7, F8, C3, C4, P3, P4, T3, T4, T5, T6, O1, O2, common reference), tibial and chin EMG, electrooculogram, oronasal flow, respiratory effort, oxymetry, heart rate, body position, recording of sounds with microphone, and video recording. Sleep stages classification was performed following the current criteria [1]. RBD was diagnosed according to the Classification of Sleep Disorders [2] which requires detection of REM sleep without atonia and episodes of vocalization and/or motor behavior during REM sleep during polysomnography.
Systematic review and meta-analysis
This meta-analysis was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[25]. Articles on RBD and MSA were identified by searches of PubMed and EMBASE through September 1, 2014. Only articles in English were included. The following search strategy was used: (“Shy-Drager” AND sleep) OR (“multiple system atrophy” AND sleep) OR (“striatonigral degeneration” AND sleep) OR (“olivopontocerebellar” AND sleep) OR (MSA AND sleep) OR (“autonomic failure” AND sleep).
In addition, a manual search of bibliographies of included trials and related reviews was carried out for additional references. No unpublished data or data from abstracts were encountered or used. Articles were evaluated independently by two reviewers (J.A.P. and C.F.C.) who extracted the following data from each study: first author, year of publication, and prevalence of RBD in patients with definite, probable, or possible MSA according to the available criteria [17, 18, 29]. Studies were classified into those that ascertained the presence of RBD clinically (by means of questionnaires or history) and those that reported the prevalence of polysomnography-confirmed RBD. Case reports or case series (≤5 patients) were not included.
Statistical analysis
The primary outcome measure was frequency of RBD in MSA as reported in prevalence (%). The pooled prevalence of RBD and 95% confidence intervals were obtained by using a DerSimonian-Laird random-effects model with double arcsine transformation [11]. We ran a random-effect model rather than a fixed-effects model because of the high likelihood of heterogeneity between study variance. The heterogeneity of effect size estimates across studies was described with the I2 index (with values of 25%, 50%, and 75% considered low, moderate, and high, respectively) and Q statistic's p value [19]. Analyses were performed with Stata 13 (College Station, TX).
Sensitivity analysis was performed using the leave-one-out approach. Publication bias was ascertained by funnel plot, with an asymmetrical plot suggesting possible publication bias. Egger's test was used to assess funnel plot asymmetry. P < 0.05 was considered as statistically significant.
Results
Prevalence of symptoms suggesting RBD
Sixty-four patients with MSA (23 MSA-P [35%], 41 MSA-C [65%]; 36 men, 28 women; aged 61.1±8 years; disease duration: 3.4±2 years; disease onset at 57.7±8.3 years) completed sleep questionnaires. Of these, 53 (83%) reported symptoms suggesting RBD. Twenty-nine (53.7%) reported symptoms of RBD before the onset of motor deficits. Symptoms of RBD were present in 82% of MSA-P and 83% of MSA-C patients (p=0.99).
Prevalence of polysomnography-confirmed RBD
Forty-two patients with MSA (14 MSA-P [34 %], 28 MSA-C [66%]; 23 men, 19 women; aged 62.2±7.8 years; disease duration: 3.3±1.8 years; disease onset at 57.1±8.2 years) completed questionnaires and underwent polysomnography. Of those, 32 (76.1%) had symptoms suggesting RBD and 34 (81%) had signs of RBD during the polysomnography. Signs of RBD were present in 86% (13/15) of MSA-P and 78% (21/27) of MSA-C patients (p=0.48) (Supplementary Video 1).
Six (14%) patients had no signs of RBD in the polysomnography, of which 5 had MSA-C and 1 had MSA-P. These subjects were not taking benzodiazepines or any other medications known to disrupt REM sleep. Disease duration in these 6 patients was 6.7±4.1 years.
Two patients (4.7%) had no REM sleep during the study so it was not possible to ascertain the presence of RBD. All patients reporting symptoms of RBD (except the 2 patients with no REM sleep) did have signs of RBD during the sleep study.
Two patients did not report symptoms of RBD but actually had polysomnography-confirmed RBD.
Meta-analysis results
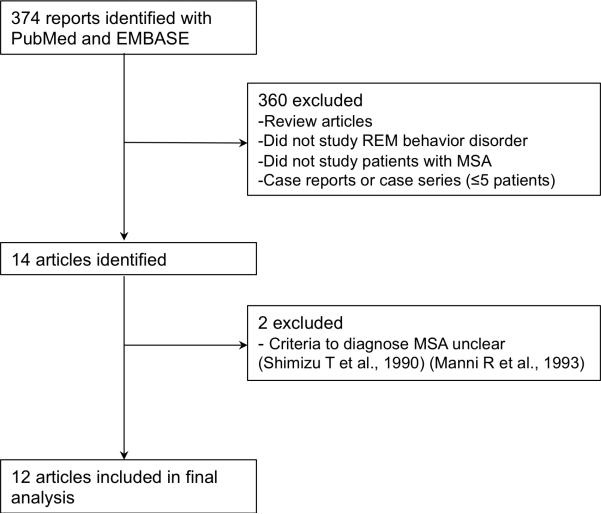
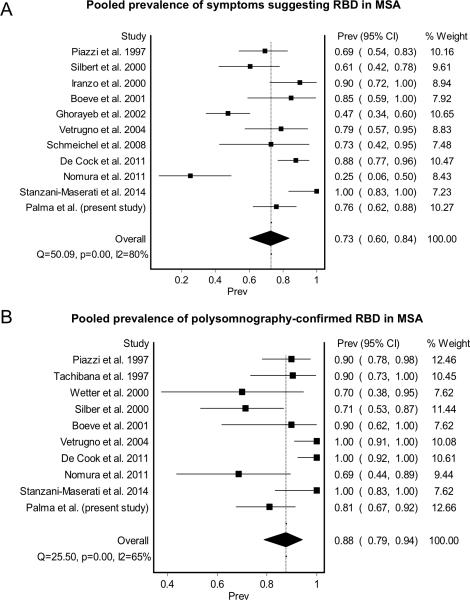
As shown in Figure 1, the primary search strategy yielded 374 articles of which 12 met the inclusion criteria. Ten of this articles included prevalence on clinically suspected RBD and nine articles included prevalence on polysomnography-confirmed RBD (Table 1). Including the results of the present study, the summary prevalence of symptoms suggesting RBD in MSA was 73% (95% CI, 62%-84%) in a pooled sample of 324 subjects (Figure 2A). Between-study heterogeneity was high (80%; p<0.001). The summary prevalence of polysomnography-confirmed RBD in MSA was 88% (95%, CI 79%-94%) in a pooled sample of 217 subjects (Figure 2B). Between-study heterogeneity was moderate (65%; p<0.001).
Figure 1.
Flow diagram of literature search to identify studies reporting the prevalence of REM sleep behavior disorder in MSA.
Table 1.
Studies reporting the prevalence of REM sleep behavior disorder in patients with MSA.
| Clinically suspected RBD | Polysomnography-confirmed RBD | |||||
|---|---|---|---|---|---|---|
| Authors | Year | Country | No of subjects studied | % with RBD | No of subjects studied | % with RBD |
| Stanzani-Maserati et al.[36] | 2014 | Italy | 10 | 100% | 10 | 100% |
| Nomura et al.[26] | 2011 | Japan | 16 | 25% | 16 | 68.8% |
| De Cock et al.[10] | 2011 | France | 49 | 91% (MSA-C) 84% (MSA-P) | 22 | 100% |
| Schmeichel et al.[32] | 2008 | USA | 11 | 72% | - | - |
| Vetrugno et al.[40] | 2004 | Italy | 19 | 79% | 19 | 100% |
| Ghorayeb et al.[16] | 2002 | France | 57 | 47.5% | - | - |
| Boeve et al. [8] | 2001 | USA | 13 | 84% | 10 | 90% |
| Iranzo et al.[22] | 2000 | Spain | 20 | 90% | - | - |
| Silber et al.[33] | 2000 | USA | 28 | 60% | 28 | 71% |
| Wetter et al.[41] | 2000 | Germany | - | - | 10 | 70% |
| Tachibana et al. [37] | 1997 | Japan | - | - | 21 | 90.5% |
| Piazzi et al.[27] | 1997 | Italy | 39 | 69% | 39 | 90% |
RBD: REM sleep behavior disorder. MSA: Multiple system atrophy.
Figure 2.
Meta-analysis results on the pooled prevalence of REM sleep behavior disorders in MSA according to symptoms (A) and polysomnography (B).
Sensitivity analysis showed unchanged results. There was no evidence of publication bias as the funnel plot was symmetrical and Egger's test was not significant (Supplementary figure 1).
Discussion
Our multicenter study confirms that RBD, as ascertained by polysomnography, is present in the vast majority of MSA patients (81%). The results of the meta-analysis, in which the prevalence of polysomnography-confirmed RBD in a sample of 225 subjects with MSA was 88%, is in keeping with the findings of our multicenter study.
The prevalence of clinically suspected RBD in MSA was lower (83% and 76.1% in the multicenter study and 73% in the meta-analysis) than the polysomnography-confirmed RBD. Conversely, patient-reported symptoms suggesting RBD accurately predicted the presence of RBD in polysomnography. Our results highlight that, although clinical recognition accurately diagnoses RBD in the majority of MSA patients, polysomnography is needed to diagnose RBD in some patients who do not report symptoms.
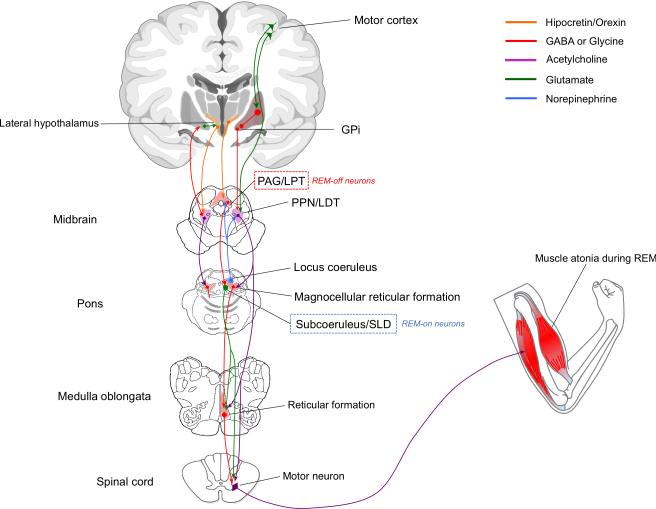
The pathways and nuclei involved in the pathophysiology of RBD are shown in Figure 3. Pontomedullary structures -including the magnocellular reticular formation (MCRF), sublaterodorsal (SLD), and pedunculopontine and laterodorsal (PPN/LDT) nuclei- are responsible for the atonia (i.e., lack of movement) during REM sleep [9]. The SLD has been identified in animals; the analog structure in humans is though to be the subcoeruleus (SC) nucleus. Lesions in SLD/SC and MCRF (even unilaterally) are thought to eliminate atonia during REM sleep, leading to dream enacting behavior [20, 24, 35, 42]. Whether depletion of locus coeruleus (LC) neurons contributes to RBD is uncertain [12].
Figure 3.
Pathways and nuclei involved in the pathophysiology of REM sleep behavior disorder. The PAG/LPT contains REM-off neurons (i.e., suppressors of REM sleep). The LC activates REM-off neurons in the PAG/LPT, thus suppressing REM sleep. The SC/SLD contains REM-on neurons (i.e., promoters of REM sleep) The SLD (or SC in humans) projects to spinal chord and likely represents the final common pathway that causes inhibition of skeletal muscle activity during REM sleep. The indirect route, denoted by the line from SLD/SC to the reticular formation to the spinal chord, probably also contributes to atonia. In humans, it is not yet known whether lesions in structures projecting to and from the magnocellular reticular formation, and lesioning the MCRF itself, affect atonia during REM sleep. GPi = globus pallidus internus, LC = locus coeruleus, LDT = laterodorsal tegmental nucleus, LPT = lateral pontine tegmentum, MCRF = magnocellular reticular formation, PAG = periaqueductal grey matter, PPN = pedunculopontine nucleus, REM = rapid eye movement, SC=subcoeruleus nucleus, SLD = sublaterodorsal nucleus.
Autopsy studies in MSA patients have shown depletion of cholinergic neurons in the PPN/LDN complex [6, 32]. The PPN may have, however, a modulatory role in REM-related phenomena rather than a primary role for the atonia during REM sleep [4, 24]. Also in MSA, neurons of the PAG [5] and the LC are depleted [6], although their specific role in RBD is unclear. In contrast to PD [15], depletion of SC neurons has not been investigated in MSA.
The absence of RBD in some patients with MSA is intriguing. Of note, the prevalence of polysomnography-confirmed RBD tended to be lower in MSA-C than in MSA-P (78% vs. 86%; p=0.49), and 4 of our 5 MSA patients with no RBD had MSA-C. However, the distribution of parkinsonian and cerebellar phenotypes in our sample was unusual as there were more MSA-C than P patients [30]. However, these patients also had longer disease duration, which suggests that RBD disappears as disease progresses, indicating degeneration of brainstem nuclei that control REM sleep as has been suggested previously[39]. Conversely, the absence of RBD in some MSA patients may be indicative of worsening rigidity [38]. Additional longitudinal studies are needed to answer this.
In our study, 54% of patients with MSA reported symptoms of RBD before the onset of motor deficits. This is in keeping with recent studies indicating that RBD represents a prodromal phase of PD, DLB and MSA [21, 23]. Further studies should determine whether the presence or absence of autonomic dysfunction in patients with idiopathic RBD predicts the development of a specific neurodegenerative disease.
In conclusion, RBD is present in up to 88% of patients with MSA, including some patients recalling no symptoms. The prevalence of RBD in MSA is significantly higher than the percentage reported in patients with PD (50-60%) [14, 34]. Our findings argue for the inclusion of RBD as a supportive feature for MSA in forthcoming consensus criteria.
Supplementary Material
Supplementary Figure 1. Publication bias. Funnel plots were symmetrical indicating no publication bias in studies assessment clinically-suspected (A) and polysomnography-confirmed (B) REM sleep behavior disorder in patients with MSA.
Supplementary Video 1. Woman with MSA-C and REM sleep behavior disorder. She moves her limbs, sleep talks, shouts, and acts as if she were fighting. This takes places during REM sleep, as ascertained by polysomnography. The patient and her spouse gave written consent to publish this video.
Acknowledgements
Funded by the National Institutes of Health (U54NS065736).
References
- 1.American Academy of Sleep Medicine . The International Classification of Sleep Disorders 2nd ed: Diagnostic and Coding Manual. American Academy of Sleep Medicine; Westchester: 2005. [Google Scholar]
- 2.American Academy of Sleep Medicine . International Classification of Sleep Disorders. Darien, IL: 2014. [Google Scholar]
- 3.Benarroch EE. Brainstem in multiple system atrophy: clinicopathological correlations. Cell. Mol. Neurobiol. 2003;23:519–526. doi: 10.1023/a:1025067912199. [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80:1148–1155. doi: 10.1212/WNL.0b013e3182886a76. [DOI] [PubMed] [Google Scholar]
- 5.Benarroch EE, Schmeichel AM, Dugger BN, Sandroni P, Parisi JE, Low PA. Dopamine cell loss in the periaqueductal gray in multiple system atrophy and Lewy body dementia. Neurology. 2009;73:106–112. doi: 10.1212/WNL.0b013e3181ad53e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- 7.Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, Ahlskog JE, Caselli RJ, Jacobson S, Sabbagh M, Adler C, Woodruff B, Beach TG, Iranzo A, Gelpi E, Santamaria J, Tolosa E, Singer C, Mash DC, Luca C, Arnulf I, Duyckaerts C, Schenck CH, Mahowald MW, Dauvilliers Y, Graff-Radford NR, Wszolek ZK, Parisi JE, Dugger B, Murray ME, Dickson DW. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14:754–762. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov. Disord. 2001;16:622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 9.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 10.De Cock VC, Debs R, Oudiette D, Leu S, Radji F, Tiberge M, Yu H, Bayard S, Roze E, Vidailhet M, Dauvilliers Y, Rascol O, Arnulf I. The improvement of movement and speech during rapid eye movement sleep behaviour disorder in multiple system atrophy. Brain. 2011;134:856–862. doi: 10.1093/brain/awq379. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp. Clin. Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Dugger BN, Murray ME, Boeve BF, Parisi JE, Benarroch EE, Ferman TJ, Dickson DW. Neuropathological analysis of brainstem cholinergic and catecholaminergic nuclei in relation to rapid eye movement (REM) sleep behaviour disorder. Neuropathol. Appl. Neurobiol. 2012;38:142–152. doi: 10.1111/j.1365-2990.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frauscher B, Ehrmann L, Zamarian L, Auer F, Mitterling T, Gabelia D, Brandauer E, Delazer M, Poewe W, Hogl B. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov. Disord. 2012;27:1673–1678. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, Carrier J, Montplaisir J. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, Pita Lobo P, Poupon C, Benali H, Arnulf I, Vidailhet M, Lehericy S. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson's disease. Brain. 2013;136:2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2002;72:798–800. doi: 10.1136/jnnp.72.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P, Litvan I, Mathias C, Oliver E, Robertson D, Schatz I, Wenning G. Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin. Auton. Res. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 18.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iranzo A, Aparicio J. A lesson from anatomy: focal brain lesions causing REM sleep behavior disorder. Sleep Med. 2009;10:9–12. doi: 10.1016/j.sleep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, Gelpi E, Vilaseca I, Sanchez-Valle R, Llado A, Gaig C, Santamaria J. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iranzo A, Santamaria J, Tolosa E. Continuous positive air pressure eliminates nocturnal stridor in multiple system atrophy. Barcelona Multiple System Atrophy Study Group. Lancet. 2000;356:1329–1330. doi: 10.1016/s0140-6736(00)02824-5. [DOI] [PubMed] [Google Scholar]
- 23.Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, Sanchez-Valle R, Vilaseca I, Lomena F, Vilas D, Llado A, Gaig C, Santamaria J. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura T, Inoue Y, Hogl B, Uemura Y, Yasui K, Sasai T, Namba K, Nakashima K. Comparison of the clinical features of rapid eye movement sleep behavior disorder in patients with Parkinson's disease and multiple system atrophy. Psychiatry Clin. Neurosci. 2011;65:264–271. doi: 10.1111/j.1440-1819.2011.02201.x. [DOI] [PubMed] [Google Scholar]
- 27.Plazzi G, Corsini R, Provini F, Pierangeli G, Martinelli P, Montagna P, Lugaresi E, Cortelli P. REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48:1094–1097. doi: 10.1212/wnl.48.4.1094. [DOI] [PubMed] [Google Scholar]
- 28.Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, Oertel W, Ju YE, Puligheddu M, Jennum P, Pelletier A, Wolfson C, Leu-Semenescu S, Frauscher B, Miyamoto M, Cochen De Cock V, Unger MM, Stiasny-Kolster K, Fantini ML, Montplaisir JY. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov. Disord. 2012;27:913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn N. Multiple system atrophy--the nature of the beast. J. Neurol. Neurosurg. Psychiatry Suppl:78-89. 1989 doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J. Neural Transm. 2014;121:507–512. doi: 10.1007/s00702-013-1133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 32.Schmeichel AM, Buchhalter LC, Low PA, Parisi JE, Boeve BW, Sandroni P, Benarroch EE. Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology. 2008;70:368–373. doi: 10.1212/01.wnl.0000298691.71637.96. [DOI] [PubMed] [Google Scholar]
- 33.Silber MH, Levine S. Stridor and death in multiple system atrophy. Mov. Disord. 2000;15:699–704. doi: 10.1002/1531-8257(200007)15:4<699::aid-mds1015>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011;77:1048–1054. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 35.St Louis EK, McCarter SJ, Boeve BF, Silber MH, Kantarci K, Benarroch EE, Rando A, Tippmann-Peikert M, Olson EJ, Mauermann ML. Lesional REM sleep behavior disorder localizes to the dorsomedial pons. Neurology. 2014;83:1871–1873. doi: 10.1212/WNL.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanzani-Maserati M, Gallassi R, Calandra-Buonaura G, Alessandria M, Oppi F, Poda R, Sambati L, Provini F, Cortelli P. Cognitive and sleep features of multiple system atrophy: review and prospective study. Eur. Neurol. 2014;72:349–359. doi: 10.1159/000364903. [DOI] [PubMed] [Google Scholar]
- 37.Tachibana N, Kimura K, Kitajima K, Shinde A, Kimura J, Shibasaki H. REM sleep motor dysfunction in multiple system atrophy: with special emphasis on sleep talk as its early clinical manifestation. J. Neurol. Neurosurg. Psychiatry. 1997;63:678–681. doi: 10.1136/jnnp.63.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tachibana N, Oka Y. Longitudinal change in REM sleep components in a patient with multiple system atrophy associated with REM sleep behavior disorder: paradoxical improvement of nocturnal behaviors in a progressive neurodegenerative disease. Sleep Med. 2004;5:155–158. doi: 10.1016/j.sleep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Vetrugno R, Alessandria M, D'Angelo R, Plazzi G, Provini F, Cortelli P, Montagna P. Status dissociatus evolving from REM sleep behaviour disorder in multiple system atrophy. Sleep Med. 2009;10:247–252. doi: 10.1016/j.sleep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Vetrugno R, Provini F, Cortelli P, Plazzi G, Lotti EM, Pierangeli G, Canali C, Montagna P. Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med. 2004;5:21–30. doi: 10.1016/j.sleep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson's disease and multiple system atrophy. Sleep. 2000;23:361–367. [PubMed] [Google Scholar]
- 42.Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med. 2009;10:143–146. doi: 10.1016/j.sleep.2007.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Publication bias. Funnel plots were symmetrical indicating no publication bias in studies assessment clinically-suspected (A) and polysomnography-confirmed (B) REM sleep behavior disorder in patients with MSA.
Supplementary Video 1. Woman with MSA-C and REM sleep behavior disorder. She moves her limbs, sleep talks, shouts, and acts as if she were fighting. This takes places during REM sleep, as ascertained by polysomnography. The patient and her spouse gave written consent to publish this video.