Abstract
Ambient light affects multiple physiological functions and behaviors, such as circadian rhythms, sleep-wake activities, and development from flies to mammals [1–6]. Mammals exhibit a higher body temperature when exposed to acute light compared to when they are exposed to dark, but the underlying mechanisms are largely unknown [7–10]. The body temperature of small ecotherms, such as Drosophila, rely on the temperature of their surrounding environment and these animals exhibit a robust temperature preference behavior [11–13]. Here, we demonstrate that Drosophila prefer a one-degree higher temperature when exposed to acute light rather than dark. This acute light response, light dependent temperature preference (LDTP), was observed regardless of the time of day, suggesting that LDTP is regulated separately from the circadian clock. However, screening of eye and circadian clock mutants suggests that the circadian clock neurons, posterior dorsal neurons 1 (DN1ps) and pigment-dispersing factor receptor (pdfr) play a role in LDTP. To further investigate the role of DN1ps in LDTP, pdfr in DN1ps was knocked down, resulting in an abnormal LDTP. The phenotype of the pdfr mutant was sufficiently rescued by expressing pdfr in DN1ps, indicating that pdfr expression in DN1ps is responsible for LDTP. These results suggest that light positively influences temperature preference via the circadian clock neurons, DN1ps, which may result from the integration of light and temperature information. Given that both Drosophila and mammals respond to acute light by increasing their body temperature, the effect of acute light on temperature regulation may be conserved evolutionarily between flies and humans.
Keywords: Temperature preference, body temperature, circadian rhythm, light, pdfr, Drosophila
RESULTS
Acute light positively influences temperature preference in Drosophila
Drosophila exhibit robust temperature preference behavior. Not only do flies avoid noxious temperatures [11, 12, 14–17], they also exhibit a temperature preference rhythm (TPR) in which preferred temperature is lower in the morning and higher in the evening [18]. We previously observed that flies entrained with light and dark (LD) cycles prefer a higher temperature than flies in free-run (constant darkness (DD)) during the daytime [18], suggesting that acute light may affect the temperature preference in flies.
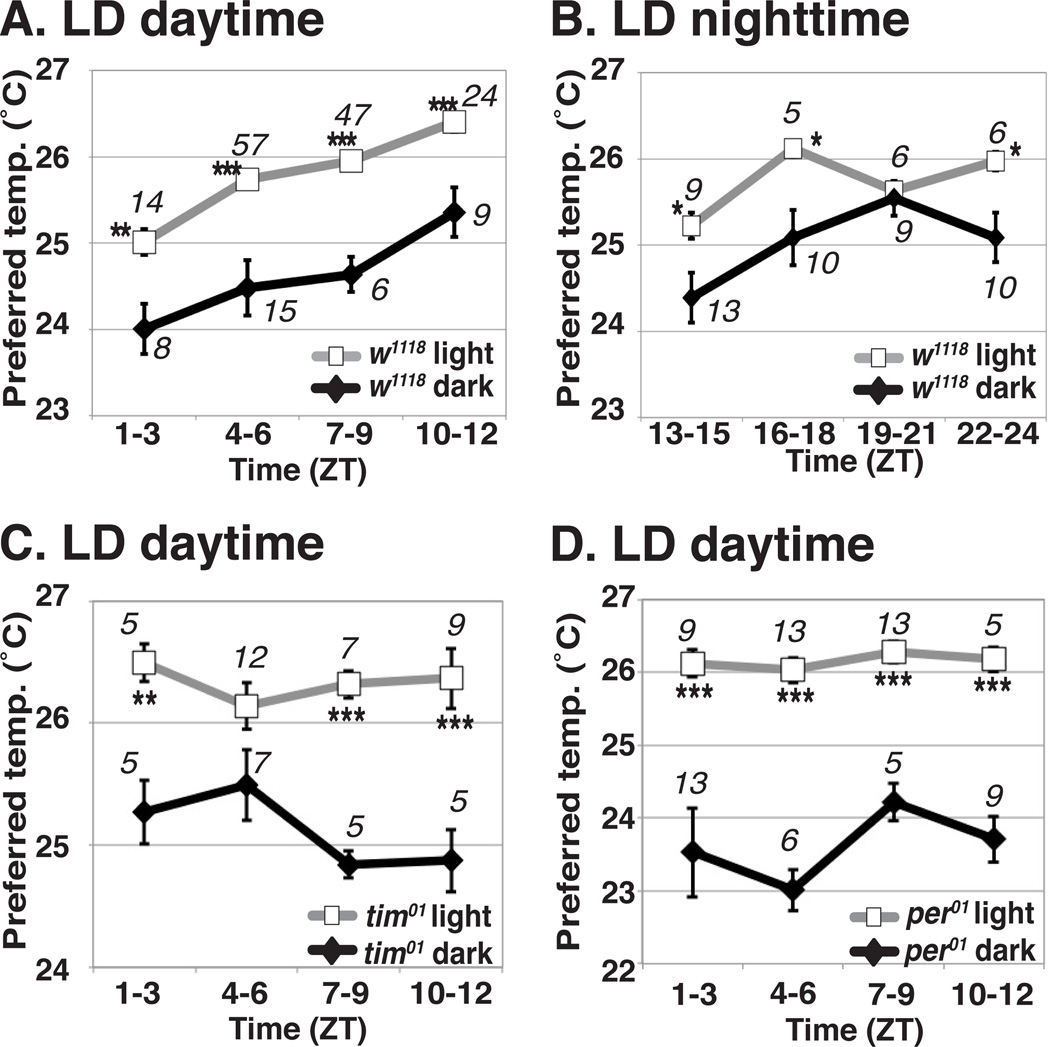
To determine whether acute light influences the selection of preferred temperature in Drosophila, we performed behavioral experiments to compare their preferred temperatures when ambient light was ON verses when ambient light was OFF. We found that wild type (w1118: WT) flies preferred ~1 °C higher temperature in the light compared to their temperature preference in the dark (Fig. 1A), suggesting that acute light positively influences the selection of preferred temperature. We refer to this behavior as light-dependent temperature preference (LDTP) and investigated the neural circuits that regulate this behavior.
Figure 1. Acute light positively influences temperature preference in Drosophila.
(A and B) Comparison of preferred temperature between light (gray line) and dark (black line) conditions for w1118 flies during the daytime (A) or nighttime (B). w1118 flies were raised in LD (light (12 h)-dark (12 h)) cycles. Ambient light was either ON or OFF when the behavioral experiments were performed for 30 min.
(C and D) Comparison of preferred temperature between light (gray line) and dark (black line) conditions for tim01 (C) and per01 (D) during the daytime. tim01 (C) and per01 (D) flies were raised in LD. Italicized numbers represent the number of assays. ZT, Zeitgeber Time (ZT0 is lights-on, ZT12 is lights-off). The same behavioral data in light (A), dark (B), light (C) and light (D) from [18] are used. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05.
Error bars are the SEM.
LDTP is controlled separately from the circadian clock
To determine whether LDTP is observed regardless of the time of day, we tested the temperature preference behavior at different time points throughout the day (Fig. 1A and B). We found that the flies consistently preferred a higher temperature throughout the day when the behavioral assays were performed in the light (Fig. 1A and B). In the same way, the flies consistently preferred a lower temperature throughout the day when the behavioral assays were performed in the dark, although preferred temperatures at ZT 19–21 were similar (Fig. 1B). These data suggest that LDTP occurs irrespective of the circadian clock.
To confirm that LDTP is independent of circadian clock function, we examined LDTP in mutants for period (per) and timeless (tim), which disrupt the circadian clock. If LDTP is independent of the circadian clock, per01 and tim01 mutants should still exhibit LDTP. We found that per01 and tim01 mutants exhibited a normal LDTP and preferred a higher temperature in the light than in the dark at all time points throughout the daytime (Fig. 1C and D), with the exception of the tim01 mutants at ZT4-6. At this time point, the tim01 mutants preferred a slightly higher temperature in the light, but the difference was not statistically significant. Thus, we concluded that LDTP is regulated separately from the circadian clock and is dependent solely on light.
glass is required for LDTP
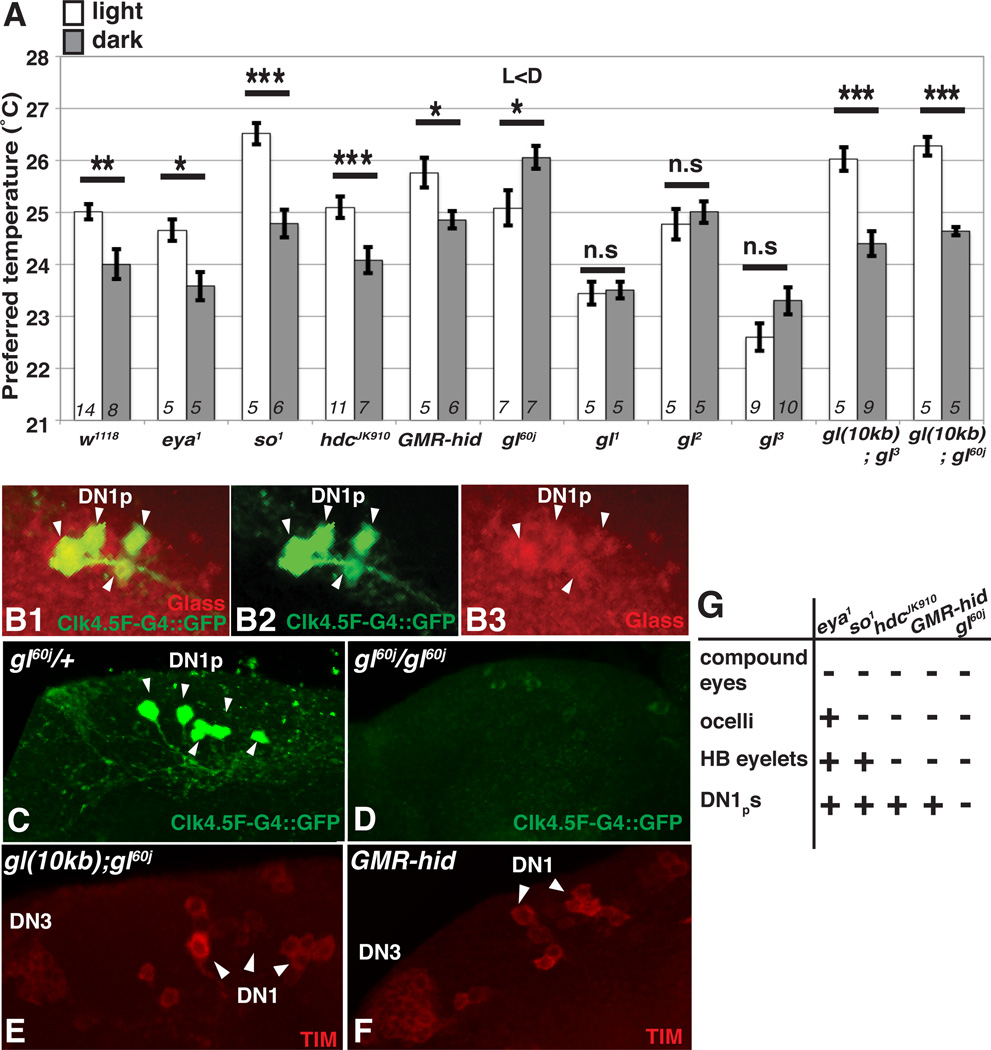
To investigate the neural circuits that regulate LDTP, we first examined the effect of eye components on LDTP. Flies have seven eye components: two compound eyes, three ocelli, and two Hofbauer-Buchner (H-B) eyelets [19]. Subsets of eye components are abnormal in the mutant fly strains eyes absent (eya1), sine oculis (so1), histidine decarboxylase (hdcJK910), and glass (gl60j), and in flies in which the proapoptosis gene hid is expressed under the control of a glass multimer response element (GMR-hid) [20, 21] (Fig. 2G). We found that eya1, so1, hdcJK910, and GMR-hid mutants all showed normal LDTP, preferring a higher temperature in the light compared to the dark (Fig. 2A). These data suggest that abnormalities in the compound eyes, ocelli and H-B eyelets do not disrupt LDTP and thus, these eye components are not essential for LDTP.
Figure 2. glass is required for LDTP.
(A) Comparison of preferred temperature of each genotype in light (white bar) and dark conditions (gray bar). The behavioral experiments were performed at ZT1-3. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
(B) The Clk4.5F-Gal4>UAS-mCD8::GFP brains were stained with anti-GFP (green) and anti-Glass (red). The GFP signals, labeled by Clk4.5F-Gal4>UAS-mCD8::GFP, overlap with the signals labeled by anti-Glass (B1). The cells for which anti-GFP and anti-Glass overlapped are shown at the arrow heads (B1-3).
(C–D) The DN1ps labeled by Clk4.5F-Gal4>UAS-mCD8::GFP with anti-GFP (green) were still present in the gl60j/+ heterozygous control (C) but were not detected in gl60j/gl60j mutants (D).
(E–F) The gl(10kb);gl60j (E) and GMR-hid (F) brains were stained with anti-TIM (red). DN1s are shown at the arrow heads.
(G) A summary of eye and DN1ps phenotypes for each eye mutant fly line. +: normal; −: abnormal.
However, we found that a null allele of glass, gl60j, had abnormal LDTP, preferring a higher temperature in the dark than in the light. Even the weak loss-offunction alleles of glass, gl1, gl2 and gl3 [22], had abnormal LDTP, in which the flies preferred a similar temperature in the light and dark (Fig. 2A). To determine whether glass is responsible for LDTP, we used the 10 KB genomic glass mini-gene to rescue the glass mutants [22]. Both of the gl(10kb); gl3 and gl(10kb); gl60j flies preferred significantly higher temperatures in the light than in the dark, indicating that the normal LDTP was restored and that glass function is required for LDTP.
Interestingly, the gl60j mutants not only have abnormal eye components but also lack a subset of circadian clock cells, the posterior dorsal neurons 1 (DN1ps) (Fig. 2G). Previous studies show that glass is expressed in DN1ps but not in the anterior dorsal neurons 1, DN1as [21, 23]. To confirm that glass is expressed in DN1ps, we used the DN1ps driver, Clk4.5F-Gal4 [24, 25], to label DN1ps in the brain (Fig. 2B). We performed immunostaining on the UAS-mCD8::GFP;Clk4.5F-Gal4 (Clk4.5FGal4>UAS-mCD8::GFP) flies using the Glass antibody and confirmed that Glass is expressed in the DN1ps (Fig. 2B). Conversely, we found that Clk4.5F-Gal4>UASmCD8:: GFP signals were not detected in gl60j/ gl60j mutants (Fig. 2D) but were still present in the gl60j /+ heterozygous control (Fig. 2C), indicating that DN1ps were ablated in the gl60j mutants. If DN1ps are key neurons for LDTP, DN1ps should be restored in the gl(10kb); gl60j flies given that gl(10kb); gl60j flies exhibit a normal LDTP (Fig. 2A). To determine this, we performed immunostaining using the Timeless (TIM) antibody and found that DN1ps were restored in gl(10kb); gl60j (Fig. 2E). In addition, as a control, we confirmed that DN1ps were present in GMR-hid flies (Fig. 2F). These data suggested that the DN1ps may be critical for LDTP.
TrpA1 and Rhodopsin 1 are not necessary for LDTP
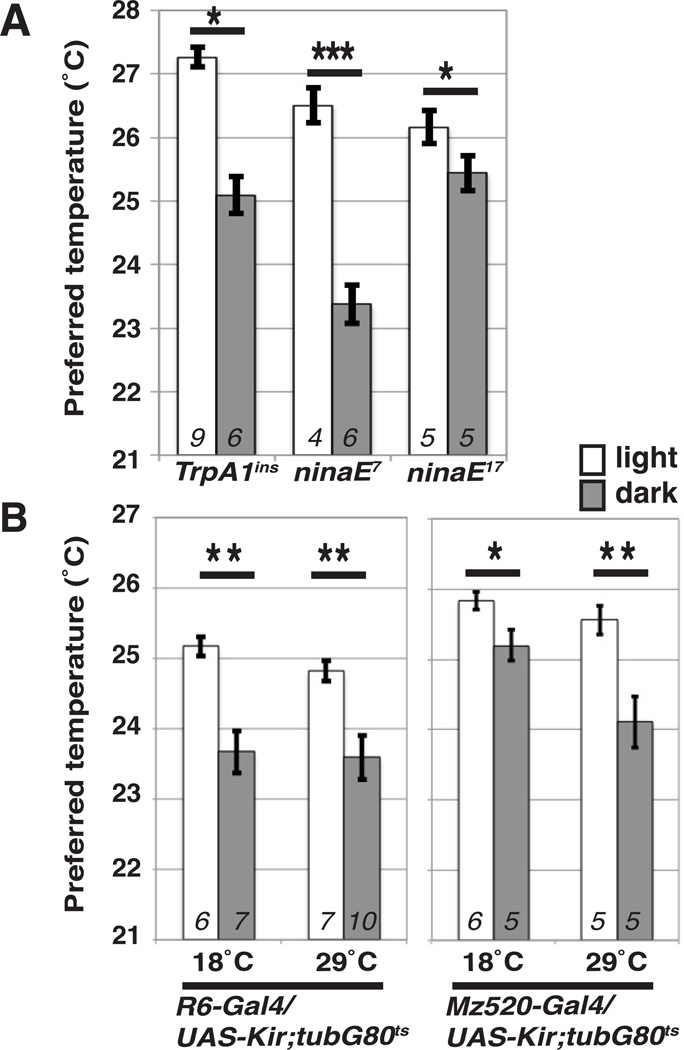
Transient receptor potential A 1 (TrpA1) is important for temperature preference behavior as flies use TrpA1 to detect and avoid warm temperatures. TrpA1 is not only a warm sensor in both larvae and adult flies [12, 26], but also is involved in light-sensing behavior in the body wall of larvae [5]. Furthermore, Rhodopsin 1 (Rh1), encoded by the neither inactivation nor afterpotential E (ninaE) gene, is a molecular light sensor and has been suggested to regulate temperature-sensing behavior in larvae [27]. Therefore, we sought to determine whether TrpA1 and Rh1 were involved in LDTP by using strong loss-of-function mutants for TrpA1 (TrpA1ins)[12] and null mutants for Rh1 (ninaE17)[28](Fig. 3A). However, both mutants showed normal LDTP, indicating that TrpA1 and Rh1 are not necessary for LDTP.
Figure 3. TrpA1, Rhodopsin 1 and sLNvs are not critical for LDTP.
(A) Comparison of preferred temperature of each genotype in light (white bar) and dark (gray bar) conditions. The behavioral experiments were performed at ZT1-3. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
(B) Comparison of preferred temperature of each genotype in light (white bar) and dark (gray bar) conditions. R6-Gal4 is expressed in sLNvs and Mz520-Gal4 is expressed in both sLNvs and lLNvs. A temperature dependent conditional repressor of Gal4, tub-Gal80ts and a mammalian inward rectifier K+ channel, Kir, were used to transiently inhibit the sLNvs depending on permissive temperature (18°C) and restrictive temperature (29°C) as adults. The behavioral experiments were performed at ZT1-3. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
LNvs are dispensable for LDTP
The clock neurons, small ventrolateral neurons (sLNvs), project to DN1s [29, 30]. sLNvs not only contact DN1ps but also receive information from the light sensors, large ventrolateral neurons (lLNvs)[31–33], and receive light inputs from the optic lobe [34]. To determine whether LNvs are involved in LDTP, we used a mammalian inward rectifier K+ channel (UAS-Kir) to genetically inhibit sLNvs with R6-Gal4 [34] as well as sLNvs and lLNvs with Mz520-Gal4 [35, 36]. Because R6-Gal4/UAS-Kir flies did not survive to adult, we used a temperature dependent conditional repressor of Gal4, tubGal80ts, to transiently inhibit the LNvs depending on the permissive temperature (18°C) and the restrictive temperature (29°C). However, at both permissive and restrictive temperatures, the R6-Gal4 and Mz520-Gal4 with UAS-Kir; tub-Gal80ts flies exhibited normal LDTP (Fig. 3B), suggesting that LNvs are not important for LDTP. As positive control using locomotor activity, we showed that Mz520-Gal4 with UAS-Kir; tub-Gal80ts flies exhibited abnormal rhythmicity at 29°C but normal rhythmicity at 18°C (Supplemental Figure S1 and Table S1).
PDFR acts in DN1ps to control LDTP
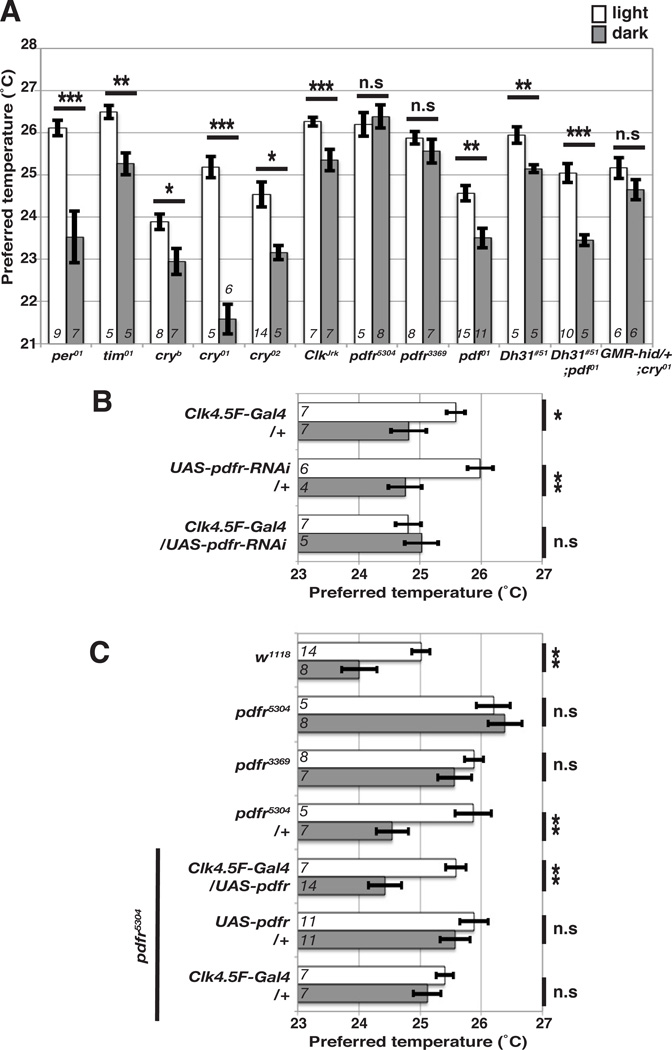
Our data suggest that DN1ps are critical for LDTP. Although the persistence of LDTP in per and tim mutants indicates that this behavior does not require a functional circadian clock (Fig. 1C and D), the DN1ps participate in circadian clock function and thus, express many clock genes. To determine which molecules might act within the DN1p cells to control LDTP, we examined the involvement of additional clock genes, including cryptochrome (cry), Clock (Clk), and pigment-dispersing factor receptor (pdfr), and tested LDTP of mutations in these genes: cryb, cry01, cry02, ClkJrk, pdfr5304 and pdfr3369 (Fig. 4A). Like per01 and tim01 mutants, cryb, cry01, cry02 and ClkJrk mutants all preferred a higher temperature in the light than in the dark, although cry01 preferred a much lower temperature in the dark. Interestingly, we found that pdfr5304 and pdfr3369 mutants displayed abnormal LDTP, in which they preferred similar temperatures in the light and the dark, suggesting that pdfr is required for LDTP (Fig. 4A). PDFR is a G-protein coupled receptor and is critical for locomotor activity and synchronization of the circadian clock [37–40].
Figure 4. PDFR in DN1ps is necessary and sufficient for LDTP.
(A) Comparison of preferred temperature of circadian clock mutants in light (white bar) and dark (gray bar) conditions. The behavioral experiments were performed at ZT1-3. ttest compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
(B) PDFR expression in DN1ps is necessary for LDTP.
Comparison of preferred temperature for flies with UAS-pdfr-RNAi in a subset of clock cells using a DN1ps driver, Clk4.5F-Gal4, and its controls. The behavioral experiments were performed at ZT1-3. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
(C) PDFR expression in DN1ps is sufficient for LDTP.
Comparison of preferred temperature of pdfr5304 mutants with UAS-pdfr in a subset of clock cells using a DN1ps driver, Clk4.5F-Gal4, and its controls. The behavioral experiments were performed at ZT1-3. t-test compared preferred temperature between light and dark conditions: ***P < 0.001, **P < 0.01 or *P < 0.05. Italicized numbers represent the number of assays. Error bars are the SEM.
To determine whether pdfr expression in DN1ps is necessary for LDTP, we knocked down pdfr in DN1ps by using UAS-pdfr-RNAi with Clk4.5F-Gal4, which is selectively expressed in subsets of DN1ps (Fig. 4B). Clk4.5F-Gal4/UAS-pdfr-RNAi flies, the flies exhibited an abnormal LDTP, showing similar preferred temperatures in the light and the dark. However, each Gal4 and UAS control fly line exhibited a normal LDTP, indicating that pdfr expression in DN1ps is necessary for LDTP (Fig. 4B).
To determine whether PDFR expression in DN1ps is sufficient to rescue the pdfr5304 mutants’ phenotype, we expressed UAS-pdfr using Clk4.5F-Gal4 in the pdfr5304 mutants. The pdfr5304 flies that expressed pdfr in DN1ps preferred a higher temperature in the light than in the dark, while the control flies did not, indicating that pdfr expression in DN1ps restored LDTP of pdfr5304 mutants. Thus, PDFR expression in DN1ps is necessary and sufficient to support pdfr’s role in LDTP (Fig. 4C).
Because Pigment-dispersing factor (PDF) and Diuretic hormone 31 (DH31) activate PDFR in vitro [37], we examined whether PDF and DH31 are involved in LDTP. We used the pdf null mutant, pdf01, and the Dh31mutant, Dh31#51, which was generated by P-element excision. Dh31#51 is a strong loss-of-function mutation, as it contains a deletion of the entire active peptide of DH31 (Supplemental Figures S2 and S3). Nonetheless, both pdf01 and Dh31#51 and even the double mutant of Dh31#51; pdf01 exhibited a normal LDTP, indicating that PDF and DH31 are not required for LDTP. These results suggest that LDTP mediated by PDFR in DN1ps is not due to the pathway activated by these known neuropeptides.
DISCUSSION
Here, we show that acute light positively affects temperature preference in Drosophila. LDTP is controlled by Pdfr expressing DN1ps independently from the circadian clock, suggesting DN1ps play an important role in integrating light and temperature information.
Although we tested several eye component mutants, abnormal light or temperature sensing mutants and cry mutants, these mutants still exhibited LDTP behavior. Because light sensors can be redundant in the eye and body wall, partial disruption of these light sensors may not be sufficient for abnormal LDTP (Fig. 2). In fact, the double mutants of GMR-hid/+; cry01, which lack the functions of the compound eye, ocelli, H-B eyelet and CRY, exhibited an abnormal LDTP (Fig. 4A). This result suggests that at least two pathways, such as the visual system and cry, act together to mediate light detection and play an important role in LDTP. Notably, in humans, 460nm light is important for an increase in body temperature during the night [10]. Therefore, it would be interesting to examine which light pathway and wavelengths are critical for LDTP.
While we show that LDTP is circadian clock independent, PDFR expression in DN1ps is critical for LDTP (Fig. 4). However, it is unclear how PDFR is activated because neither PDF nor DH31, the ligands of PDFR, are important for LDTP (Fig. 4). Therefore, our data suggest that PDFR in DN1ps is activated by other unknown mechanisms responsible for LDTP. One possible mechanism is CRY, because CRY is expressed in the clock cells, including DN1ps, and have convergent roles with PDFR for the circadian rhythm of locomotor activity [41]. CRY also antagonizes the temperature synchronization in the dorsal neurons, suggesting that CRY may be involved in the integration of light and temperature [42]. Therefore, it is possible that CRY and PDFR work to regulate LDTP. For example, DN1ps may directly receive light input via CRY, which regulates the signal cascade of PDFR.
Light is critical not only for entraining the circadian clock, but also for a behavior termed masking, in which the flies exhibit a robust increase of locomotor activity after light is turned ON or OFF [43]. The masking effect is controlled separately from the circadian clock [20, 24] and DN1ps are involved in a masking effect for locomotor activity when light is ON [24]. Given that light positively affects preferred temperature separately from the circadian clock, LDTP could be part of the masking effect. However, the light input pathways for the masking effect in locomotor activity and LDTP are not the same. This is demonstrated through evidence that shows that disruption of the compound eye is sufficient for the masking effect of locomotor activity [16], but not for LDTP (Fig. 2). Furthermore, the molecular mechanisms controlling the masking effect in locomotor activity and LDTP are different, as Pdfr mutants exhibit a normal masking effect for locomotor activity [20] but an abnormal LDTP (Fig. 4). Therefore, our data indicate that the masking effect of locomotor activity and LDTP are controlled differently.
Here, we show the positive effect of acute light on the preferred temperature in flies. Given that Drosophila adapt their body temperature to ambient temperature [13], the flies’ body temperature increases in light as a result of their temperature preference behavior. In humans, light exposure increases body temperature during the nighttime [7–9] and is dependent on light intensity [10]. While humans control body temperature through the generation of heat, ectotherms use behavioral strategies to regulate body temperature [13]. Although the mechanism of heat generation is different between humans and flies, the body temperature of both humans and Drosophila increases when exposed to light. Thus, we propose that the effect of light on temperature regulation may be evolutionarily conserved from flies to humans.
Supplementary Material
Acknowledgements
We are grateful to Dr. Patrick Emery for the Clk4.5F-Gal4, cry02 and cryb flies, Dr. Paul Taghert for the Pdf01, pdfr5304 and pdfr5304; UAS-pdfr flies, Dr. Orie Shafer for the Pdf-LexA and R6-Gal4, Dr. Charlotte Helfrich-Förster for Mz520-Gal4, Dr. Michael Rosbash for TIM antibody, Dr. Hugo Bellen for hdcJK910 and the Bloomington Drosophila fly stock center, Vienna Drosophila RNAi Center, and Developmental Studies Hybridoma Bank for the fly lines and antibodies. We thank the Hamada lab members for their comments and advice on the manuscript. This research was supported by a Trustee Grant and RIP funding from Cincinnati Children’s Hospital, JST (Japan Science and Technology)/Precursory Research for Embryonic Science and Technology (PRESTO), the March of Dimes, and NIH R01 GM107582 to F. N. H. and NIH P01 GM103770 to P.A.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
F.N.H., L.M.H., and X.T designed the research and F.N.H., L.M.H., X.T., Y.U., J.R.L., M.F., T.G., and S.E.H. performed the behavioral experiments. X.T performed immunostaining. F.N.H., E.C.C., and P.A.G. created DH31 mutants. F.N.H., L.M.H., and X.T. wrote the manuscript.
REFERENCES
- 1.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan Q, Xiang Y, Yan Z, Han C, Jan LY, Jan YN. Lightinduced structural and functional plasticity in Drosophila larval visual system. Science. 2011;333:1458–1462. doi: 10.1126/science.1207121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Myers BL, Badia P. Immediate effects of different light intensities on body temperature and alertness. Physiol Behav. 1993;54:199–202. doi: 10.1016/0031-9384(93)90067-p. [DOI] [PubMed] [Google Scholar]
- 8.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 9.Dijk DJ, Cajochen C, Borbely AA. Effect of a single 3-hour exposure to bright light on core body temperature and sleep in humans. Neurosci Lett. 1991;121:59–62. doi: 10.1016/0304-3940(91)90649-e. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 11.Hong ST, Bang S, Hyun S, Kang J, Jeong K, Paik D, Chung J, Kim J. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature. 2008;454:771–775. doi: 10.1038/nature07090. [DOI] [PubMed] [Google Scholar]
- 12.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson RD. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. The American Naturalist. 1985;126 [Google Scholar]
- 14.Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomchik SM. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2166–2176a. doi: 10.1523/JNEUROSCI.3933-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno T, Tomita J, Kume S, Kume K. Dopamine modulates metabolic rate and temperature sensitivity in Drosophila melanogaster. PloS one. 2012;7:e31513. doi: 10.1371/journal.pone.0031513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang S, Hyun S, Hong ST, Kang J, Jeong K, Park JJ, Choe J, Chung J. Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet. 2011;7:e1001346. doi: 10.1371/journal.pgen.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko H, Head LM, Ling J, Tang X, Liu Y, Hardin PE, Emery P, Hamada FN. Circadian Rhythm of Temperature Preference and Its Neural Control in Drosophila. Current biology : CB. 2012;22:1851–1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buschbeck EK, Friedrich M. Evolution of Insect Eyes: Tales of Ancient Heritage, Deconstruction, Reconstruction, Remodeling, and Recycling. Evolution: Education and Outreach. 2008;1:448–462. [Google Scholar]
- 20.Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. Journal of biological rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 21.Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moses K, Ellis MC, Rubin GM. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989;340:531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- 23.Shafer OT, Helfrich-Forster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. The Journal of comparative neurology. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Current biology : CB. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Current biology : CB. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 28.O'Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 29.Helfrich-Forster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech. 2003;62:94–102. doi: 10.1002/jemt.10357. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh DJ, Geratowski JD, Wooltorton JR, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheeba V, Gu H, Sharma VK, O'Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helfrich-Forster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. The Journal of comparative neurology. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- 35.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Amrein H. Ventral lateral and DN1 clock neurons mediate distinct properties of male sex drive rhythm in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10590–10595. doi: 10.1073/pnas.0912457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PloS one. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gentile C, Sehadova H, Simoni A, Chen C, Stanewsky R. Cryptochrome antagonizes synchronization of Drosophila's circadian clock to temperature cycles. Current biology : CB. 2013;23:185–195. doi: 10.1016/j.cub.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Page TL. Masking in invertebrates. Chronobiol Int. 1989;6:3–11. doi: 10.3109/07420528909059137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.