Abstract
Sex-specific differences in pulmonary morbidity in adults and preterm infants are well documented. Hyperoxia contributes to lung injury in experimental animals and humans. Cytochrome P450 (CYP)1A enzymes have been shown to play a mechanistic role in hyperoxic lung injury (HLI) in animal models. Whether CYP1A enzymes contribute to gender-specific differences in relation to HLI is unknown. In this investigation, we tested the hypothesis that mice will display gender-specific differences in HLI, and that this phenomenon will be altered in mice lacking the genes for Cyp1a1 or 1a2. Eight week-old male and female wild type (WT) (C57BL/6J) mice, Cyp1a1−/−, and Cyp1a2−/− mice were exposed to 72 hours of hyperoxia (FiO2>0.95). Lung injury and inflammation were assessed and pulmonary and hepatic CYP1A1 and CYP1A2 levels were quantified at the enzyme activity, protein and mRNA level. Upon exposure to hyperoxia, liver and lung microsomal proteins showed higher pulmonary CYP1A1 (apoprotein level and activity) in WT females compared to WT males and a greater induction in hepatic CYP1A2 mRNA levels and activity in WT females after hyperoxia exposure. The gender based female advantage was lost or reversed in Cyp1a1−/− and Cyp1a2−/− mice. These findings suggest an important role for CYP1A enzymes in the gender-specific modulation of hyperoxic lung injury.
Keywords: Hyperoxia, Sex-specific, CYP1A, Lung injury
1. Introduction
Sex-specific differences have been documented to play a role in the pathogenesis and outcome in various diseases in adults and children. Acute respiratory distress syndrome (ARDS) is a devastating clinical disorder in critically ill patients that causes acute lung injury and has a high mortality. Mortality in ARDS is higher in males compared to females (Moss and Mannino, 2002). Oxygen toxicity plays a role in both acute lung injury and ARDS. Hyperoxia may exacerbate or even cause acute lung injury (ALI) in mechanically ventilated patients. It leads to the production of reactive oxygen species that can in turn lead to lung injury via oxidation of cellular macromolecules including DNA, protein and lipid (Freeman and Crapo, 1981; Moss and Mannino, 2002).
The Cytochrome P450 (CYP) enzymes belong to a super family of hemeproteins, involved in the metabolism of exogenous and endogenous chemicals (Guengerich, 2004). Several groups including ours have demonstrated the protective effect of CYP1A in hyperoxic lung injury (HLI) (Lingappan et al., 2014). The CYP1A subfamily comprises 2 isoforms, i.e. CYP1A1 and 1A2. CYP1A1 is essentially an extra-hepatic enzyme (rodent and human lung, intestine, placenta and kidney) while CYP1A2 is expressed mainly in the rodent and human liver. Animals exposed to hyperoxia display upregulation of pulmonary and hepatic CYP1A1 and 1A2 expression for up to 48 hours, and by 60 hours these animals show overt respiratory distress (Couroucli et al., 2002; Moorthy et al., 1997). CYP1A inducers like beta-naphthoflavone (BNF) (Mansour et al., 1988) or 3-methylchloanthrene (3-MC) (Sinha et al., 2005) attenuate and CYP1A inhibitors like 1-aminobenzotriazole (Moorthy et al., 2000) potentiate HLI in rodents. The induction of CYP1A is thought to occur through the Aryl hydrocarbon receptor (AhR) pathway. Aryl hydrocarbon dysfunctional (AhRd) mice were more susceptible to HLI compared to wild type controls (Jiang et al., 2004). Prenatal administration of CYP1A inducer BNF attenuates HLI in newborn mice (Couroucli et al., 2011). These observations support the theory that CYP1A1 and CYP1A2 may play protective roles against oxygen-mediated lung injury.
The impact of sex and sex hormones on lung physiology and disease has been extensively studied in animal models. Gender also contributes to differential lung development prenatally and has a major role in the development of disease conditions from the neonatal (respiratory distress syndrome) to the adult period (asthma, lung cancer, interstitial lung disease) (Carey et al., 2007; Casimir et al., 2013). This is probably due to modulation by sex hormones, which may contribute to the disease pathogenesis or serve as protective factors, depending on the disease. With respect to acute lung injury due to hyperoxia, Neriishi et al (Neriishi and Frank, 1984) showed that castration prolonged tolerance of young male rats to pulmonary O2 toxicity. In other acute lung injury models, testosterone was found to increase (Card et al., 2006) and estrogen to ameliorate inflammation and injury (Speyer et al., 2005). We have shown that WT male mice are more susceptible than females to hyperoxic lung injury and that, differences in inflammatory and oxidative stress markers contribute to these gender-specific dimorphic effects (Lingappan et al., 2013).
Several endogenous factors, including hormones, can modify CYP1A induction, directly influencing CYP1A gene expression or indirectly via cross talks with other transcription factors (Monostory et al., 2009). Molecules with steroidal structure can be AhR ligands and may induce CYP1A expression in an AhR-dependent manner. Previous studies have shown that estradiol and progesterone induce CYP1A1 activity and testosterone on the other hand decreases CYP1A expression (Kojima et al., 2008; Lee et al., 1998; Monostory et al., 2009). Sex related differences in the constitutive expression of CYP1A have also been observed in animal models (Kojima et al., 2008; 2010; Rasmussen et al., 2011).
The gender-based dimorphic response in lung injury due to hyperoxia and the role of the CYP1A subfamily using knock out mouse models has not been studied. In this investigation, we tested the hypothesis that the gender-based differences in hyperoxic lung injury as observed in WT mice, will be lost in mice lacking the genes for Cyp1a1or Cyp1a2.
2. Materials and Methods
2.1. Animals
This study was conducted in accordance with the federal guidelines for the humane care and use of laboratory animals, and was approved by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine. Breeding pairs of WT mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Breeding pairs of Cyp1a2−/− mice of mixed background (C57BL/6J/Sv129) were obtained from Dr.Frank J.Gonzalez (National Cancer Institute, Bethesda, MD). The mice were back crossed with C57BL/6J mice for 12 generations to generate theoretically a >99.2% C57BL/6J background. Breeding pairs of Cyp1a1−/− mice of C57BL/6J background were obtained from Dr. D.W. Nebert (University of Cincinnati Medical Center, Cincinnati, OH). Eight-week old male and female C57BL/6J wild type (WT), Cyp1a1−/− and Cyp1a2−/− mice were maintained at Texas Children's Hospital animal facility and used for the study. Both Cyp1a1−/− and Cyp1a2−/− mice are phenotypically similar to the WT mice and have normal growth (Dalton et al., 2000; Peterson et al., 2004). They were fed standard mice food (Purina Rodent Lab Chow 5001 from Purina Mills, Inc., Richmond, IN) and water ad libitum. Animals were maintained in 12-h day/night cycles.
2.2. Chemicals
Tris, sucrose, NADPH, bovine serum albumin, ethoxyresorufin, methoxyresorufin were purchased from Sigma Chemical Co. (St. Louis, MO). Buffer components for electrophoresis and Western blotting were obtained from Bio-Rad laboratories (Hercules, CA). The primary monoclonal antibody to CYP1A1, which cross-reacts with CYP1A2, was obtained from Dr. P. E. Thomas (Rutgers University, Piscataway, NJ). Goat anti-mouse IgG conjugated with horseradish peroxidase was from Bio-Rad laboratories (Richmond, CA). All Real Time RT-PCR reagents were from Applied Biosystems (Foster City, CA).
2.3. Protocols
Briefly, we used a total of 10 animals per sex per genotype (WT, Cyp1a1−/− and Cyp1a2−/−) in the study. The mice were then maintained in either room air (21% oxygen) in standard laboratory mouse cage or exposed to hyperoxic (95–100% oxygen) environment using pure O2 at 5 l/min for 24 , 48 and 72 h in a sealed Plexiglas chamber, as reported previously (Gonder et al., 1985). After sealing, the oxygen concentration in the Plexiglas chamber was checked frequently by an analyzer (Getronics, Kenilworth, New Jersey). Purified tap water and food (Purina Rodent Lab Chow 5001 from Purina Mills, Inc., Richmond, IN) were available ad libitum. After hyperoxia exposure, the animals were immediately anesthetized with sodium pentobarbital (200 mg/kg i.p.) and euthanized by exsanguination while under deep pentobarbital anesthesia. Three animals from each group were used for lung histopathology and from the seven remaining animals in each group; lung and liver tissues were harvested for the isolation of mRNA, protein, and microsomes. Lung weight and body weight, histology, immunohistochemistry for neutrophil infiltration was reported at 72 h as the effect of hyperoxia on earlier time points was not significant as previously reported (Moorthy et al., 1997). mRNA expression was assessed at earlier time points as transcription usually precedes changes in protein levels.
2.4. Lung weight and body weight (BW) measurements
The mice were weighed immediately after being anesthetized. Lung weights were measured after sacrifice and harvesting to evaluate the severity of lung edema.
2.5. Preparation of tissues for histology, histopathology and immunohistochemistry for neutrophil infiltration
A separate group of animals were used for these analyses. Lungs were fixed for histological analysis. Liver tissue from these animals was harvested for further downstream protein and RNA extraction. Tracheotomy was performed on the anesthetized mice and the lung tissue was fixed by intratracheal instillation of 10% zinc formalin at constant pressure of 25 cm of H2O (Couroucli et al., 2002). Samples were left in solution for 24 h in 10% zinc formalin, and then transferred to 70% ethanol for long-term storage. Routine histology was performed on lung tissues from individual animals following staining of the paraffin sections with hematoxylin and eosin. For quantification of lung injury we used the guidelines published in the American Thoracic Society workshop report for the measurement of acute lung injury in experimental animals (Matute-Bello et al., 2011). Briefly, 20 random high-power fields were independently scored in a blinded fashion for five histological findings: neutrophils in the alveolar space, neutrophils in the interstitial space, hyaline membranes, proteinaceous debris filling the airspaces and alveolar septal thickening were graded using a three tiered schema resulting in a score between zero and one. For assessment of neutrophil infiltration, 5 μM deparaffinized lung sections were immunostained with rat anti-mouse neutrophil antibody (Serotec, Raleigh, NC; MCA771G, dilution 1:200) for neutrophils, followed by staining with biotinylated secondary antibodies (Vector Laboratories Burlingame, CA). To analyze the degree of pulmonary neutrophil infiltration, the positively stained cells were counted in 20 non-adjacent areas per mouse under 40x magnification. A pulmonary pathologist, who was blinded to the treatment of mice with various regimens, evaluated the histopathology and immunohistochemistry slides.
2.6. Preparation of microsomes and enzyme assays
Lung and liver samples at the time of dissection were frozen immediately with liquid nitrogen and maintained at a temperature of – 80°C until preparation of microsomes. Liver microsomes were isolated by the calcium chloride precipitation method (Moorthy et al., 1997). Lung microsomes were isolated by differential centrifugation, as published previously {Matsubara:1974ty}. Protein concentration was measured by the Bradford dye binding method. Ethoxyresorufin O-deethylase (EROD) (CYP1A1) activities in lung and liver microsomes and methoxyresorufin O-demethylase (MROD) (CYP1A2) activities in liver microsomes were assayed as described previously (Moorthy et al., 1997).
2.7. Western blotting
Liver microsomes (10 μg of protein) prepared from individual animals were subjected to SDS polyacrylamide gel electrophoresis in 10% acrylamide gels. The separated proteins on the gels were transferred to polyvinylidene difluoride membranes, followed by western blotting. For the western blot analysis, a monoclonal antibody to CYP1A1, which cross-reacts with CYP1A2 was used as a primary antibody. The primary antibody was detected by incubation with the horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody. Glucose-regulated protein (GRP, 78 kDa), a microsomal protein was used as the housekeeping protein. Lung whole protein (20 μg of protein) prepared from individual animals in Milliplex MAP lysis buffer (EMD Millipore) was used for apoprotein estimation. This was done at 48 h, because at 72 h there was significant protein degradation in lung isolates. For loading controls, the membranes were stripped and incubated with antibodies against β-actin, followed by electrochemical detection of bands.
2.8. Real Time RT-PCR assays
Total mRNA was isolated using a modification of the procedure from Chomczynski et al. (Chomczynski and Sacchi, 1987)and treated with RQ1 RNase-free DNase (Promega) to eliminate genomic DNA contamination. RNA (50 ng), isolated as above, was subjected to one step real time quantitative TaqMan® RT-PCR using 7900HT Fast Real- Time PCR System (Applied Biosystems, Foster City, CA). Gene-specific primers (CYP1a1-Mm00487217_m1; TNF-α Mm00443258_m1; 18SrRNA-Hs99999901_s1) in the presence of TaqMan® reverse transcription reagents and RT reaction mix (Applied Biosystems, Foster City, CA) were used to reverse transcribe RNA, and TaqMan®Gene Expression probes and TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA) were used for PCR amplification. For CYP1A2, the forward primer was: TCCTGGACTGACTCCCACAAC and the reverse primer was: GAACGCCATCTGTACCACTGAA. 18SrRNA was used as the reference gene. Following an RT hold for 30 minutes at 48°C, the samples were denatured at 95°C for 10 minutes. The thermal cycling step was for 40 cycles at 95°C for 15 s, and 40 cycles at 60°C for 1 minute. The ΔΔCt method was used to calculate the fold change in mRNA expression: ΔCt = Ct (target gene)- Ct (reference gene), ΔΔCt = ΔCt (treatment) - ΔCt (control), fold change = 2(-ΔΔCt) (Jiang et al., 2004).
2.9. Data analysis
The comparison between male and female mice, the three genotypes, and the effect of hyperoxia exposure was done using two-way ANOVA, followed by Bonferroni post-hoc tests, and p ≤ 0.05 was considered significant. The statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA)
3. Results
3.1. Effect of hyperoxia on Lung weight (LW) and Body weight (BW)
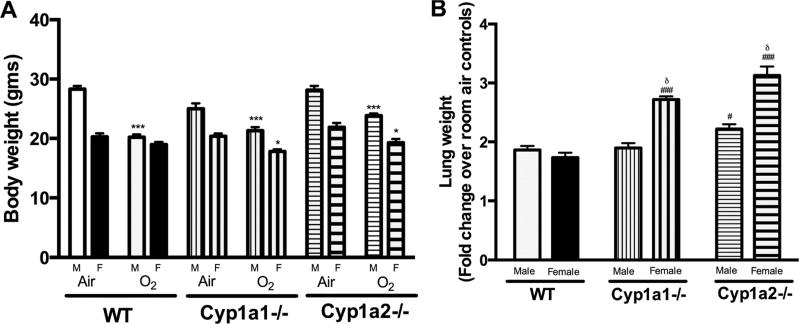
Figure 1A shows the effect of hyperoxia on body weight of male and female animals after exposure to hyperoxia. WT females showed no significant weight loss similar to our previous findings (Lingappan et al., 2013). The weight loss was significant in all other groups and was more significant in males than females. Figure 1B represents the fold change in lung weight from respective room air controls after exposure to hyperoxia. Since differences existed in lung weights between male and female animals even at room air conditions, we expressed the ratio as fold change after exposure to hyperoxia (72 h) compared to the corresponding room air breathing control animals. Exposure to hyperoxia increased the lung weights in all the groups. Cyp1a1 and Cyp1a2−/− animals showed gender-specific changes with females showing greater lung weight after 72 hours of hyperoxia exposure. The increases in lung weights were also greater in Cyp1a1−/− and Cyp1a2 −/− females when compared to respective WT females and in Cyp1a2−/− males when compared to WT males.
Figure 1.
Effect of hyperoxia (72 h) on lung weight and body weight: 1A: Effect of hyperoxia on body weights of WT, Cyp1a1−/− and Cyp1a2−/− mice. Values are means ± SEM from at least 5 individual animals. 1B: Fold change in lung weight following hyperoxia exposure in male and female WT, Cyp1a1−/− and Cyp1a2−/− mice. Values are means ± SEM from at least 3 individual animals. Significant differences between male and female mice are indicated by δ, p <0.05, between WT and knock-out (Cyp1a1−/− or Cyp1a2−/−) mice within the same sex are indicated by #, p <0.05 and ###, p<0.001, and between room air and hyperoxia exposed mice are indicated by *, p<0.05 and ***, p<0.001.
3.2. Lung histopathology
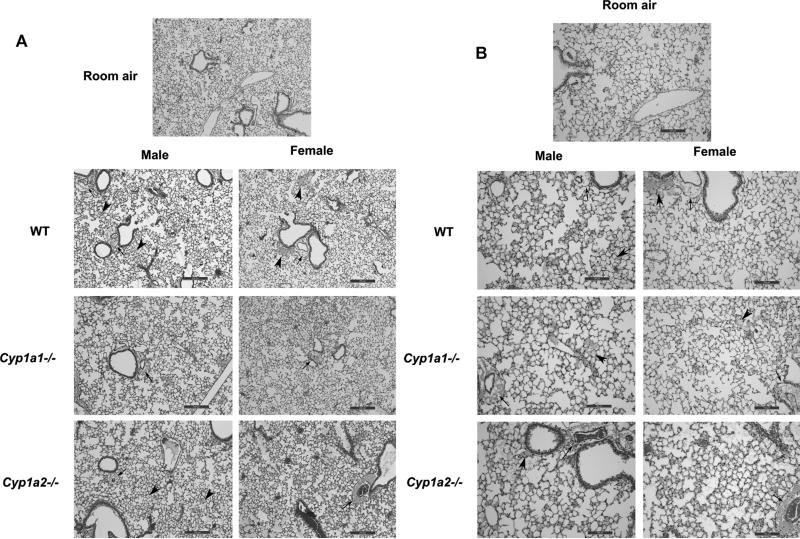
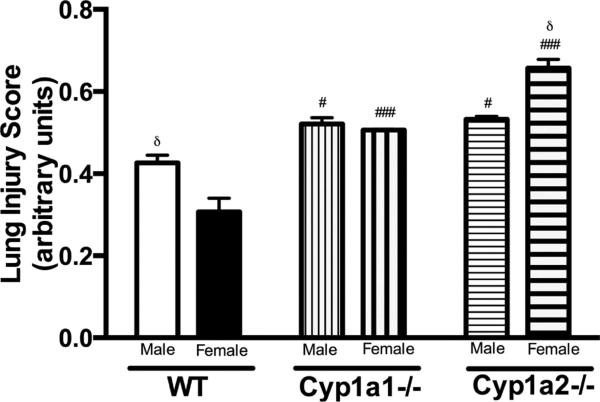
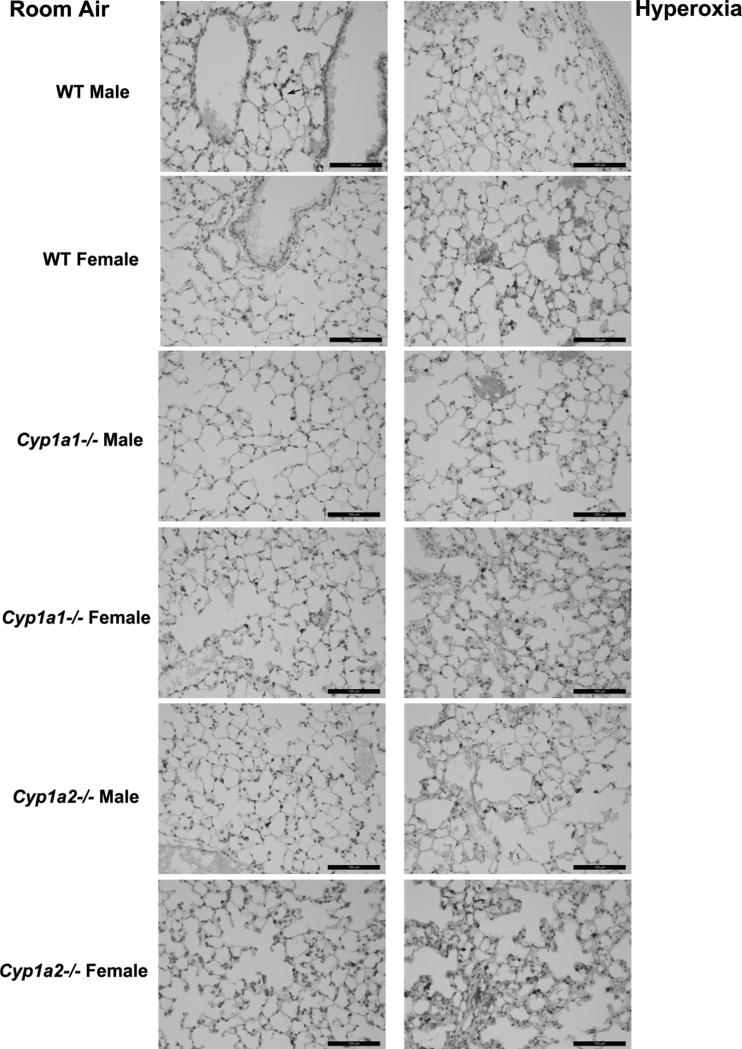
Histopathological examination of the lung sections after exposure to hyperoxia for 72 h showed greater perivascular, bronchiolar edema and alveolar hemorrhage in male WT mice when compared to female mice (Fig. 2A and 2B). The room air control images from the animals are shown in Supplementary figure 1. There was no difference in lung histology between the genotypes at baseline. In Cyp1a2−/− mice, females showed more signs of lung injury when compared to males. Lung injury was similar among male and female mice in Cyp1a1−/− mice. This was quantified objectively (Figure 3) using the American Thoracic Society guidelines for the measurement of acute lung injury in experimental animals as described in Materials and Methods (Matute-Bello et al., 2011). After 72 h of hyperoxia exposure, WT males showed greater lung injury compared to females and the reverse was observed in Cyp1a2−/− animals. There were no differences between males and female mice in the Cyp1a1 −/− group. Loss of CYP1A (1A1 or 1A2) led to increased lung injury in both males and female animals, with females showing a more significant effect.
Figure 2. Effect of hyperoxia on lung histopathology.
Representative hematoxylin eosin stained images from the lungs of WT and Cyp1a1−/− and Cyp1a2−/− mice (n=5 mice per group). WT, Cyp1a1−/− and Cyp1a2−/− mice were exposed to hyperoxia (72 h) at 4 x (2A) and 10x (2B) magnification of the lung fields. WT males show more perivascular, bronchiolar edema and alveolar hemorrhage when compared to female mice. Cyp1a2−/− females show greater signs of lung injury than males. Thick arrows point to areas of alveolar edema. Thin arrows point to regions showing perivascular edema.
Figure 3. Lung injury in WT, Cyp1a1−/− and Cyp1a2−/− male and female mice.
Lung injury scores in 20 random high-power fields (400× total magnification, n = 3 mice per group) in hematoxylin and eosin stained lung sections obtained from male and female mice of all three genotypes after exposure to hyperoxia (72 h). Significant differences between male and female mice are indicated by δ, p <0.05, between WT and knock-out (Cyp1a1−/− or Cyp1a2−/−) mice within the same sex are indicated by #, p <0.05 and ###, p<0.001
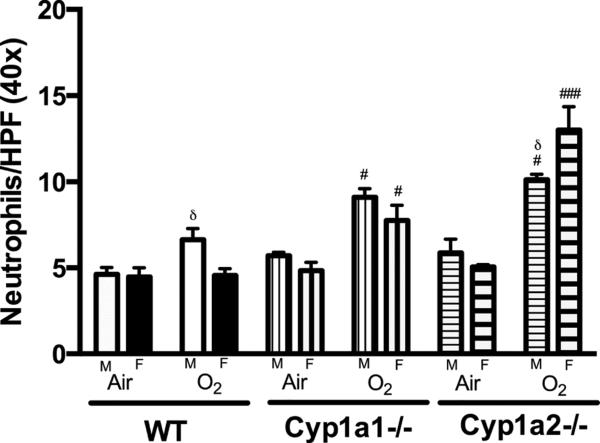
3.3. Neutrophil Infiltration
In order to assess whether increased lung injury after hyperoxia is accompanied by exacerbation of inflammation, we determined the extent of neutrophil recruitment in lung of mice exposed to hyperoxia. Upon exposure to hyperoxia for 72 h, lungs showed neutrophil infiltration in response to the injury as shown in Figure 4. Representative images from room air controls and animals exposed to hyperoxia are shown. Figure 5 shows the quantitative assessment of neutrophil infiltration in the lungs. There was no difference at room air conditions. After hyperoxia exposure, WT males showed higher neutrophil infiltration in the lungs when compared to females (Fig 5) (P<0.05). There were no differences among Cyp1a1−/− animals between males and females. Among Cyp1a2−/− animals, female mice showed significantly higher neutrophil infiltration compared to males after exposure to hyperoxia (p<0.05). Similar to the results with lung injury, there was significantly more neutrophil infiltration in Cyp1a1−/− and Cyp1a2−/− animals than WT mice.
Figure 4.
Representative immunostained images for lung neutrophils. Hyperoxia-induced neutrophil recruitment was determined by immunohistochemistry with anti-neutrophil antibodies in WT, Cyp1a1−/− and Cyp1a2−/− mice (n=4 mice per group) at room air and after 72 h of hyperoxia. Arrow points to brown-staining neutrophils.
Figure 5.
Representative quantitative analysis of neutrophil infiltration in the lungs at room air and after exposure to 72 h of hyperoxia (FiO2>95%). Neutrophil count per high power (40x magnification) was done as described under materials and methods. Data represent means ± SEM from at least 3 individual animals in each group. Significant differences between male and female mice are indicated by δ, p <0.05. Significant differences between WT and knock-out (Cyp1a1−/− or Cyp1a2−/− mice) within the same sex are indicated by #, p <0.05 and ###, p<0.001.
3.4. TNF- α mRNA expression in the lungs after hyperoxia exposure
Figure 6 shows the expression of TNF-α mRNA in the lungs after 24, 48 and 72 h of hyperoxia expressed as fold change over room air levels. Figure 6A, 6B and 6C shows the trend of TNF-α mRNA between male and female mice in WT, Cyp1a1−/− and Cyp1a2−/− mice, respectively. Among the Cyp1a2−/− mice, females showed a significantly higher expression at 24 h (P<0.05) compared to males. WT males displayed higher expression at 72 h compared to females but this trend was not statistically significant. Cyp1a2−/− male mice showed increased expression at 48 (P<0.01) and 72 h (P<0.001) compared to room air levels. WT and Cyp1a1−/− males showed increased expression (P<0.05) at 72 hours compared to room air controls. Cyp1a2−/− female mice (Figure 6B) had increased expression at 24 h (P<0.001), 48 h (P<0.01) and 72 h (P<0.001) compared to room air controls. There were no differences in the baseline expression of TNF-α between any of the groups. Cyp1a2−/− mice of either sex showed increased expression compared to WT mice (data not shown).
Figure 6.
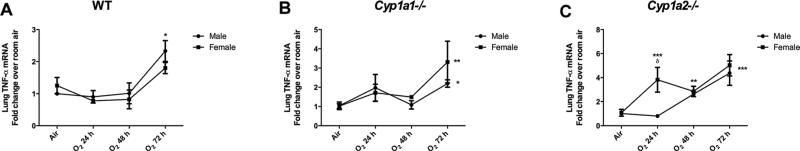
Real time PCR analysis showing the fold increase in lung TNF- α mRNA expression in over baseline room air levels after 72 h of hyperoxia exposure. Values are means ± SEM from at least 3 individual animals. Figures 6A, 6B and 6C show the changes in male and female mice in WT, Cyp1a1−/− and Cyp1a2−/− mice. Significant differences between male and female mice are indicated by δ, p <0.05. Significant differences form room air controls are indicated by *, p <0.05; **, p<0.01 and ***, p<0.001.
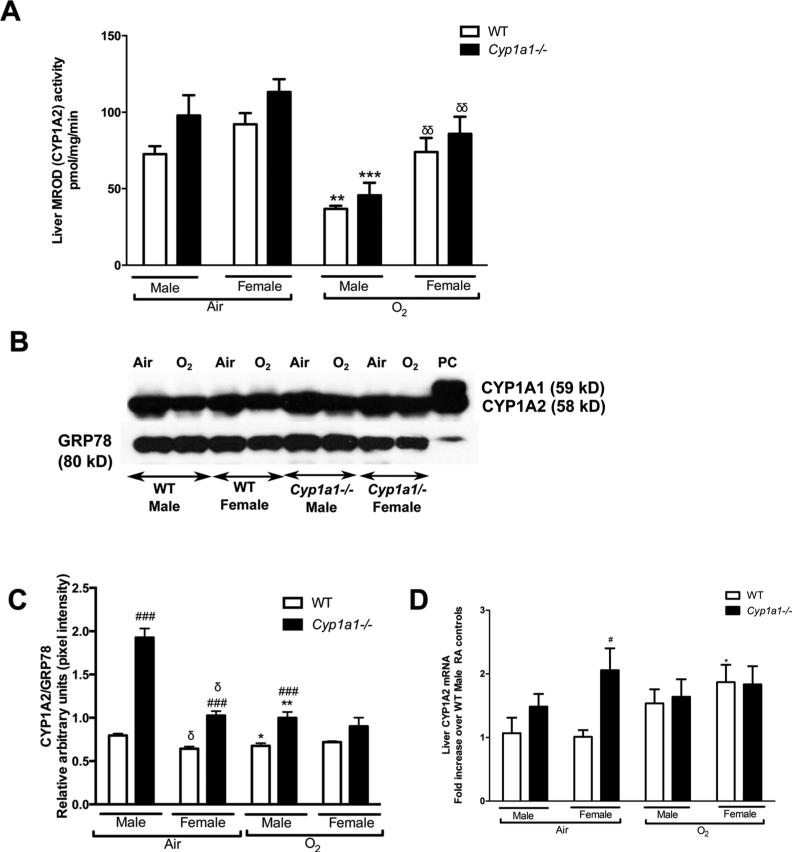
3.5. Effect of hyperoxia on hepatic CYP1A activity and expression
Hepatic CYP1A2 enzyme activity was measured using the MROD assay. Female mice (WT and Cyp1a1−/−), showed higher CYP1A2 enzyme activity as measured by the MROD assay (Fig 7A) after exposure to hyperoxia (p<0.01) compared to male mice. The decline in CYP1A2 activity compared to baseline room air levels after 72 h hyperoxia exposure was significant in male mice (WT and Cyp1a1−/−) (p <0.01), and was better preserved in female mice. Next, we determined CYP1A protein expression in the liver microsomal protein fraction under room air and after hyperoxia exposure (72 h) in WT and Cyp1a1−/− animals. Western blot assay (Fig 7B) and densitometric analysis (Fig 7C) revealed that males have greater decline in the protein after hyperoxia exposure than females (p value<0.05 in WT males; p value<0.01 in Cyp1a1−/− males). Also, Cyp1a1−/− mice showed higher CYP1A2 protein levels compared to WT mice in room air. We performed RT-PCR with liver tissues at room air and after 24 h of hyperoxia exposure to look for the expression of CYP1A2 mRNA between WT and Cyp1a1−/− animals. The expression at 24 h (Fig 7D) was expressed as fold increase from WT male room air levels. In WT animals, females showed a significant induction in CYP1A2 mRNA expression at 24 h compared to room air levels (p<0.05), which is consistent with the higher activity and protein expression in females as described before. Cyp1a1−/− females showed higher baseline expression in room air compared to WT females.
Figure 7.
Hepatic CYP1A activity and expression 7A: MROD (CYP1A2) activity in the liver as measured by the MROD assay at room air (Air) and after 72 h of hyperoxia exposure (O2). Data represent means±SEM from at least 4 individual animals in each group. 7B: Representative western blot assays showing hepatic CYP1A2 (apoprotein) expression in liver microsomes isolated from WT and Cyp1a1−/− mice at room air (Air) and after 72 h of hyperoxia exposure (O2). GRP-78 (glucose regulated protein; 78kDa) was used as the loading control. The positive control (labeled “PC”) was 0.5 μg of liver microsomes from mice treated with 3-methylcholanthrene. 7C: Densitometric analysis of hepatic CYP1A2 immunoblots. 7D: Real-time RT-PCR analysis for CYP1A2 mRNA (Liver) in WT and Cyp1a1−/− mice. Values are means±SEM from at least 3 individual animals. Liver CYP1A2 mRNA expression at 24 h of hyperoxia exposure expressed as fold change over expression level in WT males at room air. Significant differences between male and female mice are indicated by δ, p <0.05 and δδ, p <0.01. Significant differences between WT and knock-out (Cyp1a1−/− mice) within the same sex are indicated by #, p <0.05 and ###, p<0.001. Significant differences form room air controls are indicated by *, p <0.05; **, p<0.01 and ***, p<0.001.
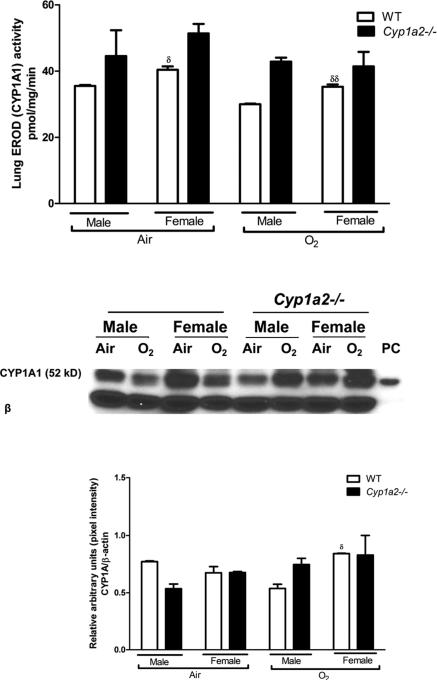
3.6. Effect of hyperoxia on pulmonary CYP1A activity and protein levels
WT male mice had lower CYP1A1 activity at room air (p<0.05) and after exposure to hyperoxia for 72 h (p<0.01) when compared to WT females (Fig 8A). This sex specific difference was not seen in Cyp1a2−/− mice. Next, we determined the effect of hyperoxia on CYP1A1 protein expression in lung total protein fractions. Western blot assay of lung protein (Fig 8B) and densitometric analysis (Fig 8C) of CYP1A1 protein expression in WT and Cyp1a2−/− mice at room air and after 48 h of hyperoxia exposure showed that there was a decline in CYP1A1 protein level in WT males, while Cyp1a2−/− males showed an increase in protein level (p<0.05). In the lung, RT-PCR for CYP1A1 expression showed no sex-specific differences (data not shown).
Figure 8.
Pulmonary CYP1A1 activity and western blot assay 8A: CYP1A1 activity in the lung as measured by the EROD assay at room air (Air) and after 72 h of hyperoxia exposure (O2). Data represent means±SEM from at least 4 individual animals in each group. Significant differences between male and female mice are indicated by δ, p <0.05 and δδ, p <0.01. 8B: Whole lung proteins (20μg) were subjected to Western blotting using monoclonal antibodies raised against CYP1A1, as described in “Materials and methods”. Whole lung proteins were isolated from WT and Cyp1a2−/− mice at room air (Air) and after 48 h of hyperoxia exposure (O2). Under each sample lane is the corresponding β-actin blot to assess for protein loading. The positive control (labeled “PC”) was 0.5 μg of liver microsomes from mice treated with 3-MC. 8C: Densitometric analysis of pulmonary CYP1A1 immunoblots at room air and after 48 h of hyperoxia exposure (O2). . Significant differences between male and female mice are indicated by δ, p <0.05.
4. Discussion
In this study, we demonstrated the gender based dimorphic response in lung injury due to hyperoxia and its possible correlation with the differences in expression of CYP1A1 and CYP1A2 in male and female mice. WT male mice were more susceptible to hyperoxic injury compared to female mice. The WT male mice had more pulmonary edema, lung injury and neutrophil infiltration after hyperoxia exposure and this effect was either lost or reversed in Cyp1a1 and Cyp1a2−/− mice.
The protective effect of CYP1A system against hyperoxic lung injury has been well documented. Induction of the CYP1A system, using agents like 3-MC and BNF (Mansour et al., 1988; Sinha et al., 2005) attenuates, while treatment with the CYP1A inhibitor 1-aminobenzotriazole (Moorthy et al., 2000) exacerbates lung injury in hyperoxic conditions. Since CYP1A1, but not CYP1A2 is expressed in lung, we only analyzed pulmonary expression of CYP1A1. Although CYP1A2 is mainly present in the liver and rarely expressed in the extra-hepatic tissues, Cyp1a2−/− mice are more susceptible to hyperoxic lung injury (unpublished results), and show evidence of increased ROS formation under hyperoxic conditions (Shertzer et al., 2004). This indicates that CYP1A2 may have extra-hepatic protective effects under these conditions. The induction of CYP1A by inducers like 3MC and BNF is thought to be mediated via the AhR pathway and we have observed that the AhR-deficient mice were refractory to CYP1A1 induction by hyperoxia and were more sensitive to lung injury than wild-type mice (Jiang et al., 2004).
Sex related differences in the constitutive expression of CYP1A have been observed in pigs (Kojima et al., 2010; 2008; Rasmussen et al., 2011). mRNA, protein and enzyme activities were higher in female and castrate male pigs suggesting down regulation of CYP1A expression by androgens. Testosterone treatment decreased the expression of CYP1A in both sexes of immature pigs (Kojima et al., 2008). Both estradiol and progesterone increased CYP1A1 activity and expression in mouse hepatoma Hepa1c1c7 cells (Lee et al., 1998). A decrease in AhR expression and Ah-responsiveness for the induction of CYP1A1 was observed in MCF-7 breast cancer cells grown in medium with low concentration of estradiol, which was reversed with estradiol supplementation (Spink et al., 2003). On the other hand testosterone has been shown to decrease CYP1A expression (Monostory et al., 2009).
To test whether CYP1A expression was different between male and female mice, which could correlate with the differences in lung injury, we conducted several experiments. Analysis of liver CYP1A2 mRNA by real time RT-PCR showed greater induction at 24 h of hyperoxia exposure in females compared to males. This indicates that exposure to hyperoxia leads to greater transcriptional activation of the Cyp1a2 gene in the liver in female mice compared to males, presumably via AhR-dependent mechanism. In the liver, we measured CYP1A2 activity using the MROD assay and, protein level using Western blot at baseline and after 72 h of hyperoxia exposure. WT males showed decreased hepatic CYP1A2 activity and a greater decline in the CYP1A2 activity and protein level after exposure to hyperoxia than females. Since CYP1A2 is protective against hyperoxic lung injury, this could explain the attenuation of lung injury in females compared to males. Our findings were contrary to Macak-Safranko et al (Mačak-Šafranko et al., 2011) where they exposed 1 month old CBA/Hr mice to 18 h of hyperoxia and measured CYP1A2 mRNA levels in male and female mice, and found that expression was decreased in females. The discrepancies in our observations may be due to different time points of measurement, different strain of mice etc. However, since the increase in mRNA expression at 24 h is accompanied with the relatively increased CYP1A2 activity in females in this study, and the fact that our unpublished data show the protective effects of CYP1A2 induction in hyperoxic lung injury, we conclude that there is a differential mechanistic relationship in males and females in regard to CYP1A2 expression and hyperoxic lung injury.
In the lungs, female WT mice had greater constitutional activity of CYP1A1, as measured by the EROD assay. After exposure to hyperoxia female mice have greater CYP1A1 activity and protein level when compared to males. The mRNA expression was not significant between the two groups. This may suggest a post-translational mechanism (e.g. protein stabilization) leading to increased enzyme activity and protein level in the females.
To further elucidate the role of CYP1A in the gender-based differences, we subjected male and female Cyp1a1−/− and Cyp1a2−/− mice of the same background to hyperoxia. Interestingly, in the knockout animals females lose the gender advantage and show greater lung injury than males. Pulmonary edema (as measured by change in lw/bw) was worse in females compared to their male counterparts. Lung histology showed more signs of injury in female mice compared to male mice. Cyp1a2−/− females showed greater neutrophil infiltration and TNF-α mRNA expression in their lungs compared to males. Induction of TNF-α in hyperoxic lung injury has been shown previously (Jensen et al., 1992). There have also been reports of sex-specific differences in its function (Kadokami et al., 2000) and its role in the repression of CYP1A1 and CYP1A2 under inflammatory conditions (Muntané-Relat et al., 1995). The increased lung injury and inflammation in Cyp1a1−/− and Cyp1a2−/− animals was similar to previously published research suggesting antioxidant activity of CYP1A2 (Shertzer et al., 2004). These findings suggest a gender-specific modulation of CYP1A in the setting of hyperoxic conditions in WT mice, which is lost in the Cyp1a1−/− and Cyp1a2−/− mice. Our results suggest an interaction between the CYP1A system and a female-specific mechanism involved in protection against hyperoxic lung injury. One possibility is the generation of 2-methoxyestradiol from 17- β estradiol by CYP1A1/A2 (hydroxylation) and subsequent methylation by catechol-O-methyl transferase (COMT) (Pribluda et al., 2000). The effects of 2-methoxyestardiol are independent of estrogen receptors alpha and beta (LaVallee et al., 2002). 2-methoxyestradiol has been shown to have anti-inflammatory effects (Duncan et al., 2012; Quezada et al., 2013; Stubelius et al., 2014). In the absence of CYP1A, in the knock-out mice, this protective metabolite is not produced in female mice compared to WT female mice, which may explain the reversal or loss of better outcomes in Cyp1a1−/− and Cyp1a2−/− female mice. The reversal of sex-specific differences is more pronounced in the Cyp1a2−/− mice. This is an interesting finding as CYP1A2 is a liver-specific enzyme, suggesting the protective role of extra pulmonary organs like the liver in hyperoxic lung injury, especially in female mice.
In this study, we did not time our experiments with the estrous cycle in female mice, which could have led to some of the variability in our findings. However, consistent results across multiple experiments prove the validity of our findings. Other mechanisms have been proposed explaining the resistance of female mice to hyperoxia induced oxidative stress such as upregulated expression of heme oxygenase-1 and other cytochrome enzymes such as Cyp2a5 (Mačak-Šafranko et al., 2011). AhR also induces phase II enzymes such as NAD(P)H quinone reductase, glutathione S-transferase-α and aldehyde dehydrogenase, the beneficial effect of these enzymes cannot be excluded. Other antioxidant enzymes like superoxide dismutase could also have contributed towards the gender based differences observed (Park and Rho, 2002). Although hormones like estradiol (E2) and testosterone are a major contributor to sex-specific outcomes, there may be other mechanisms underlying these differences (Casimir et al., 2013). Sex-specific differences in apoptosis have been shown in neuronal ischemia, heart failure and renal ischemia. Divergent cell death pathway activation could be one of the reasons leading to this phenomenon (Lang and McCullough, 2008).
5. Conclusions
Our findings showing sex-specific differences in hyperoxic lung injury indicate a mechanistic role for CYP1A enzymes in the gender differences in oxygen injury. Since drugs can modulate CYP1A enzymes, we strongly believe that further research could lead to novel approaches for the prevention and/or treatment of acute lung injury.
Supplementary Material
Highlights.
Cytochrome P450 (CYP)1A enzymes show gender-specific changes in hyperoxic lung injury.
WT female mice show higher CYP1A expression in hyperoxia compared to males.
The female advantage is lost or reversed in Cyp1a1−/− and Cyp1a2−/− mice.
Acknowledgement
This work was supported by grants from National Institutes of Health [R01-HL-112516, R01-HL-087174, R01-ES-019689, and R01-ES-009132 to B.M., and R01-HL-088343 to XIC].
Abbreviations
- AhR
aryl hydrocarbon receptor
- AhRd mice
aryl hydrocarbon receptor dysfunctional mice
- BNF
Beta-naphthoflavone
- BPD
bronchopulmonary dysplasia
- CYP
cytochrome P450
- EROD
ethoxyresorufin-o-deethylase
- 3-MC
3- methylcholanthrene
- MROD
methoxyresorufin-o-demethylase
- O2
Oxygen
- ROS
reactive oxygen species
- RDS
Respiratory Distress Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol. Metab. 2007;18:308–313. doi: 10.1016/j.tem.2007.08.003. doi:10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir GJ, Lefèvre N, Corazza F, Duchateau J. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol Sex Differ. 2013;4:16. doi: 10.1186/2042-6410-4-16. doi:10.1186/2042-6410-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. doi:10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Couroucli XI, Liang Y-HW, Jiang W, Wang L, Barrios R, Yang P, Moorthy B. Prenatal administration of the cytochrome P4501A inducer, B-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol. Appl. Pharmacol. 2011;256:83–94. doi: 10.1016/j.taap.2011.06.018. doi:10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol. Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem. Biophys. Res. Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. doi:10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Brenner D, Tusche MW, Brüstle A, Knobbe CB, Elia AJ, Mock T, Bray MR, Krammer PH, Mak TW. 2-Methoxyestradiol inhibits experimental autoimmune encephalomyelitis through suppression of immune cell activation. Proceedings of the National Academy of Sciences. 2012;109:21034–21039. doi: 10.1073/pnas.1215558110. doi:10.1073/pnas.1215558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- Gonder JC, Proctor RA, Will JA. Genetic differences in oxygen toxicity are correlated with cytochrome P-450 inducibility. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6315–6319. doi: 10.1073/pnas.82.18.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P450: what have we learned and what are the future issues? Drug Metab. Rev. 2004;36:159–197. doi: 10.1081/dmr-120033996. doi:10.1081/DMR-120033996. [DOI] [PubMed] [Google Scholar]
- Jensen JC, Pogrebniak HW, Pass HI, Buresh C, Merino MJ, Kauffman D, Venzon D, Langstein HN, Norton JA. Role of tumor necrosis factor in oxygen toxicity. J. Appl. Physiol. 1992;72:1902–1907. doi: 10.1152/jappl.1992.72.5.1902. [DOI] [PubMed] [Google Scholar]
- Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J. Pharmacol. Exp. Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. doi:10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J. Clin. Invest. 2000;106:589–597. doi: 10.1172/JCI9307. doi:10.1172/JCI9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Sekimoto M, Degawa M. A novel gender-related difference in the constitutive expression of hepatic cytochrome P4501A subfamily enzymes in Meishan pigs. Biochem. Pharmacol. 2008;75:1076–1082. doi: 10.1016/j.bcp.2007.10.030. doi:10.1016/j.bcp.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kojima M, Sekimoto M, Degawa M. Androgen-mediated down-regulation of CYP1A subfamily genes in the pig liver. Journal of Endocrinology. 2010;207:203–211. doi: 10.1677/JOE-10-0160. doi:10.1677/JOE-10-0160. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. doi:10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62:3691–3697. [PubMed] [Google Scholar]
- Lee SS, Jeong HG, Yang KH. Effects of estradiol and progesterone on cytochrome P4501A1 expression in Hepa 1c1c7 cells. Biochem. Mol. Biol. Int. 1998;45:775–781. doi: 10.1080/15216549800203192. [DOI] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Couroucli XI, Barrios R, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: implications for acute and chronic lung disease in humans. Toxicol. Appl. Pharmacol. 2013;272:281–290. doi: 10.1016/j.taap.2013.06.007. doi:10.1016/j.taap.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Wang G, Couroucli XI, Shivanna B, Welty SE, Barrios R, Khan MF, Nebert DW, Roberts LJ, Moorthy B. Mice Deficient in the Gene for Cytochrome P450 (CYP)1A1 are More Susceptible than Wild Type to Hyperoxic Lung Injury: Evidence for Protective Role of CYP1A1 Against Oxidative Stress. Toxicol. Sci. 2014 doi: 10.1093/toxsci/kfu106. doi:10.1093/toxsci/kfu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mačak-Šafranko Z, Sobočanec S, Sarić A, Balog T, Sverko V, Kušić B, Marotti T. Cytochrome P450 gender-related differences in response to hyperoxia in young CBA mice. Exp. Toxicol. Pathol. 2011;63:345–350. doi: 10.1016/j.etp.2010.02.009. doi:10.1016/j.etp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Mansour H, Brun-Pascaud M, Marquetty C, Gougerot-Pocidalo MA, Hakim J, Pocidalo JJ. Protection of rat from oxygen toxicity by inducers of cytochrome P-450 system. Am. Rev. Respir. Dis. 1988;137:688–694. doi: 10.1164/ajrccm/137.3.688. doi:10.1164/ajrccm/137.3.688. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. An Official American Thoracic Society Workshop Report: Features and Measurements of Experimental Acute Lung Injury in Animals. American Journal of Respiratory Cell and Molecular Biology. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. doi:10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monostory K, Pascussi J-M, Kóbori L, Dvorak Z. Hormonal regulation of CYP1A expression. Drug Metab. Rev. 2009;41:547–572. doi: 10.1080/03602530903112284. doi:10.1080/03602530903112284. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol. Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J. Pharmacol. Exp. Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979-1996). Crit. Care Med. 2002;30:1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Muntané-Relat J, Ourlin JC, Domergue J, Maurel P. Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology. 1995;22:1143–1153. [PubMed] [Google Scholar]
- Neriishi K, Frank L. Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am. J. Physiol. 1984;247:R475–81. doi: 10.1152/ajpregu.1984.247.3.R475. [DOI] [PubMed] [Google Scholar]
- Park EY, Rho HM. The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Mol. Cell. Biochem. 2002;240:47–55. doi: 10.1023/a:1020600509965. [DOI] [PubMed] [Google Scholar]
- Peterson TC, Peterson MR, Wornell PA, Blanchard MG, Gonzalez FJ. Role of CYP1A2 and CYP2E1 in the pentoxifylline ciprofloxacin drug interaction. Biochem. Pharmacol. 2004;68:395–402. doi: 10.1016/j.bcp.2004.03.035. doi:10.1016/j.bcp.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Pribluda VS, Gubish ER, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- Quezada M, Alvarez M, Peña OA, Henríquez S, d' Alençon CA, Lange S, Oliva B, Owen GI, Allende ML. Antiangiogenic, antimigratory and antiinflammatory effects of 2-methoxyestradiol in zebrafish larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013;157:141–149. doi: 10.1016/j.cbpc.2012.10.008. doi:10.1016/j.cbpc.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Zamaratskaia G, Ekstrand B. Gender-related differences in cytochrome P450 in porcine liver--implication for activity, expression and inhibition by testicular steroids. Reprod. Domest. Anim. 2011;46:616–623. doi: 10.1111/j.1439-0531.2010.1714.x. doi:10.1111/j.1439-0531.2010.1714.x. [DOI] [PubMed] [Google Scholar]
- Shertzer HG, Clay CD, Genter MB, Schneider SN, Nebert DW, Dalton TP. Cyp1a2 protects against reactive oxygen production in mouse liver microsomes. Free Radic Biol Med. 2004;36:605–617. doi: 10.1016/j.freeradbiomed.2003.11.013. doi:10.1016/j.freeradbiomed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol. Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. doi:10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am. J. Physiol. Cell Physiol. 2005;288:C881–90. doi: 10.1152/ajpcell.00467.2004. doi:10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- Spink DC, Katz BH, Hussain MM, Pentecost BT, Cao Z, Spink BC. Estrogen regulates Ah responsiveness in MCF-7 breast cancer cells. Carcinogenesis. 2003;24:1941–1950. doi: 10.1093/carcin/bgg162. doi:10.1093/carcin/bgg162. [DOI] [PubMed] [Google Scholar]
- Stubelius A, Erlandsson MC, Islander U, Carlsten H. Immunomodulation by the estrogen metabolite 2-methoxyestradiol. Clinical Immunology. 2014;153:40–48. doi: 10.1016/j.clim.2014.03.011. doi:10.1016/j.clim.2014.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.