Abstract
The μ1 opioid receptor (OPRM1) genetic variant A118G results in decreased μ-receptor binding potential in the brain and increases morphine requirement. We hypothesized that OPRM1 A118G polymorphism will affect morphine-induced respiratory depression (MIRD) risk in children receiving morphine. A prospective genotype-blinded study was conducted in 88 healthy adolescents (11–18 years; 67% female, 85% Caucasian) who underwent spine fusion for scoliosis. They were followed for 48 h postoperatively for MIRD, pain scores, morphine consumption and use of analgesic adjuvants. Patients were genotyped for OPRM1 A118G variant—76% were wild type (AA) and 24% heterozygous/homozygous for variant (AG/GG). Multivariable logistic regression showed that the risk of MIRD in patients with AA genotype was significantly higher (odds ratio 5.6, 95% CI: 1.4–37.2, P = 0.030). Presence of G allele was associated with higher pain scores (effect size 0.73, P = 0.045). This novel association is an important step toward predicting MIRD susceptibility and personalizing morphine use.
INTRODUCTION
Morphine is a commonly used opioid for the treatment of moderate–severe perioperative pain in children and adults. Owing to unpredictable large inter-patient variability in responses and narrow therapeutic indices of the drug, postoperative pain relief remains suboptimal and central side effects like respiratory depression occur often (up to 41% incidence) in postsurgical patients.1–3 In fact, 50% of postoperative respiratory failure events involve patients receiving opioids.4,5 Respiratory depression could potentially lead to hypoxic brain injury and fatality.6 In addition, medical fear of opioid-related respiratory depression leads to under-treated pain and humanitarian concerns of unnecessary suffering,7 and subsequent postoperative pulmonary complications.8 These concerns are especially true in children, who differ from adults with respect to developmental physiology and pharmacology, pain assessment and likely increased sensitivity to opioid’s adverse effects.9,10
Variability in morphine response is multifactorial.11 Genetics may account for up to 30% of variability in respiratory depression and 60% of variability in analgesia from opioid use.12,13 Hence, investigating the role of genetics in opioid responses is crucial. The μ opioid receptor is the main target of both endogenous and clinically used opioids, such as morphine, and an important mediator of opioid-induced respiratory depression. Specifically, in knockout mice lacking μ opioid receptors, administration of morphine and other opioids failed to induce respiratory depression in contrast to mice with active μ opioid receptors.14,15 The μ1 opioid receptor gene (OPRM1) that codes for this receptor has a functionally significant and common variant called A118G (rs1799971). This single nucleotide polymorphism (SNP) causes substitution of an adenine (A) with a guanine (G) at base 118, which in turn causes the amino acid exchange at position 40 of the μ opioid receptor protein from asparagine to aspartic acid (N40D), leading to the loss of a N-glycosylation site in the extracellular region of the receptor.16 Thus, based on this variant, it is possible to have three genotypes at this locus, namely, AA (homozygous wild type), AG (heterozygous) and GG (homozygous minor allele). Several studies show that having at least one copy of the G allele (AG or GG) is associated with lower pain threshold and higher opioid consumption in postoperative patients,17–20 although a meta-analysis of its effects have been inconclusive.21 A few studies have shown an association between the variant and opioid-induced side effects, like, protective effects of the G allele for nausea/vomiting,19 but to our knowledge, no clinical studies have evaluated its effect on the most serious adverse effect, respiratory depression.
We conducted a prospective study in adolescents undergoing spine surgery for idiopathic scoliosis, with the aim of determining if OPRM1 variant A118G affects susceptibility to morphine-induced respiratory depression (MIRD). We hypothesized that the presence of OPRM1 G allele decreases sensitivity to morphine response; the corollary being that, a patient with no G allele (AA genotype) will have increased sensitivity to morphine and have higher incidence of MIRD and lower pain scores with comparable or lower morphine doses. The ability to predict a priori susceptibility to MIRD based on OPRM1 genetics will be a founding step toward formulation of preventive strategies and personalization of analgesia.
METHODS
Study design and setting
This is a prospective, genotype-blinded, clinical observational study conducted in a cohort of otherwise healthy children/adolescents undergoing spine fusion surgery with standard perioperative anesthetic, surgical and nursing care between 2008 and 2013. The study was approved by the institutional review board. Written informed consent was obtained from parents and assent was obtained from children before enrollment.
Participants
Subjects 10–18 years with a diagnosis of idiopathic scoliosis and/or kyphosis undergoing elective spine fusion surgery were recruited. Healthy children with an American Society of Anesthesiologists (ASA) physical status less than or equal to two were included. Exclusion criteria included known hypersensitivity to morphine, pregnant or breastfeeding females, use of opioids in the past 6 months or history of chronic pain, developmental delay and liver or renal diseases.
Standard anesthesia protocol
All participants received uniform perioperative care, including a standardized surgical technique and general endotracheal anesthesia with intraoperative use of titrated propofol/remifentanil infusions and midazolam for anesthesia. All patients received fentanyl 0.5–1 mcg/kg at the beginning of surgery and incremental doses of morphine 0. 05 mg/kg at the end of surgery titrated to respiratory rate (RR) of 16–18 per min after discontinuation of remifentanil infusion. All patients received two intravenous lines and arterial line, and were monitored with standard ASA monitors. All patients received prophylactic ondansetron intraoperatively. Depth of anesthesia was monitored using Bispectral Index (BIS) XP (Aspect Medical Systems, Norwood, MA, USA).22 Intraoperative doses of propofol, remifentanil and morphine were collected.
Standard postoperative care and data collection
All patients were admitted to the postoperative care unit immediately after surgery after their tracheas were extubated in the operating room. Morphine was administered postoperatively in the PACU via a ‘smart’ pump with wireless capability, thus enabling real-time patient controlled analgesia (PCA) infusion rates and boluses administered to be downloaded. Initial PCA setting was standardized to morphine doses of 20-μg/kg boluses with lockout interval of 8–10 min with or without basal infusion of 10 μg/kg/h, as per physician preference. Patients were advised on the appropriate use of the PCA to maintain satisfactory analgesia. They were followed for 48 h postoperatively. The nurses adjusted PCA settings on the floor according to the physician’s orders, based on patient needs. Specific data from the PCA, including the amount of morphine received as loading doses, the initial PCA settings and eventual modifications, and the number of total and successful attempts was recorded. Amount of morphine used in the postoperative period was recorded in mg for first hour after surgery, second hour and then every eight hours for 48 h. From the electronic medical record, doses and administration times of other analgesics like acetaminophen, ketorolac and diazepam were recorded.
As per hospital protocol, all patients receiving PCA were monitored using continuous electrocardiography/RR module (for heart rate and RR by transthoracic impedence pneumography) and pulse oximetry (for oxygen saturation), with set alarm threshold of 8 per min for RR. Moreover, nurses also evaluated and recorded the patient’s vital signs (pulse, RR and depth, blood pressure and oxygen saturation) and Ramsay sedation score every 4 h on the floor. The continuous monitor data were captured in real-time on the Epic-based electronic medical record (EMR) (Epic Systems Corporation, Verona, WI, USA) in 1-min intervals and available for review. Whenever there was an alarm due to low RR, the nurse was alerted and corrective measures (such as repeated stimulation, PCA dose reduction, oxygen administration or increase, bag-mask ventilation, naloxone or escalation of care to ICU) were documented in Epic (Epic Systems Corporation). Epic records (Epic Systems Corporation) were reviewed to determine whether the low RR was for >3 min. Subjective pain assessments were recorded by nurses per PCA protocol every 4–6 h using a 0–10 numerical rating scale (NRS).23
Clinical outcome measures
The primary outcome was clinical MIRD occurring anytime from 2 to 48 h after the surgery. MIRD was defined as RR <8/min for >3 min. The analgesic effectiveness outcomes included average pain scores and morphine usage (mg/kg) over postoperative days 1 and 2.
Genetic analysis
Blood was drawn for DNA in the operating room upon intravenous line placement for genotyping of OPRM1 polymorphism A118G. DNA was isolated on the same day, frozen at − 20 °C and tested for rs1799971 by TaqMan SNP genotyping assays (Life Technologies, Applied Biosystems, Forest City, CA, USA).
Statistical analysis
Prior to the analyses, quality of the data was examined. Clinical data was reviewed for biologic plausibility and genetic data was assessed for Hardy–Weinberg equilibrium (HWE) by means of goodness of fit χ2 test. Descriptive statistics were generated for demographic and clinical data using mean, standard deviation and range for continuous variables and percentage and frequency for categorical variables.
Given the multifactorial nature of morphine response, we first examined the relationship between morphine consumption, use of analgesic adjuncts and outcomes. To understand how the use of analgesic adjuncts may be associated with pain score, morphine use, and occurrence of MIRD, we compared individuals who did and did not receive acetaminophen, ketorolac and diazepam separately, using t-tests (pain and morphine amount) and χ2 tests (MIRD). We also tested the effect of using basal infusion on PCA, remifentanil doses and duration of surgery on pain scores. We also examined the relationship between morphine amount and average pain score using simple linear regression. As pain was collected longitudinally, we evaluated the pain scores and morphine consumption at each time point by the presence or absence of the G allele (AA versus AG, GG).
To determine whether the OPRM1 variant was associated with MIRD and pain, we performed both unadjusted and adjusted analyses. Unadjusted analyses were used to detect associations between not only the OPRM1 variant and our outcomes, but to also test for associations between our outcomes and demographic variables (age, sex, race). Unadjusted analyses included χ2/Fisher exact test for two categorical variables (e.g. MIRD vs sex or race), t-tests for continuous versus categorical (e.g. pain vs sex, race and MIRD vs age) and linear regression for two continuous variables (e.g. pain vs age). We then performed adjusted analyses. To build the model for the adjusted analyses, we first identified possible covariates and evaluated a saturated model with all those covariates not including the OPRM1 variant. For MIRD, we included age, sex, race (dichotomized into Caucasian Y/N), diazepam (mg/kg), morphine consumption (mg/kg) and use of basal PCA infusion as covariates. For pain, morphine consumption (mg/kg), sex, age, race, use of intravenous acetaminophen, ketorolac and diazepam, use of basal PCA infusion, duration of surgery and intraoperative remifentanil dose were considered as covariates. MIRD was modeled using logistic regression, whereas pain was modeled with linear regression. To select the best fitting model (e.g. final covariates to include) prior to genetic analyses, the P-value in the saturated model and log likelihood, Akaike and Bayesian Information criterion were compared between models including and excluding the covariate. Additionally, residuals were examined. To ensure that the covariates were not also associated with our outcomes, we then performed χ2 tests or t-test as appropriate. To assess the A118G variant association with the outcomes, we used dominant models, in which the genotypes were coded as AA = 0 and AG_GG = 1. P-value, Odds’ Ratios (OR), and 95% confidence intervals were calculated. Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC, USA).
Power analysis
Statistical a priori power analysis was done using Quanto.24 Dominant model for genetic variant with minimum allele frequency 0.125 was assumed for effect size. Our analysis showed that sample size of 88 would allow us to detect OR 4 or greater for MIRD and beta 0.9 or greater for Pain score with power 80% and significance level 0.05.
RESULTS
Patient demographics and perioperative descriptives
Eighty-eight participants met inclusion criteria for the study (Table 1). Overall the majority of participants were white (85%) with a small number of African-American (10%) and other races (5%). Given the small numbers of African-American and other races, these were collapsed into a single group for the analyses. Majority of subjects (76%) belonged to the AA genotype for the A118G SNP. The SNP was in HWE (P = 0.71). Relevant pre-, intra-and postsurgical details for the study cohort are provided in Table 1. The overall incidence of MIRD was 31% (27/88) over 48 h postoperatively. Majority of MIRD events prompted changes in PCA doses and/or their PCA button taken away until they recovered (42%), some of them required oxygen therapy through nasal cannula or face mask (33%) or increase in oxygen administration (10%); few patients required frequent tactile/verbal stimulation (23%). Only one patient needed a brief assistance with bag-mask ventilation and none needed naloxone or escalation of care to the critical care unit. Two events of MIRD occurred after with diazepam administration while on PCA. continuous basal infusion on PCA was initiated in the majority of patients (73/88, 83%). The average pain scores of the population during the 48-h postoperative period was 4.6 (range: 0.6–8.7), indicating moderate pain with a mean morphine consumption of 1.87 mg/kg (range: 0.2–4.3 mg/kg). The broad ranges for both analgesic parameters (0.6–8.7 for pain scores and 0.2–4.3 mg/kg for morphine consumption) reinforce the high interindividual variability in response to morphine and pain sensitivity that is expected. No association was detected between patient demographic characteristics (race, sex and age) and occurrence of MIRD or average pain scores (Table 2).
Table 1.
Demographics and distribution of variables in the study population (N =88)
| Variable | Number (%) | ||
|---|---|---|---|
| Sex (F/M) | 59/29 (67/33) | ||
| Race | |||
| Caucasian | 75 (85) | ||
| African-American | 9 (10) | ||
| Others | 4 (5) | ||
| OPRM1 Genotypes | |||
| AA | 67 (76.1) | ||
| AG | 20 (22.7) | ||
| GG | 1 (1.1) | ||
| Basal infusion on PCA (number of patients, %) | 73 (83) | ||
| Mean | Range | SD | |
|
| |||
| Age (years) | 14.59 | 11–18.9 | 1.89 |
| Levels (vertebrae) | 11.3 | 1–13 | 2.5 |
| Curve (degrees) | 56.4 | 30–85 | 11.7 |
| Pain scores (numerical rating scale)a | |||
| POD1 | 4.78 | 0.40–8.67 | 1.82 |
| POD2 | 4.27 | 0.00–8.25 | 1.46 |
| POD1 & 2 | 4.56 | 0.58–8.67 | 1.49 |
| Morphine consumption (mg/kg)a | |||
| POD1 | 1.13 | 0.18–2.43 | 0.45 |
| POD2 | 0.75 | 0.00–1.94 | 0.35 |
| POD1 and 2 | 1.87 | 0.18–4.31 | 0.72 |
| Propofol doseb mg/kg | 88.9 | 42.9–148.3 | 21.2 |
| Remifentanil doseb μg/kg (entire population) | 130 | 69–295 | 44 |
| AA genotype | 131 | 45 | |
| AG+GG genotypes | 128 | 44 | |
| Surgical duration (minutes) (entire population) | 314 | 165–576 | 72 |
| AA genotype | 313 | 64 | |
| AG+GG genotypes | 329 | 90 | |
Abbreviations: NRS, numerical rating scale; POD, postoperative day.
Values from the postoperative period.
Cumulative intraoperative doses.
Table 2.
Relationship between patient demographic characteristics and morphine-induced respiratory depression and pain outcomes over postoperative days 1 and 2
| Demographic VARIABLES | MIRD (N = 27) | No MIRD (N = 61) | P-value |
|---|---|---|---|
| Demographic characteristics and morphine-induced respiratory depression (MIRD) | |||
| Age (years); Mean ±SD | 14.9 ±2 | 14.5 ± 1.5 | 0.35 |
| Sex | |||
| Female (N) | 17 | 42 | 0.59 |
| Male (N) | 10 | 19 | |
| Race | |||
| Caucasian (N) | 26 | 49 | 0.06 |
| African-American (N) | 0 | 9 | |
| Others (N) | 1 | 3 | |
| Demographic variables | Mean pain score | SD | P-value |
|---|---|---|---|
| Demographic characteristics and postoperative pain scores (numerical rating scale) | |||
| Age (years) | β =0.04a | 0.62 | |
| Sex | |||
| Female (N =59) | 4.6 | 1.6 | 0.63 |
| Male (N =29) | 4.5 | 1.2 | |
| Race | |||
| Caucasian (N =75) | 4.5 | 1.5 | 0.26 |
| African-American (N =9) | 5.3 | 1.3 | |
| Others (N =4) | 4.2 | 0.6 | |
Abbreviations: N, number; s.d., standard deviation.
Per year of age from 10–18 years, pain score increased by 0.04 (clinically and statistically insignificant).
Relationship between morphine consumption, use of analgesic adjuncts and outcomes
Morphine consumption was significantly higher in patients not administered acetaminophen intravenously (P = 0.0008), but pain scores and occurrence of MIRD was similar in the two groups. Use of intravenous ketorolac and diazepam did not independently affect occurrence of MIRD, the pain scores or morphine consumption (Table 3). Diazepam doses were similar in patients who had MIRD (0.18 ± 0.12 mg/kg) and those who did not (0.21 ± 0.15 mg/kg) over the first two postoperative days (P = 0.42). There was no significant association between morphine requirement and occurrence of MIRD (1.74 ± 0.7 mg/kg and 1.92 ± 0.74 mg/kg, for with and without MIRD respectively; P = 0.8). Though there was a trend towards higher pain scores and higher morphine requirement, this was not statistically significant (P = 0.06). Use of continuous PCA infusion, remifentanil dose used intraoperatively and surgical duration did not significantly affect pain scores (P = 0.85, 0.86 and 0.90, respectively).
Table 3.
Association of analgesic adjuncts with respiratory depression, pain outcomes and morphine consumption
| Variable | Acetaminophen n = 44 (50%) | No Acetaminophen n = 44 (50%) | P-value |
|---|---|---|---|
| Pain score, mean ±s.d. | 4.44 ±1.37 | 4.68 ±1.59 | 0.43 |
| Morphine mg/kg, mean ±s.d. | 1.62 ±0.60 | 2.13 ±0.75 | 0.0008 |
| Number of patients with MIRD | 16 | 11 | 0.25 |
| Ketorolac n =33 (38%) | No Ketorolac n =55 (62%) | ||
| Pain score, mean ±s.d. | 4.65 ±1.45 | 4.51 ±1.52 | 0.67 |
| Morphine mg/kg, mean ±s.d. | 1.88 ±0.77 | 1.86 ±0.70 | 0.93 |
| MIRD | 10 | 17 | 0.95 |
| Diazepam n =74 (84%) | No diazepam n =14 (16%) | ||
| Pain score, mean ±s.d. | 4.68 ±1.38 | 3.92 ±1.9 | 0.17 |
| Morphine mg/kg, mean ±s.d. | 1.87 ±0.69 | 1.88 ±0.90 | 0.95 |
| MIRD | 21 | 6 | 0.28 |
Abbreviation: MIRD, morphine-induced respiratory depression. Acetaminophen group includes patients who received intravenous acetaminophen (10 mg/kg to a maximum of 1000 mg per dose every 6 h); Ketorolac group includes patients who received ketorolac 0.5 mg/kg (maximum 30 mg) every 6 h; and diazepam group includes patients who received intravenous diazepam 0.05 mg/kg every 4 h as needed for muscle spasm.
Unadjusted analyses for association between A118G and MIRD and Pain
Of the patients who had MIRD, 93% (25/27) were homozygous for the A allele. On comparison of genotype groups AA vs AG+GG, we found that the genotype was significantly associated with MIRD (P = 0.016) (Figure 1). There was a significant difference in mean pain scores between the groups (P = 0.024) with AG and GG having higher pain scores (5.09 ± 1.06) relative to the AA group (4.39 ± 1.56) (Figure 2).
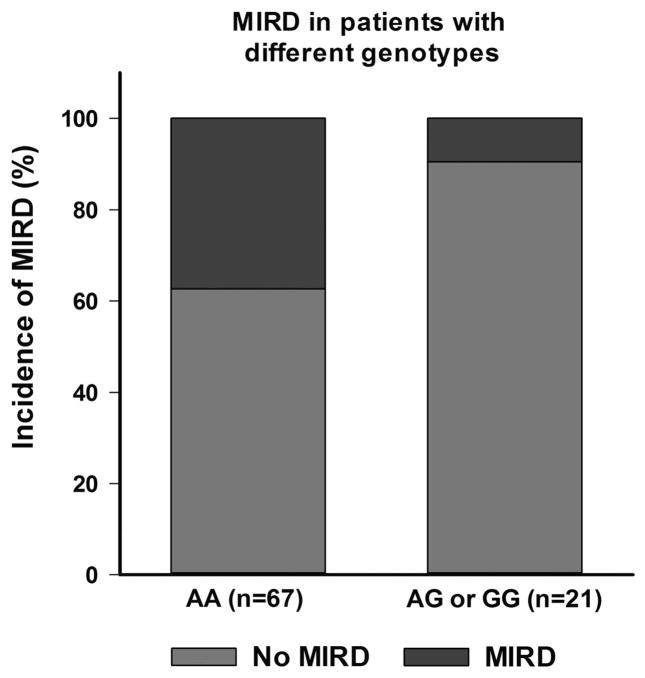
Figure 1.
Incidence of morphine-induced respiratory depression in the two genotype groups (AA and AG+GG) for A118G polymorphism of the OPRM1 gene. The incidence was significantly higher in the AA subgroup (25/67 or 37%) compared with AG+GG subgroup (2/21 or 0.09%) (P =0.016).
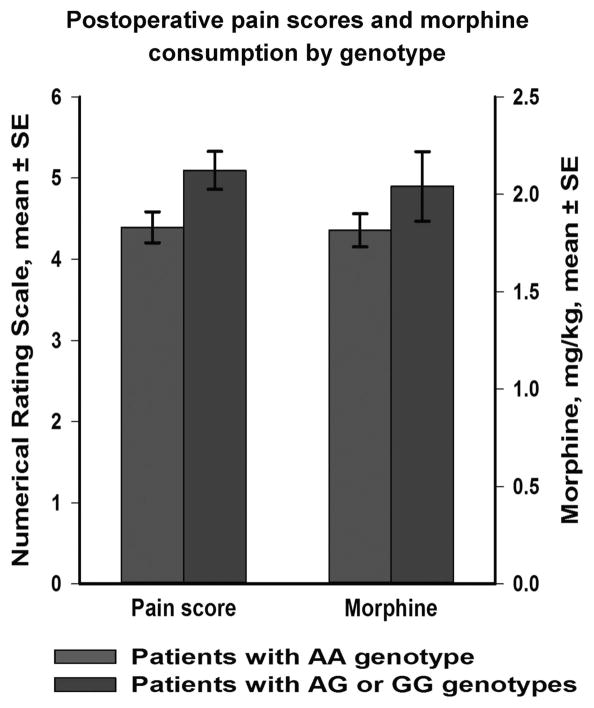
Figure 2.
Comparison of the OPRM1 genotype subgroups (AA versus AG+GG) at the A118G variant with respect to pain scores and morphine requirements in the first two postoperative days after spine surgery. Mean pain scores were significantly higher in the AG +GG subgroup (P =0.024).
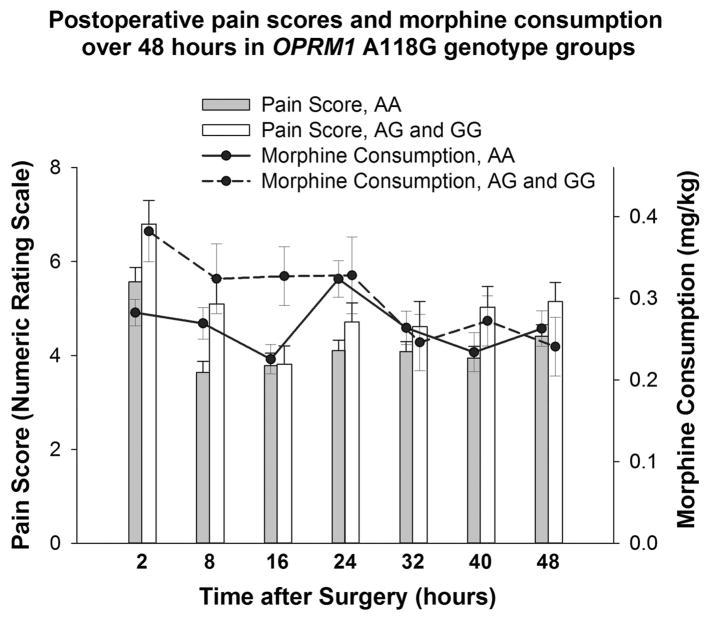
When looking across time, pain was highest 2 hours after surgery, and it was higher in individuals carrying a G allele consistently (Figure 3). Differences in morphine consumption by genotype were greatest between 2 and 16 h after surgery.
Figure 3.
Mean pain scores and morphine consumption over time epochs along with standard error of means (tails) are presented by OPRM1 genotype for the A118G variant. The data at each time point (in hours) describes the cumulative measure collected during that interval after surgery. Pain scores were significantly higher for AG +GG group (compared with AA) at 2 h (P =0.04), 8 h (P =0.02) and 40 h (P =0.04); and morphine consumption was also significantly higher for AG+GG at 2 h (P =0.02) and 16 h (0.02).
Associations between A118G and covariates
There was no association between genotype and race (P = 0.5). Individuals with a G allele used marginally higher morphine doses (2.04 ± 0.82 mg/kg) than the AA group (1.82 ± 0.68 mg/kg), though not statistically significant (P = 0.26). Use of diazepam was similar in both groups (0.18 ± 0.17 mg/kg in AG+GG, compared with 0.16 ± 0.13 mg/kg in AA, P = 0.57). Intraoperative propofol and remifentanil doses were not significantly different among the two genetic groups (P = 0.68 and 0.78, respectively).
Adjusted regression models for association between MIRD and analgesia outcomes and A118G
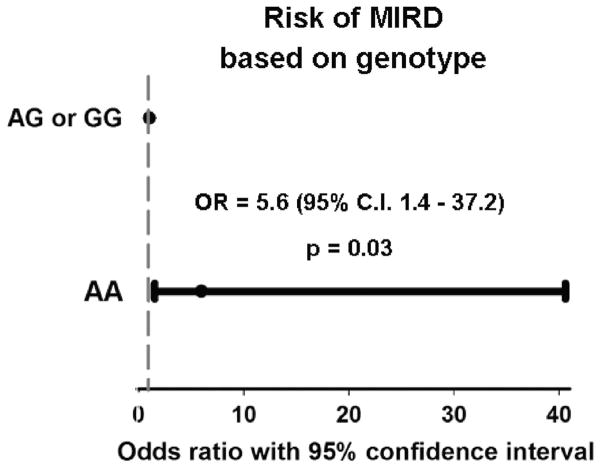
The saturated and final multivariable regression models were presented in Table 4. After adjusting for covariates the odds for patients with AA genotype developing MIRD was 5.6 times more than that of the AG–GG genotype group (P = 0.030). (Figure 4). Caucasians had increased MIRD risk compared with African-Americans, though not statistically significant (P = 0.09). After adjusting the analyses for covariates, the presence of the AG and GG genotype was associated with higher pain (β = 0.73, P = 0.045) or in corollary, presence of AA was associated with less pain scores (β = − 0.37, P = 0.045).
Table 4.
Multivariate regression results for morphine induced respiratory depression (MIRD) and pain outcomes
| Variable | Saturated model | Final model | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Results of regression for MIRD outcome | ||||
| Sex (female vs male) | 1.00 (0.55–1.88) | 0.98 | — | — |
| Race (Caucasian vs other) | 2.77 (1.15–12.08) | 0.061 | 6.24 (1.09–118.17) | 0.091 |
| Age (years) | 1.24 (0.91–1.72) | 0.19 | — | — |
| Morphine consumption/kga | 0.57 (0.26–1.14) | 0.13 | — | — |
| Use of basal infusion on PCA | 1.21 (0.62–2.54) | 0.59 | — | — |
| Diazepam dose/kga | 0.52 (0.01–15.96) | 0.71 | — | — |
| OPRM1 (AA vs AG+GG) | — | — | 5.57 (1.42–37.22) | 0.030 |
| Variable | Saturated Model | Final Model | ||
|---|---|---|---|---|
| Beta ± standard error | P-value | Beta ± standard error | P-value | |
| Results of regression with pain scores as outcome | ||||
| Sex (female vs male) | 0.11 ±0.21 | 0.59 | — | — |
| Race (Caucasian vs other) | − 0.25 ±0.24 | 0.31 | — | — |
| Age (years) | 0.07 ±0.11 | 0.51 | — | — |
| Morphine consumption/kga | 0.40 ±0.25 | 0.11 | — | — |
| Use of basal infusion on PCA | 0.02 ±0.23 | 0.92 | — | — |
| Diazepam (Y/N)a | − 0.35 ±0.23 | 0.13 | − 0.40 ±0.21 | 0.061 |
| Intraoperative Remifentanil/kg | − 0.25 ±0.45 | 0.57 | — | — |
| Surgical duration | − 0.001 ±0.003 | 0.65 | — | — |
| Intravenous Tylenol (Y/N)a | 0.07 ±0.20 | 0.73 | — | — |
| Intravenous Ketorolac (Y/N)a | − 0.08 ±0.18 | 0.65 | — | — |
| OPRM1 (AA vs AG+GG) | — | — | − 0.37 ±0.18 | 0.045 |
Abbreviations: CI, confidence interval; PCA, patient controlled analgesia.
Over postoperative days 1 and 2.
Figure 4.
Risk of morphine-induced respiratory depression in the first two postoperative days for the two genotype groups (AA and AG+GG) of the OPRM1 A118G polymorphism. Odds ratio and 95% confidence intervals are given. This is a dominant genetic model assuming OR of 1 for the AG+GG genotypes.
DISCUSSION
Our prospective genotype blinded clinical study in adolescents undergoing spine surgery shows significant associations between OPRM1 A118G variant on MIRD and analgesia. Our findings confirm our hypothesis that the presence of G allele of this SNP (GG or AG genotypes) decreases sensitivity to morphine resulting in less respiratory depression and less analgesia in the setting of acute postoperative pain. Earlier studies have focused on the effect of the variant on analgesic outcomes in postoperative patients.17–19,25,26 To our knowledge, there have previously been only a handful of small studies in volunteers investigating the variant’s effect on this important central depressant effect of morphine, and none in children or adults undergoing surgery, where opioids are frequently used with concerns about respiratory depression. Hence, the genotype–phenotype association we have found is novel in a clinical setting.
Of the two studies reported in volunteers, our findings mirror those of Oertel et al.27 They conducted an open-label study in 20 human volunteers with known OPRM1 A118G genotypes who received a computerized alfentanil infusion. They found that a hypercapnic challenge produced a similar degree of respiratory depression at 10–12 times higher concentrations of alfentanil in 118G homozygous carriers, compared with heterozygotes or AA homozygotes.27 The other study, a population pharmacokinetic-pharmacodynamic study by Romberg et. al,28 was conducted in 16 volunteers using isocapnic acute hypoxic ventilatory response as the respiratory depression outcome to morphine-6-glucuronide infusion. They did not find any difference in Emax or C50 between the genotypes and hence concluded that the G variant did not protect against opioid-induced respiratory depression despite reduced analgesic response. Appropriately, they acknowledged that their sample size was small. The differences in their findings could also possibly relate to the dissimilar experimental methods of quantifying respiratory depression used in the two studies. Our study has the distinct advantages of having larger sample sizes of healthy children undergoing one type of major invasive surgery with standard perioperative care with significant postoperative pain needing intravenous morphine for at least 2 days. Also of note is that although a meta-analysis of the relevance of OPRM1 gene in pain21 concluded that the available evidence refuted significant effects of OPRM1 on opioid effects, the meta-analysis did not include the volunteer studies mentioned above. The only postoperative studies that were available to the meta-analysis either did not describe respiratory depression as an outcome19,29,30 or had no respiratory depression events in their cohort at all.17,18
Our results confirm previous observations of resistance to morphine analgesia conferred by the presence of the G allele. Similar to studies in postsurgical Asian adult patients,17,19,20 carriers of the G allele in our study had significantly higher pain scores and higher morphine requirements than the AA genotypes. Although the differences in morphine consumption were significant between the genotype groups on postoperative day 1, they were not very different on postoperative day 2. Our observations are similar to those of an adult study in Taiwanese patients receiving morphine following total abdominal hysterectomy, wherein individuals with GG genotype were found to require more morphine 24 h following surgery, but not at 48 h.17 A potential explanation for this phenomenon could be that the higher pain intensities experienced in the immediate postoperative period allow observation of exaggerated differences in morphine consumption in the different genotype groups. Moreover, possible development of acute morphine tolerance in patients with the 118G SNP may be an explanation although there is no direct evidence. Differences in levels of cell surface μ opioid receptor binding capacity as well as agonist-induced accumulation of cyclic adenosine monophosphate for several opioid agonists have been elucidated, based on this OPRM1 SNP.31 Furthermore, agonist-dependent uncoupling, internalization and possible recycling or degradation of the receptor, could be altered by the A118G polymorphism.32,33 Since adenyl cyclase type 5 is an essential mediator of morphineaction34 and acute morphine tolerance is related to opioid receptor activation, endocytosis, resensitization and desensitization,35 we can deduce that this SNP might play a role in the development of acute morphine tolerance. Of course, this does not rule out the effect of other SNPs or splice variants that were not investigated in this study, which could have an effect on immediate morphine effects along with the SNP studied here. Further mechanistic studies are needed to evaluate underlying mechanisms behind our associations and possible explanations for our study findings. Of note, our study focused on average pain across two days; clearly, average pain may not capture the complex and dynamic nature of postsurgical pain, which may help explain why studies have such disparate results. Thus, future studies may need to collect additional data points so that the response of pain to morphine can be modeled. Moreover, although we included morphine consumption in the regression for pain, we would like to point out that morphine requirement and pain scores had high correlation. Increased morphine consumption may be a result of increased pain sensitivity or because of the decreased effect of morphine, which needs to be investigated further.
Mechanisms by which this variant causes alteration of μ opioid receptor protein stability and reduced receptor expression by an epigenetic mechanism have been elucidated.16,36,37 In vitro studies have shown lower cell-surface receptor binding site availability for morphine in cells expressing the G variant compared with the A variant;31,38 less efficient agonist-induced receptor signaling in postmortem human brain tissue in 118G carriers39 and 1.5–2.5-fold increased mRNA expression in 118A human brain cells compared with the G variant.40 Other studies show conflicting results which suggest that the biological consequences at the molecular level are dependent on the cellular environment.26 In the only in vivo study to date investigating the effects of A118G genotype on μ opioid receptor binding potential in human brains using carfentanil positron emission tomography, Ray et al41 found that smokers homozygous for the wild-type OPRM1 A allele (AA genotype) exhibited significantly higher levels of receptor binding potential than smokers carrying the G allele in bilateral amygdala, left thalamus and left anterior cingulate cortex. The above mechanisms could explain the increased sensitivity to morphine effects in AA genotypes and reduced effect of morphine in the G variants (AG and GG genotypes) consistent with the clinical association that we have found in this study. Bond et. al42 showed that the variant receptor had a three-fold higher binding affinity for endogenous β-endorphins which would be expected to affect pain sensitivity but not analgesic response to opioids.
Use of basal PCA infusions have been shown to not be safe in adults, whereas, in children, it has been shown to be safe and effective.43 The use of PCA continuous infusion did not affect risk of MIRD in our study. While the use of sedative adjuncts like diazepam may be a confounding factor for oversedation and respiratory depression,44 use of diazepam did not affect occurrence of MIRD in our cohort, likely because diazepam is not given within two hours of morphine boluses in our practice. Some studies support that intravenous acetaminophen reduces morphine consumption in patients undergoing major surgery,45 while others do not;46 in our cohort, the use of acetaminophen did not improve pain scores or the occurrence of MIRD. Use of remi-fentanil infusion had been implicated in the development of opioid-induced hyperalgesia after spine surgery;47 we did not find an association between remifentanil dose and pain scores. Although duration of surgery could affect development of persistent postoperative pain, we did not find it to be a risk factor for increased pain in the immediate postoperative period.
The importance of our findings stem from the high frequency of 118G in the Caucasian population (11–17%) that implicates immediate clinical relevance for a large part of the population.48 The overall frequency of the 118G allele is about 10.5%, but varies between different ethnic groups (African-American, 0.016; Caucasian, 0.115; Hispanic, 0.142).42 Our cohort was mainly Caucasian (85%) and African-Americans comprised a minority (10%). Since the allelic distribution was not significantly different among patients from different races in our cohort, we did not stratify by race for analyses. We did note however a tendency towards increased risk of MIRD in Caucasians compared with other races, though not statistically significant. These findings are similar to the effect of race on morphine related side-effects that we have reported earlier in younger children undergoing tonsillectomy.11
The clinical implications for the findings that the G variant offers resistance to both analgesic and respiratory depressive effects of morphine are that patients with AA genotype might have superior analgesia with less morphine requirements but are at higher risk of respiratory depression than AG and GG genotypes. On the other hand, patients with AG and GG genotype in the absence of clinical signs of respiratory depression may benefit from higher doses of morphine for effective analgesia or use of more potent opioids for the same. Protection against toxicity had been anecdotally suggested by the report of the 118G patient who tolerated morphine well despite higher plasma morphine 6 glucuronide concentrations,49 and achievement of similar respiratory depressant effects at 10–12 times higher alfentanil concentrations by homozygous G carriers of the OPRM1 variant, compared with A carriers. Oertel et. al27 were unable to conclude the protective effect of G allele in AG heterozygotes for respiratory depression because of only four AG patients in their study; hence our conclusions from mainly AG heterozygotes lends strength to the theory that the presence of even one G allele is protective against MIRD. We would like to point out that the implications of our findings may be restricted to patients in acute postoperative pain receiving intravenous opioids, and may not be applicable to clinical situations like labor and neuraxial opioid analgesia, where the effects are dictated by the presence of high levels of endogenous β-endorphins which have higher affinity for the 118G variant.50–52 Our findings may also have extended applications in patients with cancer pain and drug addiction in whom OPRM1 A118G variant has also been found to predict survival and addiction liability.
A more comprehensive study researching the effects of numerous other variants on the OPRM1 gene and interactions with other genes that play a role in the opioid pharmacokinetic–pharmacodynamic pathways is essential to provide a more complete understanding of the role of genetics in interindividual variability to opioid response. Moreover, the low number of subjects in the GG homozygote group and non-Caucasian races makes it difficult to arrive at conclusions regarding the effect of the OPRM1 variant in these groups. Despite the above limitations, our results provide novel evidence for the role of the OPRM1 gene in individual risk for morphine-induced postoperative respiratory depression in children.
In conclusion, our observations support our hypothesis that the OPRM1 118A>G polymorphism affects the respiratory depressant and analgesic effects of morphine in otherwise healthy post-surgical adolescents. Our results provide a rational basis for future assessments of this SNP in clinical opioid therapy and will need validation in future large clinical studies with adequate sample size of all ethnic populations.
Acknowledgments
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077 through the T1 Junior Faculty Award and Clinical Research Feasibility Funds (PI: Chidambaran). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. It was also supported by the APSF/ASA Safety Scientist Career Development Award by the Anesthesia Patient Safety Foundation (PI: Chidambaran). We acknowledge the contributions of the Clinical Analytic support team at Cincinnati Children’s Hospital for their assistance with medical record data retrieval.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg. 2007;105:412–418. doi: 10.1213/01.ane.0000269489.26048.63. [DOI] [PubMed] [Google Scholar]
- 2.Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait AR, Malviya S. The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth Analg. 2008;107:70–75. doi: 10.1213/ane.0b013e318172fa9e. [DOI] [PubMed] [Google Scholar]
- 3.Sadhasivam S, Boat A, Mahmoud M. Comparison of patient-controlled analgesia with and without dexmedetomidine following spine surgery in children. J Clin Anesth. 2009;21:493–501. doi: 10.1016/j.jclinane.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Fecho K, Jackson F, Smith F, Overdyk FJ. In-hospital resuscitation: opioids and other factors influencing survival. Therapeut Clin Risk Manag. 2009;5:961–968. doi: 10.2147/tcrm.s8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overdyk FJ. Postoperative opioids remain a serious patient safety threat. Anesthesiology. 2010;113:259–260. doi: 10.1097/ALN.0b013e3181e2c1d9. [DOI] [PubMed] [Google Scholar]
- 6.Lotsch J, Dudziak R, Freynhagen R, Marschner J, Geisslinger G. Fatal respiratory depression after multiple intravenous morphine injections. Clin Pharmacokinet. 2006;45:1051–1060. doi: 10.2165/00003088-200645110-00001. [DOI] [PubMed] [Google Scholar]
- 7.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105:205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 8.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. 2007;104:689–702. doi: 10.1213/01.ane.0000255040.71600.41. [DOI] [PubMed] [Google Scholar]
- 9.Duarte LT, do Fernandes MC, Costa VV, Saraiva RA. The incidence of postoperative respiratory depression in patients undergoing intravenous or epidural analgesia with opioids. Br J Anestesiol. 2009;59:409–420. doi: 10.1590/s0034-70942009000400003. [DOI] [PubMed] [Google Scholar]
- 10.Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–217. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 11.Sadhasivam S, Chidambaran V, Ngamprasertwong P, Esslinger HR, Prows C, Zhang X, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129:832–838. doi: 10.1542/peds.2011-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angst MS, Lazzeroni LC, Phillips NG, Drover DR, Tingle M, Ray A, et al. Aversive and reinforcing opioid effects: a pharmacogenomic twin study. Anesthesiology. 2012;117:22–37. doi: 10.1097/ALN.0b013e31825a2a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153:1397–1409. doi: 10.1016/j.pain.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 15.Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, et al. Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology. 2001;94:824–832. doi: 10.1097/00000542-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Huang P, Chen C, Mague SD, Blendy JA, Liu-Chen LY. A common single nucleotide polymorphism A118G of the mu opioid receptor alters its N-glycosylation and protein stability. Biochem J. 2012;441:379–386. doi: 10.1042/BJ20111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, et al. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 19.Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 20.Tan EC, Lim EC, Teo YY, Lim Y, Law HY, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Mol Pain. 2009;5:32. doi: 10.1186/1744-8069-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter C, Lotsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. Pain. 2009;146:270–275. doi: 10.1016/j.pain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. 2006;102:383–388. doi: 10.1213/01.ANE.0000184115.57837.30. [DOI] [PubMed] [Google Scholar]
- 23.Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 24.Gauderman W, Morrison J. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006 [Google Scholar]
- 25.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 26.Mura E, Govoni S, Racchi M, Carossa V, Ranzani GN, Allegri M, et al. Consequences of the 118A>G polymorphism in the OPRM1 gene: translation from bench to bedside? J Pain Res. 2013;6:331–353. doi: 10.2147/JPR.S42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oertel BG, Schmidt R, Schneider A, Geisslinger G, Lotsch J. The mu-opioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet Genom. 2006;16:625–636. doi: 10.1097/01.fpc.0000220566.90466.a2. [DOI] [PubMed] [Google Scholar]
- 28.Romberg RR, Olofsen E, Bijl H, Taschner PE, Teppema LJ, Sarton EY, et al. Polymorphism of mu-opioid receptor gene (OPRM1:c. 118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hayashida M, Nagashima M, Satoh Y, Katoh R, Tagami M, Ide S, et al. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008;9:1605–1616. doi: 10.2217/14622416.9.11.1605. [DOI] [PubMed] [Google Scholar]
- 30.Huehne K, Leis S, Muenster T, Wehrfritz A, Winter S, Maihofner C, et al. High post surgical opioid requirements in Crohn’s disease are not due to a general change in pain sensitivity. Eur J Pain. 2009;13:1036–1042. doi: 10.1016/j.ejpain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 32.Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KS, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, et al. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA. 2006;103:3908–3913. doi: 10.1073/pnas.0508812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Johnson AD, Zhang Y, Papp AC, Pinsonneault JK, Lim JE, Saffen D, et al. Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: detection through allelic expression imbalance in human target tissues. Pharmacogenet Genom. 2008;18:781–791. doi: 10.1097/FPC.0b013e3283050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oertel BG, Doehring A, Roskam B, Kettner M, Hackmann N, Ferreiros N, et al. Genetic-epigenetic interaction modulates mu-opioid receptor regulation. Hum Mol Genet. 2012;21:4751–4760. doi: 10.1093/hmg/dds314. [DOI] [PubMed] [Google Scholar]
- 38.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 39.Oertel BG, Kettner M, Scholich K, Renne C, Roskam B, Geisslinger G, et al. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009;284:6530–6535. doi: 10.1074/jbc.M807030200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 41.Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, et al. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci USA. 2011;108:9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yildiz K, Tercan E, Dogru K, Ozkan U, Boyaci A. Comparison of patient-controlled analgesia with and without a background infusion after appendicectomy in children. Paediatr Anaesth. 2003;13:427–431. doi: 10.1046/j.1460-9592.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 44.Gordon DB, Pellino TA. Incidence and characteristics of naloxone use in postoperative pain management: a critical examination of naloxone use as a potential quality measure. Pain Manag Nurs. 2005;6:30–36. doi: 10.1016/j.pmn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–831. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Hiller A, Helenius I, Nurmi E, Neuvonen PJ, Kaukonen M, Hartikainen T, et al. Acetaminophen improves analgesia but does not reduce opioid requirement after major spine surgery in children and adolescents. Spine. 2012;37:E1225–E1231. doi: 10.1097/BRS.0b013e318263165c. [DOI] [PubMed] [Google Scholar]
- 47.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 48.dbSNP: the NCBI database of genetic variation. 2001 doi: 10.1093/nar/29.1.308. http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1799971database on the Internet. [cited December 5th 2013]. Available from http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1799971. [DOI] [PMC free article] [PubMed]
- 49.Lotsch J, Zimmermann M, Darimont J, Marx C, Dudziak R, Skarke C, et al. Does the A118G polymorphism at the mu-opioid receptor gene protect against morphine-6-glucuronide toxicity? Anesthesiology. 2002;97:814–819. doi: 10.1097/00000542-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Landau R, Kern C, Columb MO, Smiley RM, Blouin JL. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14. doi: 10.1016/j.pain.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Z, Du B, Wang K, Shi X. Effects of OPRM1 A118G polymorphism on epidural analgesia with fentanyl during labor: a meta-analysis. Genetic Testing and Molecular Biomarkers. 2013;17:743–749. doi: 10.1089/gtmb.2013.0282. [DOI] [PubMed] [Google Scholar]
- 52.Sprouse-Blum AS, Smith G, Sugai D, Parsa FD. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69:70–71. [PMC free article] [PubMed] [Google Scholar]