Abstract
The development of an in vivo rodent discogenic pain model can provide insight into mechanisms for painful disc degeneration. Painful disc degeneration in rodents can be inferred by examining responses to external stimuli, observing pain-related behaviors, and measuring functional performance. This study compared the sensitivity of multiple pain and functional assessment methods to disc disruption for identifying the parameters sensitive to painful disc degeneration in rats. Disc degeneration was induced in rats by annular injury with saline injection. The severity of disc degeneration, pain sensitivity, and functional performance were compared to sham and näve control rats. Saline injection induced disc degeneration with decreased disc height and MRI signal intensity as well as more fibrous nucleus pulposus, disorganized annular lamellae and decreased proteoglycan. Rats also demonstrated increased painful behaviors including decreased hindpaw mechanical and thermal sensitivities, increased grooming, and altered gait patterns with hindpaw mechanical hyperalgesia and duration of grooming tests being most sensitive. This is the first study to compare sensitivities of different pain assessment methods in an in vivo rat model of disc degeneration. Hindpaw mechanical sensitivity and duration of grooming were the most sensitive parameters to surgically induced degenerative changes and overall results were suggestive of disc degeneration associated pain.
Keywords: discogenic pain, disc degeneration, in vivo rat model, pain sensitivity, functional performance
Intervertebral discs (IVDs) are fibrocartilaginous structures that function to transmit loads and allow mobility between adjacent vertebrae. IVD degeneration is highly associated with low back pain, which affects 70–85% of the population during their lifetime,1 and is the leading cause of disability worldwide.2 Understanding the mechanism underlying discogenic back pain and developing relevant model systems capable of screening treatments for discogenic pain are a high research priority.
In vivo animal models are essential to understand the association between pain and IVD degeneration. Rat models are an important tool for the study of painful spine conditions,3–8 because they exhibit behavioral changes that can associated with painful conditions, they are anatomically similar to the human spine, and they are sufficiently large to enable many spine surgical procedures to be accurately performed. Painful behaviors in rats can be assessed by: (1) examining responses to mechanical or thermal stimuli, (2) observing pain-related behaviors, and (3) measuring functional performance. Hindpaw stimulation has been adopted to assess discogenic pain in rat3,4,9 since the hindpaw is innervated from the lumbar spine (i.e., L3-5).10 Mechanical and thermal stimulation is commonly used and behavioral changes suggestive of pain have been reported with degenerated IVDs.3,9 It is generally accepted that pain affects animal behaviors, and posterior annular puncture with facet joint removal increased grooming and “wet-dog shakes” suggesting that increased stress and spine pain.6 Spine pain can also be inferred by reduced functional performance in gait, rotarod, and inclined plane tests. Rat gait exhibited longer stance phase and shorter strides following lumbar IVD injury.7 Rotarod tests measure balance and coordination of the rats, and performance decreased after annular stab injury.11 Inclined plane tests measure rat performance standing on a steep hill, and were sensitive to spine and spinal cord injury,5,12 yet it remains unclear if this test is sensitive to discogenic pain.
Numerous methods have been utilized to assess painful spine conditions in rats, however, there are few painful IVD degeneration models and there has been no study to compare sensitivities of these pain assessments. The objective of this study was to evaluate behavioral changes in rats that relate to painful IVD degeneration using a variety of assessment methods. Painful IVD degeneration was induced via anterior annular puncture and injection. The findings provide sensitivities of multiple behavioral and functional assessment methods to IVD disruption and to identify if IVD puncture is associated with painful conditions in rats.
MATERIALS AND METHODS
Eighteen skeletally mature male Sprague-Dawley rats were used, and all experimental procedures were approved by the Institutional Animal Care and Use Committee. Rats were equally divided into three groups: PBS, sham, and näve. Lumbar IVDs of sham and PBS rats were exposed and phosphate buffered saline (PBS) was injected into the PBS group. No surgical procedure was performed on the rats in the näve group. Severity of IVD degeneration was determined using weekly radiographic IVD height, postmortem magnetic resonance imaging (MRI) and histology. Pain sensitivity was assessed using hindpaw mechanical and thermal hyperalgesia tests. Performance and behavioral changes were assessed using spontaneous behavior, rotarod, inclined plane, and gait tests. Hindpaw mechanical hyper-algesia, rotarod and spontaneous behavior tests were performed throughout the 6-week experiment. Hindpaw thermal hyperalgesia, inclined plane, and gait tests were added on day 42, when effects were expected to be the largest, in order to augment the series of measurements, compare across measurements, and better understand how this intervention affected the animal.
Surgery was performed under sterile conditions using general anesthesia (2% Isoflurane in oxygen). A midline abdominal incision opened the peritoneal cavity. L3-4, L4-5, and L5-6 IVDs were identified using the pelvic rim as an anatomic landmark and were punctured at their midline (Fig. 1A) using a 26-gauge needle at a depth of 3 μm, guided by a needle stopper. 2.5 μl of PBS was slowly injected into experimental IVDs. This volume and method was determined from preliminary in vitro and in vivo tests showing no fluid leakage, IVD swelling, or nerve root irritation. Abdominal muscles were closed using 3-0 silk sutures and the skin was closed using 4-0 nylon sutures.4 Rats were closely monitored to ensure no serious impairments. Six weeks after surgery all rats were euthanized using carbon dioxide inhalation.
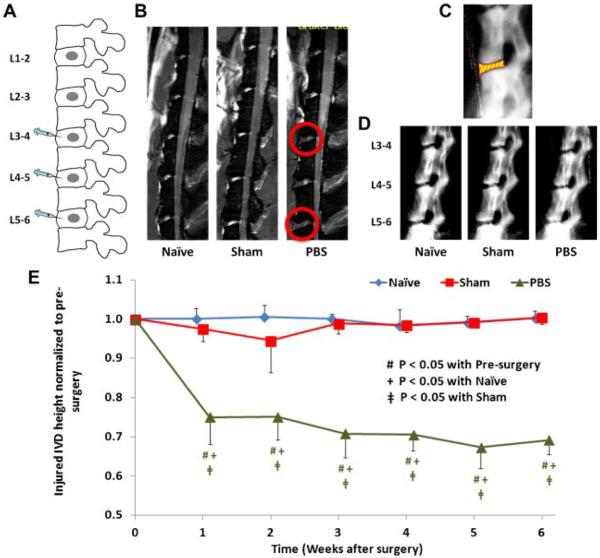
Figure 1.
(A) Schematic diagram showing surgical procedure with annular puncture at ventral portion of L3-4, L4-5, and L5-6 IVDs. (B) Representative T2-weighted MRI images of rat lumbar spines from näve, sham, and PBS groups. Injected IVDs showed decreased signal intensity (indicated with red circles). (C) Measurement of IVD height, which is defined as intervertebral space between vertebral boundaries (i.e., the yellow part with red boundary lines). (D) Representative radiographs of rat lumbar spines from näve, sham, and PBS groups. (E) The changes of injured IVD height (normalized to pre-surgery) of näve, sham, and PBS groups, the standard deviations are marked as error bars, and significant differences are indicated.
IVD heights of injected IVDs and self-control IVDs (L1-2 and L2-3) were measured using lateral radiographs. Rats were anesthetized ~10 min prior to radiography to minimize anesthetic effects due to muscle relaxation and IVD swelling.13 A step wedge was used reference for grayscale intensity and linear dimensions. Radiographs were digitized using a flatbed scanner with backlighting. Images were magnified and vertebral boundaries were identified to determine the IVD height using a custom MATLAB script (Mathworks, Inc., Natick, MA) (Fig. 1C). IVD heights were measured weekly and compared to those obtained 1 day pre-surgery.
Postmortem MRI scanning was performed using a 7-T imager (BioSpec, Bruker Corporation, Billerica, MA) with a BGA-S gradient coil. Sagittal T2-weighted images were obtained using settings determined from preliminary studies: time to repetition (TR) of 1,500 ms; time to echo (TE) of 19.4 ms; 640 × 640 matrix; field of view of 80 × 80 mm; in-plane resolution of 125 × 125 μm; total imaging time of 12.5 h. In each rat, a total of 32 slices with the thickness of 500 μm for each slice was obtained. The mid-sagittal section, with all lumbar IVDs, was identified and examined for IVD degeneration.9 The gray scale of the NP from the injected discs and self-control discs were measured, and the findings of the injected discs were normalized to the self-control discs in order to minimize the variations between animals.
Lumbar motion segments L4-6 were collected for histological analyses. The specimens were fixed, decalcified, paraffin-embedded, and sectioned sagittally at 5 μm intervals. The sections were stained with Safranin-O/fastgreen/hematoxylin for IVD morphology of nucleus pulposus (NP) and annulus fibrosus (AF), as well as glycosaminoglycan (GAG) content using bright-field microscopy.
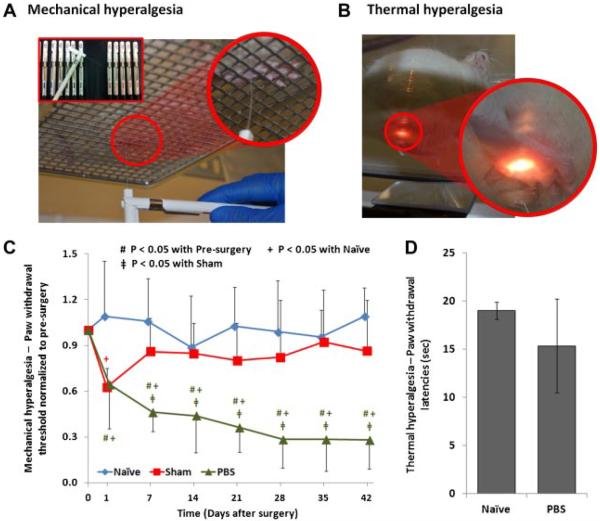
Hindpaw mechanical hyperalgesia is a measure of pain sensitivity using calibrated von Frey filaments with forces ranging from 0.4 to 26.0 g (Stoelting, Wood Dale, IL).3,4 Wire-mesh floor cages were used to allow access to the hindpaw from bottom (Fig. 3A). After 20 min of acclimation, filaments were applied to the hindpaw plantar surface in ascending order starting from 0.4 g until paw withdrawal or the 26.0 g filament had been used. The filament was applied until buckling to ensure sufficient pressure. The 50% withdrawal thresholds of both left and right side, with three trials for each side, were determined using the up-down method.14 This test was performed 1 day pre-surgery and at days 1, 7, 14, 21, 28, 35, and 42 post-surgery.
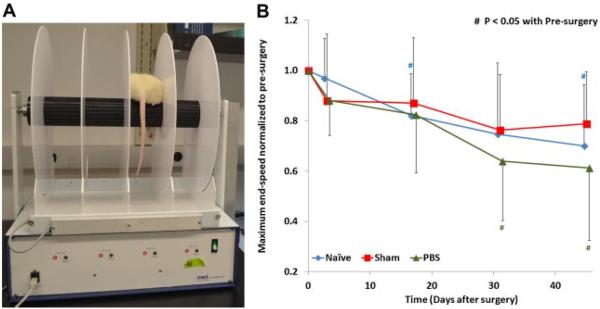
Figure 3.
(A) The experimental setup of the rotarod test. (B) The changes of the maximum end-speed (normalized to pre-surgery) of näve, sham, and PBS groups, the standard deviations are marked as error bars, and significant differences are indicated. All animal groups exhibited reduced performance with time and no difference between groups.
Thermal hyperalesia was performed using a Hargreaves Apparatus (Plantar Analgesia Meter, IITC Life Science Inc., Woodland Hills, CA). After 20 min of acclimation, a thermal stimulus was applied to hindpaw plantar surface using a heat source through a glass floor (Fig. 3B) until the rat lifted its leg away from the heat source.9 A cutoff latency of 30 s was used to prevent tissue damage. Paw withdrawal latencies of both left and right hindpaws were assessed three times separately. This test was performed on day 42 post-surgery.
The rotarod test was performed using a rotarod treadmill (Med Associates, Inc., St. Albans, VT) (Fig. 4A) with trainings prior to surgery. The rotarod started from stationary and accelerated from 4 to 40 rpm over 5 min. The maximum end-speed was recorded when the rat fell off of the treadmill.15 Three trials were performed with a 20-min break between trials. This test was performed 1 day pre-surgery and days 1, 14, 28, and 42 post-surgery.
Figure 4.
The experimental setup for (A) hindpaw mechanical hyperalgesia test and (B) hindpaw thermal hyperalgesia test. (C) The changes of paw withdrawal threshold (normalized to pre-surgery) of näve, sham, and PBS groups, the standard deviations are marked as error bars, and significant differences are indicated. (D) The paw withdrawal latencies of the näve and PBS groups (standard deviations are marked as error bars).
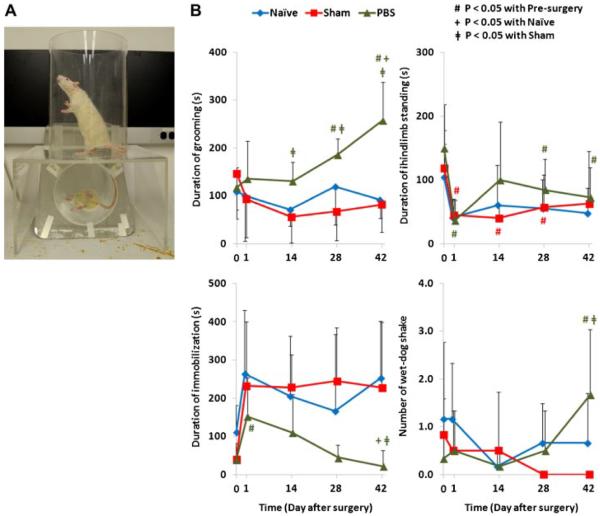
Spontaneous behavior was observed in a transparent acrylic cylinder with a diameter of 25 cm (Fig. 5A). The rats were videotaped from the side by a fixed high-speed digital video camera for 10 min. The floor was transparent with an inclined mirror to allow capture of the behavior when they were not facing the camera. Duration of grooming, immobilization, hindlimb standing, and number of “wet-dog shake” were determined during the 10 min.6 The test was performed 1 day pre-surgery and at days 1, 14, 28, and 42 post-surgery.
Figure 5.
(A) The experimental setup for the assessment of spontaneous behavior. (B) The results of spontaneous behaviors (e.g., duration of grooming, immobilization and hindlimb standing, as well as number of “wet-dog shake”) of näve, sham, and PBS groups, the standard deviations are marked as error bars, and significant differences are indicated.
The inclined plane test evaluates rat motor function by assessing its ability to support body weight on an inclined plane.5 The surface of an inclined board was covered with a grooved rubber mat and pre-set at 30° from horizontal. The rat was positioned on the mat with its head facing upslope to ensure support of the body weight was sensitive to hindlimb strength (S. Fig. 1A). The inclined angle was increased slowly until the rat could not maintain its position, which was defined as maximum inclined angle. Three trials were performed with 15 min between consecutive trials. The test was performed on day 42 after surgery.
Rat gait was studied using an advanced treadmill system with an image acquisition and analysis software (DigiGait Imaging System, Mouse Specifics Inc., Quincy, MA). Rats were trained to walk on a motorized transparent treadmill belt with walking speed at 26 cm/s16 (S. Fig. 2A), and the rat gait was videotaped using a high-speed digital camera. The paws of each limb were recognized for generating “digital paw prints” and dynamic gait signals. Gait signals are comprised of the full stride, which was used to determine the stance and swing phases of the rat gait. The gait analysis was performed on day 42 after surgery.
Results of IVD height, hindpaw withdrawal threshold (from mechanical hyperalgesia test), and maximum end-speed (from rotarod test) at each time point were normalized to those pre-surgery. Normalized results and the results from spontaneous behavior were evaluated using repeated measures analysis of variance (ANOVA) with least significant difference as post-hoc comparison to determine differences between time points. Differences between groups were evaluated using one-way ANOVA with Tukey's post-hoc comparison. All statistical tests were performed using SPSS Statistics v.20 (IBM Corporation, Armonk, NY) using p<0.05.
RESULTS
The surgical procedure was well tolerated by the rats with no intraoperative complications. The differences in body weight between näve and surgery groups (i.e., sham and PBS groups) of 7, 12, 18, and 25 g, for pre-surgery and weeks 2, 4, and 6, respectively, were small with averaged body weight about 500 g. The slightly changes in the weight difference suggested that there were no obvious effects of surgery or intradiscal injections on the rats.
Postmortem MRI showed decreased signal intensity in injected IVDs when compared to lumbar IVDs in the näve and sham groups as well as the self-control IVDs (Fig. 1B). The normalized gray scales of the injected discs were 1.24±0.12, 1.09±0.06, and 0.76±.15 for näve, sham, and PBS groups, respectively, which confirmed that the intensity of the PBS injected discs were lower than those of self-control discs as well as those of näve and sham groups. There was no evidence of IVD herniation in any of the rats.
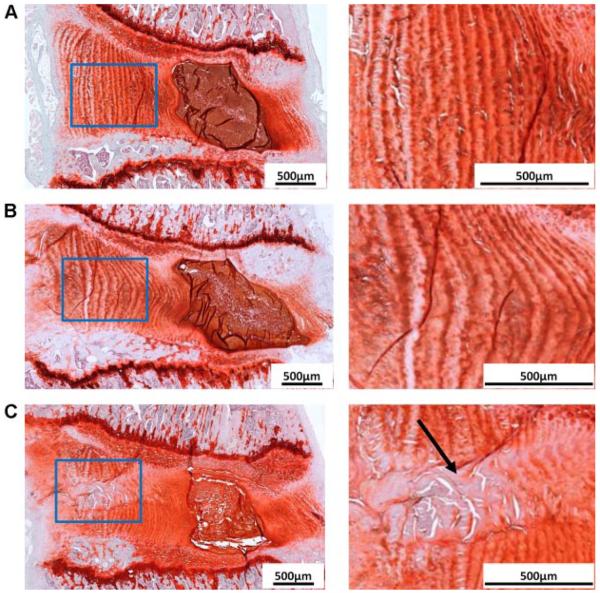
Both näve and sham groups exhibited normal IVD morphology (Fig. 2). They had well-defined GAG-rich NP with notochordal cells, which surrounded by well-organized annular collagen lamellae with fibroblastic annular cells. However, annular puncture with PBS injection affected the morphology of both NP and AF. The needle track induced during annular puncture was found. Disorganized annular lamellae with decreased GAG content and annular cells were observed along the needle track. Compared to the discs in näve and sham groups, PBS injected IVD also showed reduced IVD height, smaller NP, and less distinct NP/AF boundary (Fig. 2).
Figure 2.
Safranin-O/Fast-Green/hematoxylin staining for rat lumbar discs from all experimental groups (A for naive, B for sham, and C for PBS). The AF regions (indicated with blue rectangles) are highlighted and shown at higher magnifications. The black arrow indicates the annular disruption during annular puncture.
Self-control IVD heights did not significantly change with time or across group. Normalized heights of injured IVDs were averaged for analysis since they exhibited patterns that were not significantly different from each other. Normalized IVD heights of näve and sham remained constant for the 6 weeks (p>0.05). However, injected IVD height decreased continuously when compared to pre-surgery (p<0.05), and was significantly smaller than those of näve and sham at all time points, except pre-surgery (Fig. 1D and E).
Withdrawal thresholds from the right and left hindpaws were averaged for analysis because of similar patterns (p>0.05). The näve group showed constant withdrawal thresholds throughout the 6 weeks (p>0.05). The sham surgery induced a temporary decreased threshold, but recovered 1 week post-surgery and did not show significant difference from the näve group (Fig. 2C). The withdrawal threshold of the PBS group progressively decreased after intradiscal injection until 6 week post-surgery (p<0.05), and were also lower than those of näve and sham groups at all time points (p<0.05), except pre-surgery and 1 day post-surgery (Fig. 2C).
No tissue damage was observed after the thermal hyperalgesia test. The withdrawal latencies from the right and left paws were averaged for analysis because of similar patterns (p>0.05). Although näve and PBS groups did not exhibit significant differences, the averaged withdrawal latency of PBS group was smaller than that of näve group (Fig. 2D).
For the rotarod, all groups showed continuous decrease in maximum end-speed (p<0.05 for näve and PBS groups) with no significant difference between groups (Fig. 4B).
All spontaneous behaviors, except the hindlimb standing, did not show significant change for both näve and sham groups from pre-surgery. PBS injection altered spontaneous behaviors with significantly increased grooming and wet-dog shakes, as well as decreased hindlimb standing (Fig. 5B). Only grooming exhibited significant differences between the PBS group compared to the näve and sham groups (näve: p<0.05 at weeks 2 and 6; sham: p<0.05 at weeks 2, 4 and 6).
The inclined plane test did not show significant differences among the three groups, with the inclined angle of 72.89±2.74°, 71.50±4.37°, and 72.11±3.12° for näve, sham, and PBS groups, respectively (S. Fig. 1B).
Gait was successfully videotaped for five rats (three from näve and two from PBS groups). PBS injection altered the walking stability of hindlimbs with decreased swing duration, as well as increased percentage of stride in stance and stance-to-swing ratio (S. Fig. 2B).
DISCUSSION
Low back pain is a multifactorial disorder and disruption of spinal structures (e.g., IVDs, endplates, ligaments, facet joints and muscles) can be a major source of back pain.17,18 IVD degeneration-related pain can arise from multiple sources in human patients including: (1) chronic inflammation with up-regulation of cytokines, neurotrophins and their receptors on nerves leading to development or maintenance of painful degenerated IVDs,19,20 (2) neurovascular ingrowth of newly formed nerve fibers entering the disc through tears in the annulus fibrosus,21,22 (3) mechanical back pain with nerve irritation due to altered intradiscal pressure or irregular spinal motions, and (4) herniated nucleus pulposus with nerve root compression.17,23,24
Development of an animal model with painful IVD degeneration (that does not include herniation and radiculopathy) is a high research priority since it could provide insights into the pathophysiology of discogenic pain. The current model was suggestive of IVD degeneration-related pain since rats exhibited decreased hindpaw withdrawal threshold and increased grooming that was associated with structural changes to the IVD including height loss, annular disruption, and early degeneration. An unhealed IVD puncture can result in a loss of nucleus pressurization and decreased torsional stiffness,25 and this altered motion segment biomechanics could result in mechanical back pain. However, IVD disruption was relatively localized and degeneration mild. IVD injuries can result in persistent inflammation of the IVD26 suggesting that at least part of the pain behaviors were likely to be associated with inflammation of the IVD and spinal structures which may sensitize nerve or dorsal root ganglion. Annulus defects can create a permissive environment for neurovascular ingrowth.27 Neoinnervation was not observed histologically in our studies (despite extensive efforts), yet it cannot be excluded as a possible source of pain since nerve fibers are fine structures that can be difficult to detect histologically. Lastly, nerve root compression by herniated IVD could have occurred in our animals but we believe it remains a an unlikely source of pain since both MRI and histology showed no evidence of IVD herniation, and because there was no evidence for functional disability suggestive of radiculopathy observed on inclined plane and rotarod tests. Taken together, results are suggestive of nociceptive pain via inflammation and biomechanical dysfunction of the spine without obvious nerve injury and neuropathic pain. The key purpose of this study was to identify and validate methods for sensitively detecting pain related behaviors and loss of function due to IVD pathology and future investigations are warranted to better identify precise mechanisms for the observed pain behaviors.
In this study, painful IVD degeneration was initiated via anterior annular puncture with intradiscal injection of PBS using a procedure designed to avoid radiculopathy. Most animal models of painful IVD degeneration investigated herniation and radicular pain. Most studies investigating other sources of disc degeneration-associated pain involve anterior annular puncture (Table 1). Annular puncture alone was demonstrated to induce a temporary pain only.9 Our model added PBS injection to the puncture to induce more observable and consistent painful responses since injection is known to increase structural damage via increased intradiscal pressure and delamination.28 The current annular puncture was not a naturally occurring disc degeneration model, for example sand rat29,30 and SPARC-null mice,31 where the changes were associated with aging. However, it should be noted that the changes observed (especially the painful response) in the naturally occurring model might be systemic and might not be directly traceable to IVD disorders. The degenerative changes and pain behaviors observed in this study were associated with the injury rather than aging or other systemic factors. The injection model also facilitates future investigation the directly evaluate effects of the injected chemicals and therapeutics on IVD degeneration and painful behavior.
Table 1.
Review of In Vivo Animal Model Studies in Disc Degeneration-Associated Pain*
| Animal (Reference) | Intervention | Structural and Biological Changes | Painful Response |
|---|---|---|---|
| Sprague-Dawley rats3 | Annular puncture of L5-6 disc anteriorly (26G needle) followed by intradiscal injection of 10μL Complete Freund's adjuvant (CFA) | Decreased NP notochordal cells, and replaced by chondrocyte-like cells | Decrease in withdrawal threshold in hindpaw mechanical hyperalgesia test |
| AF collagen layers were intermingled with fibrocartolagenous portions of NP | |||
| Calcitonin gene-related peptide (CGRP) immune-positive nerve fibers were observed at the ventral parts of AF lamellae in degenerated IVDs | |||
| Upregulation of CGRP in both dorsal root ganglion (DRG) and dorsal horn | |||
| Upregulation of inducible nitric oxide synthase (iNOS) and prostaglandin E in DRG | |||
| Sprague-Dawley rats4 | Annular puncture of L4-5 and L5-6 IVDs anteriorly (0.5mm diameter microsugical drill with a depth of 2mm) followed by NP removal | Decrease in IVD height | Development of pressure hyperalgesia as measured by vocalization threshold in response to applied force upon stimulation of paravertebral tissue at L4-5 disc region |
| Increase in IVD degeneration according to Masuda grading scale (grade 12) | |||
| Proteoglycan depletion with loss and/or collapsed NP | |||
| Increase in IVD inflammatory response with increased immune reactivity of CD11b antibody | |||
| Upregulation of TNFα, toll-like receptor 4, monocyte chemoattractant protein-1 and α2δ1 expression in both DRG and spinal dorsal horn | |||
| Sprague-Dawley rats9 | Annular puncture of L4-5 IVD via anterior or posterior approach (21G needle with a depth of 3 mm and stayed for 30 s) | Decrease in MRI signal intensity | Decrease in withdrawal threshold in hindpaw mechanical hyperalgesia test (posterior stronger than anterior) |
| Decrease in NP size and extracellular matrix production, and disorganized collagen fiber in AF | Decrease in withdrawal latency in hindpaw thermal hyperalgesia test (posterior stronger than anterior) | ||
| Increase in TNFα, interleikin-1β, interleukin-6, substance P and iNOS in NP | |||
| Increased immunoreactivity of TNFα and interleikin-1β in DRG following annular puncture (posterior stronger than anterior) | |||
| SPARC-null mice31 | Naturally occur | Disc herniation with increased deep nerve ingrowth in older SPARC-null mice | SPARC-null mice were hypersensitive to cold stimuli, and exhibited significant impairment in grip force test and shorter duration of immobility in tail suspension assay |
| Age-dependent upregulation of CGRP and neuropeptide-Y in DRG | |||
| Age-dependent upregulation of CGRP, microglia and astrocytesin spinal cord dorsal horn |
Spine pain models involving herniation and radiculopathy are excluded.
The assessment of painful IVD degeneration in animals is challenging since the pain can only be assessed via their changes in function performance and behaviors. Intradiscal injection induced IVD degenerative changes with progressively decreased IVD heights, reduced MRI T2 signal intensity, disorganized AF with decreased GAG content, more fibrous NP and less distinct NP/AF boundary. The rats with degenerated IVDs exhibited behavioral changes demonstrating animal discomfort without disability, suggestive of a localized pain response. Behaviors indicative of a localized painful response included decreased hindpaw mechanical and thermal sensitivities, increased grooming, and altered walking gait patterns with longer stance phases and shorter swing phases. No evidence for herniation was observed, and no lasting behavioral changes for näve or sham animals, suggesting the changes were representative of discogenic pain from the degenerated IVDs induced by anterior injection.
Rats with degenerated IVDs had significantly reduced mechanical withdrawal thresholds and a trend towards shorter thermal withdrawal latency, similar to prior studies.3,9 Li et al. found that annular puncture with facet joint removal significantly affected both mechanical and thermal hyperalgesia after surgery although only mechanical hyperalgesia was significantly different from the control.9 Together, results suggest the mechanical test might be more sensitive than the thermal test in assessing painful IVD degeneration in rats, which might be associated with differences in sensitivity between mechanical and thermal nociceptors.32 Hindpaw sensitivity is likely to be associated with lumbar dorsal root ganglion involvement that may have been associated with IVD disruption and inflammatory processes in the lumbar region, or loss of IVD height and biomechanical changes at these levels.
PBS injection altered spontaneous behaviors with increased grooming, similar to findings from Olmarker6 who reported significantly increased grooming and “wet-dog shakes” after posterior IVD puncture. Together, these studies suggest that duration of grooming might be associated with discomfort and pain associated with degenerated IVDs. However, both studies showed that rats without surgery were less active with increased immobilization and decreased hindlimb standing. These findings suggest PBS injection heightened discomfort and/or pain that limited the animal's rest time, but the IVD degeneration associated pain was relatively mild and rats continued normal activities. They also suggest that the physical activity level of the individual animal and duration of videotaping are important considerations when analyzing spontaneous behaviors.
Changes in gait were similar to the study by Miyagi et al.,7 who found longer stance phase in rats with injured IVDs. There is limited literature to evaluate the association between gait and discogenic back pain. In humans, obese females with back pain had longer stance duration and shorter step length when compared to non-symptomatic obese and healthy females.33 The altered gait might be an adaptation to increased trunk stiffness34 and altered coordination of trunk muscles with increased activation amplitudes of anterior muscles and reduced activation amplitudes of posterior muscles.35,36 In our study, no statistical test was performed due to limited sample size with low success rate for capturing rat gait. It is likely that gait is sensitive to IVD degeneration associated pain, and increased sample size with longer acclimation to enhance capture success would be necessary to provide more definitive rat gait results.
For the rotarod test, all experimental groups demonstrated a continuous decrease in maximum end-speed suggesting an animal training effect that was not sensitive to the intervention. Kim et al.4 also showed similar findings in their rats after annular puncture with nucleotomy. However, Rousseau et al.11 found significantly decreased rotarod performance following annular stab with 11-blade at a depth of 1.5 mm, suggesting the test is associated with size in injury. In our experience, potential differences in rotarod performance in rats with mild injuries can be dominated by training effects, boredom, and changed body weight.
This is the first study to adopt the inclined plane test in assessing the pain associated with degenerated IVDs. No significant differences between groups were noted and we believe this is because there was no evidence for radiculopathy or other pathologies that would lead to diminished function. However, the outcome and sensitivity of this test might be associated timing of injury or with the friction of the rubber mat. A mat with less friction might increase the challenge for the rats to maintain their positions, which might enhance the sensitivity of this test. A repeated measures study design would also reduce inter-specimen variance. The inclined plane test has been used to evaluate the pain associated with facet joint distraction and nerve root compression in rat,5 providing more evidence of the potential sensitivity of this test to spinal injury.
The rat model is a widely accepted to study painful IVD degeneration since the rat spine is structurally similar to human spine, and the rat lumbar IVD sufficiently large in size to perform spine surgical procedures accurately. Most importantly, compared to other animal models, rats exhibit behavioral changes that can be associated with specific painful conditions. Pain was not associated with the open surgical procedure in this study. Despite early and extensive efforts to use a percutaneous surgical approach with image guidance, we used an open surgical approach to more precisely locate and puncture the disc with the needle (26G needle ~½ of the IVD height) without risking herniation (and risking radiculopathy) or disrupting the posterior elements (and risking acute mechanical instability) due to the small size of the IVD (IVD height<1 mm), facet orientation and surrounding spinal nerves and muscles of the rat lumbar spine. However, sham experiments indicated the rats healed rapidly from the open surgery procedure exhibiting no pain behaviors after 1 week and almost no detectable scarring at the end of the 6-week experiment justifying the open surgical procedure.
Pain in this model was related to the IVD injection, yet biological differences between rats and humans must be considered in interpreting the mechanisms of this painful IVD degeneration model which involves injury, repair and degeneration processes. Rat IVDs exhibit different NP cell phenotypes (i.e., notochordal cells) and their smaller size enables enhanced nutrient transport from endplate and annulus routes, suggesting greater healing potential. The IVD injury in our rat model did not heal with stable IVD height loss (>3 weeks) and increased pain sensitivity (>4 weeks) that remained through the 6-week experiment. Furthermore, our annular puncture model did not exhibit Modic changes which are known to correlate with painful conditions in humans. With the aid of these sensitive pain assessment techniques, future studies can investigate injection of other agents besides PBS (e.g., pro-inflammatory cytokines), in order to study their effects on IVD degeneration and painful response in vivo. Future studies can also investigate histology at multiple time points in order to better characterize different stages of injury, attempted repair, and degeneration and mechanisms driving these IVD degeneration-related painful behaviors.
CONCLUSION
The anterior annular puncture with PBS injection induced IVD degeneration, increased pain sensitivity, and resulted in behavioral modifications. IVD degeneration was observed as loss of IVD height, annular disorganization with decreased proteoglycan, more fibrous NP with decreased NP cells and MRI signal intensity without any evidence for herniation. The increased sensitivity of the hindpaw to mechanical and thermal hyperalgesia along with increased grooming suggested that the animals exhibited discomfort and a `painful' response, likely to be associated with lumbar IVD degeneration. Lack of sensitivity for the rotarod test, inclined plane test, and lack of differences in general health or weight gain between groups suggests that the animals are still largely behaving normally. Together, we consider this a repeatable model of IVD degeneration associated with painful response, although future studies are required to assess the precise biological mechanisms for the observed pain and behavioral changes.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Venetia Zachariou for technical support in the hindpaw thermal hyperalgesia test, Dr. Chenjian Li for the technical support in rotarod test and spontaneous behavior observation, Dr. Cheuk Ying Tang, Dr. Junqian Xu and Mr. Lazar Fleysher for the technical support in MRI scanning and image processing, and Dr. Nelly Andarawis-Puri for the technical support in gait analysis.
Grant sponsor: NIAMS/NIH; Grant number: R01AR064157; Grant sponsor: Orthopaedic Research and Education Foundation.
Footnotes
SUPPORTING INFORMATION Additional supporting information may be found in the online version of this article at the publisher's web-site.
REFERENCES
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M, Kim BJ, Lim EJ, et al. Complete Freund's adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth Analg. 2009;109:1287–1296. doi: 10.1213/ane.0b013e3181b31f39. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Kroin JS, Li X, et al. The rat intervertebral disk degeneration pain model: relationships between biological and structural alterations and pain. Arthritis Res Ther. 2011;13:R165. doi: 10.1186/ar3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunk NM, Nicholson KJ, Winkelstein BA. Impaired performance on the angle board test is induced in a model of painful whiplash injury but is only transient in a model of cervical radiculopathy. J Orthop Res. 2011;29:562–566. doi: 10.1002/jor.21272. [DOI] [PubMed] [Google Scholar]
- 6.Olmarker K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine (Phila Pa 1976) 2008;33:850–855. doi: 10.1097/BRS.0b013e31816b46ca. [DOI] [PubMed] [Google Scholar]
- 7.Miyagi M, Ishikawa T, Kamoda H, et al. Assessment of pain behavior in a rat model of intervertebral disc injury using the CatWalk gait analysis system. Spine (Phila Pa 1976) 2013;38:1459–1465. doi: 10.1097/BRS.0b013e318299536a. [DOI] [PubMed] [Google Scholar]
- 8.Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Liu H, Yang H, et al. Both expression of cytokines and posterior annulus fibrosus rupture are essential for pain behavior changes induced by degenerative intervertebral disc: an experimental study in rats. J Orthop Res. 2014;32:262–272. doi: 10.1002/jor.22494. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Nakajima Y, Sakamoto T. Dermatome mapping in the rat hindlimb by electrical stimulation of the spinal nerves. Neurosci Lett. 1994;168:85–88. doi: 10.1016/0304-3940(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau MA, Ulrich JA, Bass EC, et al. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976) 2007;32:17–24. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 12.Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- 13.Lai A, Chow DH, Siu WS, et al. Reliability of radiographic intervertebral disc height measurement for in vivo rat-tail model. Med Eng Phys. 2007;29:814–819. doi: 10.1016/j.medengphy.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Piel MJ, Kroin JS, van Wijnen AJ, et al. Pain assessment in animal models of osteoarthritis. Gene. 2014;537:184–188. doi: 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen RK, Samson KL, Simonsen D, et al. Effect of early and late rehabilitation onset in a chronic rat model of ischemic stroke—assessment of motor cortex signaling and gait functionality over time. IEEE Trans Neural Syst Rehabil Eng. 2013;21:1006–1015. doi: 10.1109/TNSRE.2013.2279375. [DOI] [PubMed] [Google Scholar]
- 17.Adams MA, Bogduk N, Burton K, et al. The biomechanics of back pain. 2nd ed. vii. Elsevier; Edinburgh Churchill Livingston: 2006. p. 316. [316] p. of plates p. [Google Scholar]
- 18.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 19.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 21.Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 22.Freemont AJ, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 23.Adams MA, McNally DS, Dolan P. `Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 24.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 25.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 2010;19:2110–2116. doi: 10.1007/s00586-010-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulrich JA, Liebenberg EC, Thuillier DU, et al. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–2819. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 27.Stefanakis M, Al-Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976) 2012;37:1883–1891. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 28.Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine. 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 29.Gruber HE, Johnson T, Norton HJ, et al. The sand rat model for disc degeneration: radiologic characterization of age-related changes: cross-sectional and prospective analyses. Spine (Phila Pa 1976) 2002;27:230–234. doi: 10.1097/00007632-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Moskowitz RW, Ziv I, Denko CW, et al. Spondylosis in sand rats: a model of intervertebral disc degeneration and hyperostosis. J Orthop Res. 1990;8:401–411. doi: 10.1002/jor.1100080312. [DOI] [PubMed] [Google Scholar]
- 31.Miyagi M, Millecamps M, Danco AT, et al. ISSLS Prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 2014;39:1345–1354. doi: 10.1097/BRS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 32.Janig W, Grossmann L, Gorodetskaya N. Mechanoand thermosensitivity of regenerating cutaneous afferent nerve fibers. Exp Brain Res. 2009;196:101–114. doi: 10.1007/s00221-008-1673-5. [DOI] [PubMed] [Google Scholar]
- 33.Cimolin V, Vismara L, Galli M, et al. Effects of obesity and chronic low back pain on gait. J Neuroeng Rehabil. 2011;8:55. doi: 10.1186/1743-0003-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Hoorn W, Bruijn SM, Meijer OG, et al. Mechanical coupling between transverse plane pelvis and thorax rotations during gait is higher in people with low back pain. J Biomech. 2012;45:342–347. doi: 10.1016/j.jbiomech.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Lamoth CJ, Meijer OG, Daffertshofer A, et al. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J. 2006;15:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanada EY, Johnson M, Hubley-Kozey C. A comparison of trunk muscle activation amplitudes during gait in older adults with and without chronic low back pain. PM R. 2011;3:920–928. doi: 10.1016/j.pmrj.2011.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.