Abstract
Environmental obesogens are a newly recognized category of endocrine disrupting chemicals that have been implicated in contributing to the rising rates of obesity in the United States. While obesity is typically regarded as an increase in visceral fat, adipocyte accumulation in the bone has been linked to increased fracture risk, lower bone density, and osteoporosis. Exposure to environmental toxicants that activate peroxisome proliferator activated receptor γ (PPARγ), a critical regulator of the balance of differentiation between adipogenesis and osteogenesis, may contribute to the increasing prevalence of osteoporosis. However, induction of adipogenesis and suppression of osteogenesis are separable activities of PPARγ, and ligands may selectively alter these activities. It currently is unknown whether suppression of osteogenesis is a common toxic endpoint of environmental PPARγ ligands. Using a primary mouse bone marrow culture model, we tested the hypothesis that environmental toxicants acting as PPARγ agonists divert the differentiation pathway of bone marrow-derived multipotent mesenchymal stromal cells towards adipogenesis and away from osteogenesis. The toxicants tested included the organotins tributyltin and triphenyltin, a ubiquitous phthalate metabolite (mono-(2-ethylhexyl) phthalate, MEHP), and two brominated flame retardants (tetrabromobisphenol-a, TBBPA, and mono-(2-ethylhexyl) tetrabromophthalate, METBP). All of the compounds activated PPARγ1 and 2. All compounds increased adipogenesis (lipid accumulation, Fabp4 expression) and suppressed osteogenesis (alkaline phosphatase activity, Osx expression) in mouse primary bone marrow cultures, but with different potencies and efficacies. Despite structural dissimilarities, there was a strong negative correlation between efficacies to induce adipogenesis and suppress osteogenesis, with the organotins being distinct in their exceptional ability to suppress osteogenesis. As human exposure to a mixture of toxicants is likely, albeit at low doses, the fact that multiple toxicants are capable of suppressing bone formation supports the hypothesis that environmental PPARγ ligands represent an emerging threat to human bone health.
Keywords: osteoblast, PPARγ, organotin, tetrabromobisphenol-a, mono-(2-ethylhexyl) phthalate, mono-(2-ethylhexyl) tetrabromophthalate
1. Introduction
The bone marrow is a specialized microenvironment containing both bone and adipose cells. Because the bone tissue is undergoing near constant homeostatic remodeling, any perturbation in the balance of adipogenesis and osteogenesis could lead to alterations in bone maintenance. Bone ageing is associated with increased adiposity, reduced osteoblast function, and increased osteoclast activity, giving rise to osteoporotic pathologies such as reduced bone mass, altered bone structure, and increased risk of fracture (Rosen and Bouxsein, 2006). Bone loss that occurs with aging also is associated with an increase in peroxisome proliferator active receptor γ (PPARγ)1 expression in the marrow (Moerman et al., 2004).
The plasticity of bone marrow multipotent mesenchymal stromal cells (BM-MSCs) allows them to differentiate into either adipocytes or osteocytes. Adipocyte formation is dependent on PPARγ activation (Tontonoz et al., 1994), whereas bone formation is chiefly controlled by runt related transcription factor 2 (Runx2) (Ducy et al., 1997). MSC differentiation is controlled by the balance of PPARγ and Runx2 transcriptional activation and co-regulated by signaling through the Wnt/β-catenin pathway, which modulates the expression and function of both PPARγ and Runx2 (Bennett et al., 2005; Jeon et al., 2003; Kang et al., 2007; Moldes et al., 2003).
Activation of PPARγ by the therapeutic ligand rosiglitazone drives differentiation toward adipogenesis and suppresses osteogenesis, a result observed both in vitro and in vivo (Lecka-Czernik et al., 1999; Rzonca et al., 2004). Conversely, decreasing expression of PPARγ (e.g. by molecular knockdown) results in reduced adipogenesis and increased bone mass (Akune et al., 2004). Human treatment with therapeutic PPARγ ligands (e.g. thiazolidinediones) is associated with an increased risk of fracture in bone (Aubert et al., 2010; Bilik et al., 2010; Schwartz et al., 2006).
While there is a reciprocal relationship between bone formation and adipogenesis in response to PPARγ activation by rosiglitazone, these functions of PPARγ (along with insulin sensitization) are distinct and separable. For example, Rahman et al. (2012) demonstrated the anti-osteogenic capability of PPARγ to be independent of its pro-adipogenic activity. Phosphorylation of PPARγ can increase insulin sensitivity independently of adipogenesis (Choi et al., 2014). PPARγ ligands can selectively activate the multiples functions of PPARγ, with the distinct abilities to activate adipogenesis and suppress osteogenesis not necessarily being correlated (Lecka-Czernik et al., 2002; Lazarenko et al., 2006; Kolli et al., 2014).
A growing number of environmental contaminants, including organotins and phthalates, are being recognized for their ability to activate PPARγ and therefore are members of the environmental obesogen class of toxicants (Grun and Blumberg, 2006). While organotins primarily have been used as antifouling agents and fungicides, their pervasive distribution is indicated by their presence in house dust (Kannan et al., 2010). Significant human exposure is indicated by the presence of organotins in liver and blood (0.1–450 nM) (Antizar-Ladislao, 2008). Despite being structurally distinct from several other PPARγ ligands, multiple organotins are capable of activating PPARγ and its heterodimerization partner, retinoid X receptor, and act as potent and efficacious adipogenic agents in pre-adipocyte and MSC models (Carfi et al., 2008; Grun et al., 2006; Yanik et al., 2011).
Phthalates are well known environmental PPARγ ligands (Feige et al., 2007; Hurst and Waxman, 2003). Over 18 billion pounds of phthalates are produced yearly, and humans are regularly exposed to significant levels (~10 µg/kg bw/day) of di-(2-ethylhexyl) phthalate (DEHP) (Koch et al., 2003). Substantial exposures occur during acute medical procedures, resulting in blood DEHP concentrations ranging from 50–350 µM (Tickner et al., 2001). MEHP, the active metabolite of DEHP, directly activates PPARγ and promotes adipogenesis in NIH 3T3L1 cells (Bility et al., 2004; Feige et al., 2007; Hurst and Waxman, 2003) and has been measured in human blood samples at µM concentrations (Li et al., 2013).
Newly recognized environmental PPARγ ligands include tetrabromobisphenol-A (TBBPA) (Riu et al., 2011) and mono-(2-ethylhexyl)tetrabromophthalate (METBP) (Springer et al., 2012). TBBPA is a component of the mass produced brominated flame retardants (over 150,000 tons annually (de Wit et al., 2010)), is detectable in home and office dust samples (Ali et al., 2011; D'Hollander et al., 2010), and in human breast milk and serum (at levels as high as 649 ng/g lipid weight) (Cariou et al., 2008). Di-(2-ethylhexyl)tetrabromophthalate, a component of Firemaster® 550, has been found at ppm levels in house dust and at 260 ng/g lipid weight in humans (He et al., 2013), and its metabolite METBP is capable of increasing lipid accumulation as well as activating expression of the PPARγ gene target fatty acid binding protein 4 (Fabp4) in NIH 3T3-L1 cells (Springer et al., 2012).
PPARγ-mediated suppression of bone formation and the substantial human exposure to multiple environmental PPARγ ligands highlight the need to understand their contribution to bone loss. Furthermore, it is unknown if the pro-adipogenic effects of these environmental PPARγ ligands are associated with or are distinct from anti-osteogenic effects, an important question because divergent effects have been shown for some natural and synthetic ligands (Lecka-Czernik et al., 2002; Lazarenko et al., 2006; Kolli et al., 2014). Studies described herein were designed to test the hypothesis that environmental PPARγ ligands, in general, selectively induce adipogenesis at the expense of osteogenesis. Accordingly, we examined the potential for a structurally diverse set of environmental PPARγ ligands (TPhT, MEHP, TBBPA, METBP) to induce adipogenesis and suppress osteogenesis in mouse-derived bone marrow MSCs (BM-MSCs) in comparison to PPARγ ligands known to suppress bone formation (rosiglitazone and tributyltin (TBT)). Surprisingly, despite the structural dissimilarities of the ligands, the efficacy of osteogenesis suppression by treatment was strongly negatively correlated with efficacy of induction of adipogenesis. The organotins were distinct in their exceptional ability to suppress osteogenesis. The data are consistent with the conclusion that suppression of osteogenesis is a common toxic effect of environmental toxicants that are capable of activating PPARγ.
2. Materials and Methods
2.1 Materials
Rosiglitazone was from Cayman Chemical (Ann Arbor, MI). DMSO was from American Bioanalytical (Natick, MA). Insulin, Nile Red, p-nitrophenyl phosphate (pNPP) reagent, TBT chloride, TPhT chloride, and TBBPA were from Sigma-Aldrich (St. Louis, MO). MEHP was from TCI America (Portland, OR). METBP was synthesized by AsisChem (Waltham, MA). All other reagents were from Thermo Fisher Scientific (Suwanee, GA).
2.2 Cell culture
Bone marrow was isolated from 9-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Boston University. All animals were treated humanely and with regard for alleviation of suffering. Mice were housed 4 per cage, with a 12 hour light cycle. Water and food (2018 Teklad Global 18% Protein Rodent Diet, Irradiated, Harlan Laboratories, Indianapolis, IN) were provided ad libitum. Animals were euthanized for collection of bone marrow two days after arrival. After euthanasia (cervical dislocation under terminal euthanasia followed by pneumothorax), limbs were aseptically dissected, and soft tissue was removed from the bone. Marrow was flushed from the femur, humerus, and tibia bones, strained through a 70 um cell strainer, and suspended in MSC medium consisting of α-MEM, 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin-B. Cells from 2–4 animals were pooled and plated so that each pool represented an experimental n. Cells were plated either in 6-well (12 million cells in 2 mls per well) or 12-well plates (6 million cells in 1 ml per well). Half of the medium was replaced 5 days after plating. At day 7, the medium was replaced with osteoinductive medium consisting of α-MEM, 12.5 µg/ml l-ascorbate, 8 mM β-glycerol phosphate, 0.5 µg/ml insulin, and 10 nM dexamethasone. Prior to the day 7 medium change, a naïve, undifferentiated well was harvested for gene expression analysis as described below. Cells received no treatment (Naïve) or were dosed with vehicle (DMSO, 0.1% final concentration) or with the chemical of interest. Following treatment, cells were cultured for 7 days (mRNA expression) or 10–11 days (adipocyte and bone phenotype, cell viability). During these periods, medium was changed and the cultures were re-dosed 2 times for mRNA expression or 3 times for phenotype analysis.
2.3 Cell viability assay
Viability was assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) labeling for 3 hrs by standard methods. Absorbance measurements were determined using a Synergy2 plate reader (BioTek, Winooski, VT). Absorbance in experimental wells was normalized by dividing by the absorbance in untreated cultures and reported as “Fold Change from Medium.”
2.4 Lipid accumulation
Cells were washed with one volume of PBS and incubated with Nile Red (1 µg/ml in PBS). Fluorescence (excitation 485 nm, 20 nm bandwidth; emission 530 nm, 25 nm bandwidth) was measured using a Synergy2 plate reader. The fluorescence in all experimental wells was normalized by subtracting the fluorescence measured in Naïve cells (those that received osteogenic medium alone) and reported as “RFUs.”
2.5 Osteogenesis assays
Following Nile Red staining, cells were rinsed with PBS and fixed in paraformaldehyde (2% in PBS). To quantify alkaline phosphatase activity, cells were incubated in pNPP solution. After quenching with NaOH (final concentration: 0.75 M), absorbance (405 nM) was measured using a Synergy2 multifunction plate reader. The absorbance in all experimental wells was normalized by dividing by the absorbance measured in wells that received osteogenic medium but were not treated and reported as “Fold Change from Medium.” Following the pNPP assay, cells were stained with Alizarin Red (Osteogenesis Quantitation Kit, Millipore, Billerica, MA). Cells were extensively washed and then photographed using the UVP Bioimaging System (UVP, Inc., Upland, CA). The resulting images were analyzed for bone nodule count using Image-Pro Plus (MediaCybernetics, Bethesda, MD), and the number of nodules per square cm is reported. Following image capture, Alizarin Red staining was quantified as indicated in the manufacturer’s instructions. Absorbance (405 nM) was measured and normalized as described above.
2.6 Gene expression analysis
Total RNA was extracted and genomic DNA was removed using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was prepared from total RNA using the GoScript™ Reverse Transcription System (Promega), with a 1:1 mixture of random and Oligo (dT)15 primers. All qPCR reactions were performed using the GoTaq® qPCR Master Mix System (Promega). Validated primers were purchased from Qiagen (see Supplemental Table 1). qPCR reactions (in duplicate) were performed using a 7300 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA): Hot-Start activation at 95°C for 2 min, 40 cycles of denaturation (95°C for 15 sec) and annealing/extension (55°C for 60 sec). Relative gene expression was determined using the Pfaffl method (Pfaffl, 2001) to account for differential primer efficiencies. The Cq value for 18s ribosomal RNA (Rn18s) was used for normalization. The Cq value for naïve, undifferentiated cultures was used as the reference point, and the data are reported as “Fold Change from Naive.”
2.7 Protein expression
Cells were collected and lysed in Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA) followed by sonication. The lysates were cleared by centrifugation, and the supernatants were used for protein expression analyses. Protein concentrations were determined by the Bradford method. Total proteins (20 µg) were resolved on 10% polyacrylamide gels, transferred to a 0.2 µm nitrocellulose membrane, and incubated with monoclonal rabbit anti-perilipin (3470, Cell Signaling Technology (Beverly, MA)). Immunoreactive bands were detected using HRP-conjugated goat-anti rabbit secondary antibodies (Biorad, Hercules, CA) followed by enhanced chemiluminescence. To control for equal protein loading, blots were re-probed with a β-actin-specific antibody (A5441, Sigma).
2.8 Reporter assays
Cos-7 cells (in 96 well plates) were transiently transfected with vectors containing mouse Pparg1 (plasmid 8886; Addgene, Cambridge, MA) or mouse Pparg2 (plasmid 8862; Addgene), with human RXRA (plasmid 8882; Addgene) (Tontonoz et al., 1994), PPRE x3-TK-luc (plasmid 1015; Addgene) (Kim et al., 1988), and CMV-eGFP using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transfected cultures were incubated overnight. The medium was replaced, and cultures received no treatment (Naïve) or were treated with Vh (DMSO, 0.1%), rosiglitazone, TBT, TPhT, METBP, MEHP, TBBPA at concentrations ranging from 0.1 nM to 400µM and incubated for 24 hrs. Cells were lysed in Glo Lysis Buffer (Promega, Madison, WI), to which Bright Glo Reagent (Promega) was added. Luminescence and fluorescence were determined using a Synergy2 plate reader. Luminescence was normalized by the GFP fluorescence in the same well. The normalized luminescence for each well was then divided by the normalized luminescence measured in transfected but untreated wells to determine the “Fold Change from Naive.”
2.9 Statistical Analyses
Statistical analyses were performed with Prism 5 (GraphPad Software Inc., La Jolla, CA). Data are presented as means ± standard error (SE). Gene expression data were log transformed prior to analysis. One-way ANOVAs with the Dunnett’s post hoc test and Pearson’s correlations were performed where noted. All analyses were performed at α = 0.05.
3. Results
3.1 Activation of PPARγ1 and PPARγ2
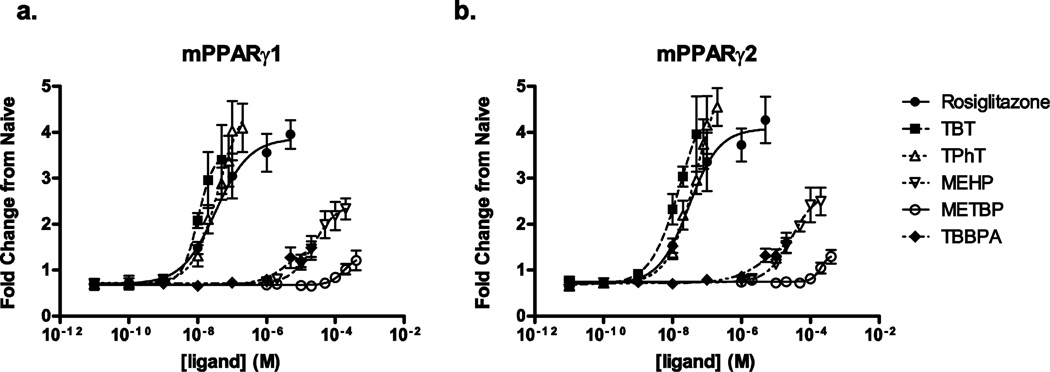
Whereas each of the toxicants being investigated in this study has been identified as a PPARγ ligand, they have not been characterized for their potency and efficacy all in the same study. Thus, we began by characterizing the dose-response of each individual ligand with respect to activation of mouse PPARγ1 and PPARγ2 using a PPRE-luciferase reporter in Cos-7 cells (Fig. 1). Curve maxima, EC50s, and Hill coefficients are reported in Table 1. Rosiglitazone, the positive control, was potent and efficacious at activating PPARγ1 and 2. The organotins, TBT and TPhT, had similar potencies and efficacies as rosiglitazone. MEHP, TBBPA, and METBP each acted as partial agonists, with lower efficacies and potencies compared to rosiglitazone.
Figure 1. Environmental toxicantss activate PPARγ1 and PPARγ2 with differing potencies and efficacies.
Cos-7 cells transfected with mouse PPARγ1 or PPARγ2 and a PPRE-luciferase reporter plasmid were treated with the indicated compounds. Luminescence normalized to GFP fluorescence was divided by the normalized luminescence of untreated cultures to calculate fold change from untreated. n = 4–6 independent transfections.
Table 1.
EC50, maximal activation values, and Hill coefficients for mPPARγ activation by selected toxicants.
| mPPARγ1 | mPPARγ2 | |||||
|---|---|---|---|---|---|---|
| Ligand | EC50 (M) | Max | Hill coeff. | EC50 (M) | Max | Hill coeff. |
| Rosiglitazone | 3.4 × 10−8 | 3.9 | 0.9 | 3.0 × 10−8 | 4.1 | 1.0 |
| TBT | 1.0 × 10−8 | 3.6 | 1.9 | 1.7 × 10−8 | 5.0 | 1.0 |
| TPhT | 4.0 × 10−8 | 4.8 | 1.2 | 5.0 × 10−8 | 5.5 | 1.1 |
| MEHP | 2.4 × 10−5 | 2.4 | 1.3 | 2.9 × 10−5 | 2.7 | 1.2 |
| METBP | 1.8 × 10−4 | 1.3 | 2.1 | 2.0 × 10−4 | 1.4 | 3.3 |
| TBBPA | 3.9 × 10−6 | 1.5 | 1.2 | 7.8 × 10−6 | 2.0 | 0.8 |
Values are fit estimates from 4-parameter Hill plot using Prism (GraphPad software, Inc. La Jolla, CA). Maximal fold change in PPARγ activation (Max) represents the difference between estimated curve maximum and minimum for each individual chemical. n = 4–6 separate transfection experiments.
3.2 Induction of adipogenesis in bone marrow derived MSCs
Our studies and others have demonstrated that both rosiglitazone and TBT are potent inducers of adipogenesis in the mouse-derived bone marrow MSC lines BMS2 and U33/γ2, as well as adipose-derived stromal cells (Kirchner et al., 2010; Lecka-Czernik et al., 2002; Yanik et al., 2011). Furthermore, rosiglitazone has been shown to concurrently suppress osteogenesis as it induces adipogenesis (Lecka-Czernik et al., 1999). Here, we tested the hypothesis that environmental PPARγ agonists would divert differentiation toward adipogenesis in primary bone marrow MSCs grown in osteogenic conditions.
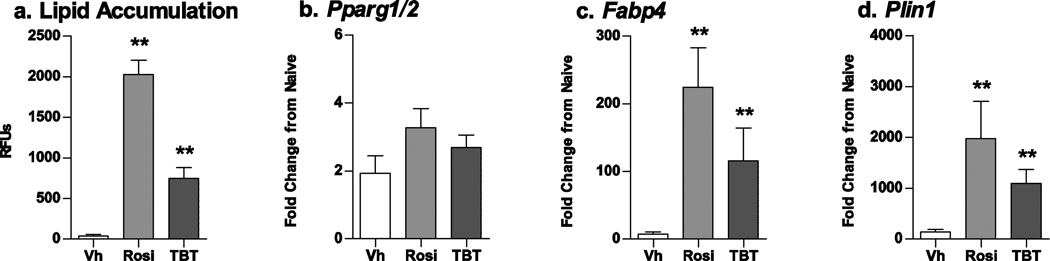
We established the maximal adipogenic response in our system using two known differentiation modulators, rosiglitazone and TBT. Primary bone marrow cultures were prepared from male C57BL/6J mice and treated with Vh (DMSO, 0.1%), TBT (100 nM), or rosiglitazone (100 nM) in the presence of osteoinductive medium. Cultures were assessed for lipid accumulation (10–12 days) and adipogenic gene expression (7 days). As expected, TBT and rosiglitazone induced significant lipid accumulation compared to Vh-treated cells (Fig. 2A), although TBT was less efficacious than rosiglitazone. Whereas TBT and rosiglitazone did not induce expression of Pparg1/2, they strongly induced its target genes Fabp4 and perilipin (Plin1) (Fig. 2B–2D).
Figure 2. Known modulators of adipogenesis increase lipid accumulation and expression of adipogenic genes in mouse BM-MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with vehicle (Vh, DMSO), rosiglitazone (Rosi, 100 nM) or TBT (100 nM) in the presence of osteoinductive media for 7 (gene expression) or 11 days (lipid accumulation). (A) Lipid accumulation was quantified by Nile Red staining. (B–D) mRNA expression was quantified by RT-qPCR. Data are presented as means ± SE (n = 4–8 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s). Vehicle-treated cells showed increases in expression of adipocyte-related genes relative to undifferentiated cells (1.9-fold for PPARγ, 7.7-fold for Fabp4, and 144.4-fold for Plin1).
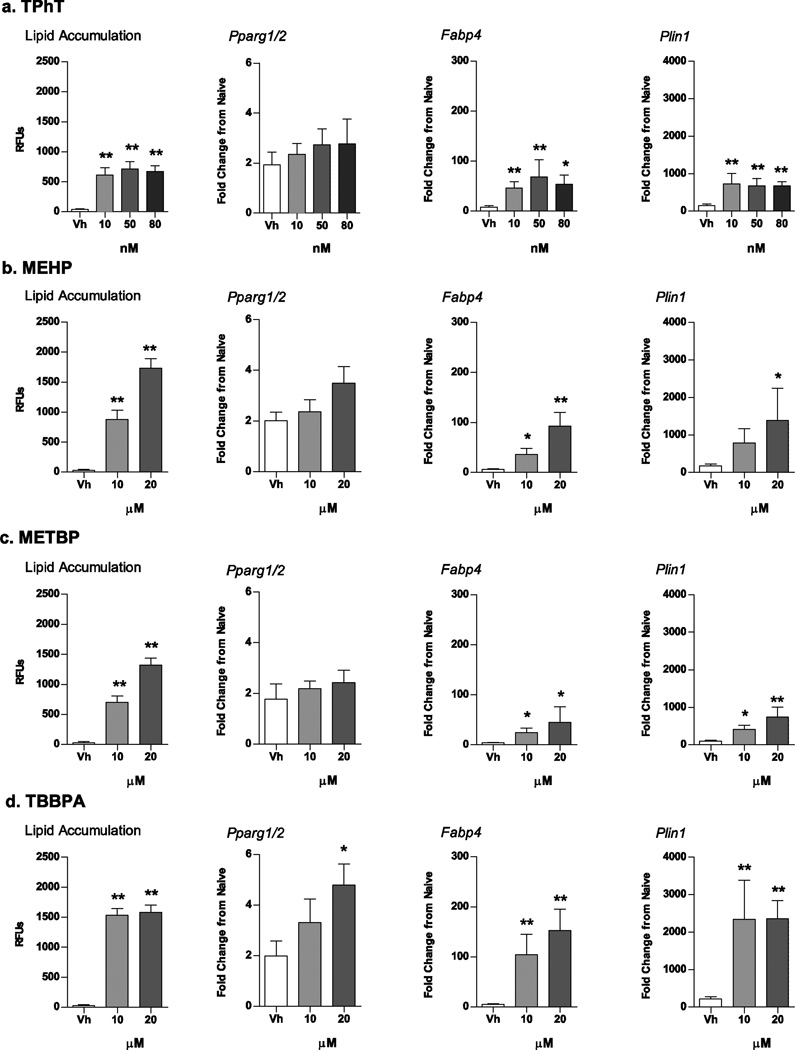
Next, we tested the ability of several structurally distinct environmental PPARγ ligands to induce adipogenesis in BM-MSCs. Established bone marrow cultures were treated with Vh (DMSO, 0.1%), TPhT (10, 50, 80 nM), MEHP, METBP or TBBPA (10, 20 µM), in the presence of osteoinductive medium. The high concentration in each case was the maximal sub-toxic concentration in this model (Supplemental Fig. 1). At all concentrations, TPhT significantly increased lipid accumulation compared to Vh-treated cells, with an efficacy similar to 100 nM TBT (Fig. 3A). While there was only a trend toward an increase in expression of Pparg1/2 mRNA, expression of the PPARγ target genes Fabp4 and Plin1 was increased significantly by all concentrations of TPhT (Fig. 3A). A similar pattern of effect was observed with MEHP and TBBPA, with significant increases occurring in lipid accumulation and PPARγ-target gene expression (Fig. 3B and D). METBP showed more limited increases in lipid accumulation, Fabp4 expression and Plin1 expression (Fig. 3C).
Figure 3. Structurally distinct environmental PPARγ ligands induce lipid accumulation and adipogenic gene expression in mouse BM-MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with Vh (DMSO), TPhT (10–80 nM; A), MEHP (10–20 µM; B), METBP (10–20 µM; C) or TBBPA (10–20 µM; D) in the presence of osteoinductive media for 7 (gene expression) or 11 days (lipid accumulation). Lipid accumulation was quantified by Nile Red staining. mRNA expression was quantified by RT-qPCR. Data are presented as means ± SE (n = 4–8 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s).
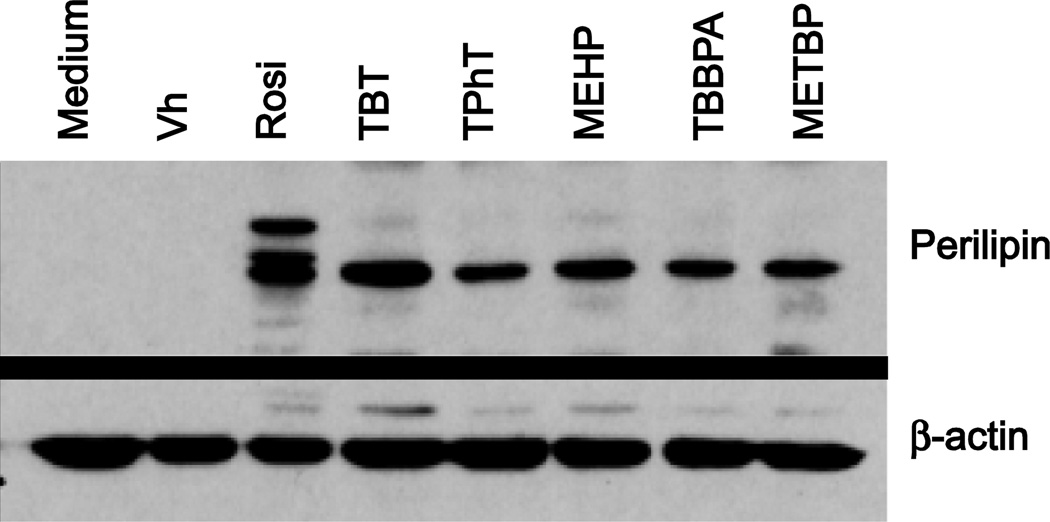
Terminal adipocyte differentiation was confirmed by analyzing perilipin protein expression. Established cultures were treated with Vh (DMSO, 0.1%), rosiglitazone (100 nM), TBT (100 nM), TPhT (50 nM), MEHP, TBBPA or METBP (20 µM each), in the presence of osteoinductive medium for 7 days. All of the chemicals induced the expression of perilipin protein (Fig. 4). Rosiglitazone alone induced formation of multiple bands. Perilipin is a phosphoprotein, thus the multiple bands observed are likely phosphorylated and nonphosphorylated forms of the protein (Greenberg et al., 1991).
Figure 4. Environmental PPARγ ligands induce perilipin protein expression in mouse BM–MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with Vh (DMSO), rosiglitazone (Rosi, 100 nM), TBT (100 nM), TPhT (50 nM), MEHP, TBBPA, or METBP (20 µM) in the presence of osteoinductive media. Cells were harvested after 7 days. Perilipin and β-actin expression were determined in whole cell lysates by immunoblot. Image is representative of 4 separate experiments.
These data illustrate that structurally diverse environmental PPARγ ligands induce adipocyte differentiation and PPARγ signaling in primary bone marrow MSCs, but with different potencies and efficacies.
3.3 Environmental PPARγ ligands show differential efficacies of bone suppression in primary bone marrow MSCs
A number of studies have demonstrated that therapeutic PPARγ agonists suppress osteoblast differentiation and bone formation both in vitro (Jeon et al., 2003; Lecka-Czernik et al., 1999) and in vivo (Lazarenko et al., 2007; Rzonca et al., 2004). Similarly, TBT inhibits differentiation of osteoblast-like (ROB) cells (Tsukamoto et al., 2004) and suppresses osteogenesis in multipotent adipose-derived stromal cells by a PPARγ-mediated mechanism (Kirchner et al., 2010). Here, we tested the hypothesis that environmental PPARγ-agonists would suppress osteogenic differentiation in BM-MSCs.
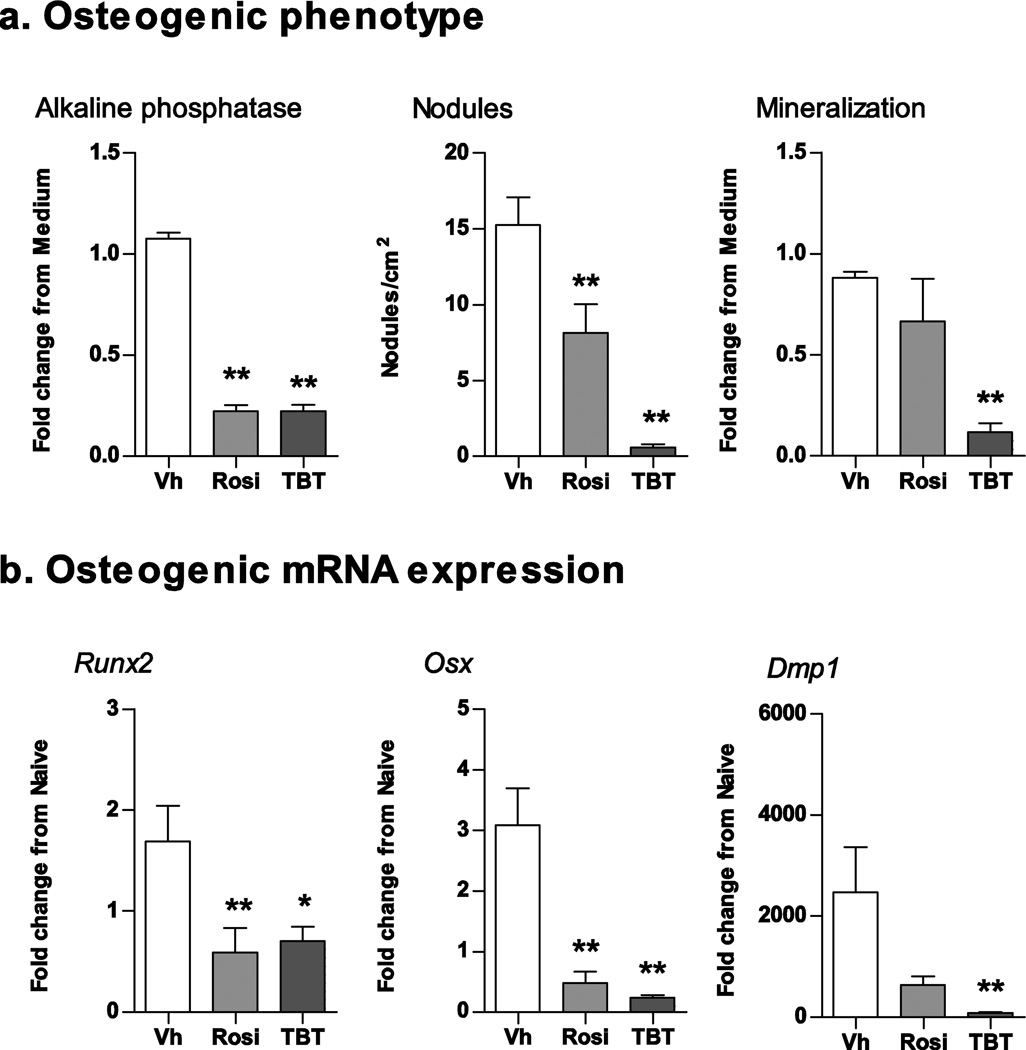
Again, we began by establishing the effect of rosiglitazone and TBT in our model system. Primary bone marrow cultures were prepared from male C57BL/6J mice and treated with Vh (DMSO, 0.1%), TBT (100 nM), or rosiglitazone (100 nM) in the presence of osteoinductive medium. Cultures were assessed for changes in alkaline phosphatase activity, mineralization, and nodule number (10–12 days) and osteogenic gene expression (7 days). Both TBT and rosiglitazone significantly decreased alkaline phosphatase activity and the average number of bone nodules per square cm (Fig. 5A). TBT also significantly suppressed calcium deposition (Fig. 5A). Suppression of osteogenesis also was reflected in decreased expression of osteogenic genes (Fig. 5B). TBT and rosiglitazone significantly suppressed expression of Runx2, an essential transcription factor regulating osteogenesis, as well as expression of osterix (Osx), a direct gene target of Runx2 (Fig. 4B). Only TBT significantly suppressed expression of dentin matrix phosphoprotein 1 (Dmp1), a gene expressed in mineralizing osteocytes (Bonewald, 2011).
Figure 5. Rosiglitazone and TBT suppress osteogenesis in mouse BM-MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with vehicle (Vh, DMSO), rosiglitazone (Rosi, 100 nM) or TBT (100 nM) in the presence of osteoinductive media for 7 (gene expression) or 11 days (osteogenesis assays). (A) Osteogenesis was assessed via alkaline phosphatase activity, alizarin staining and bone nodule counting. (B) mRNA expression was quantified by RT-qPCR. Data are presented as means ± SE (n = 5–8 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s).
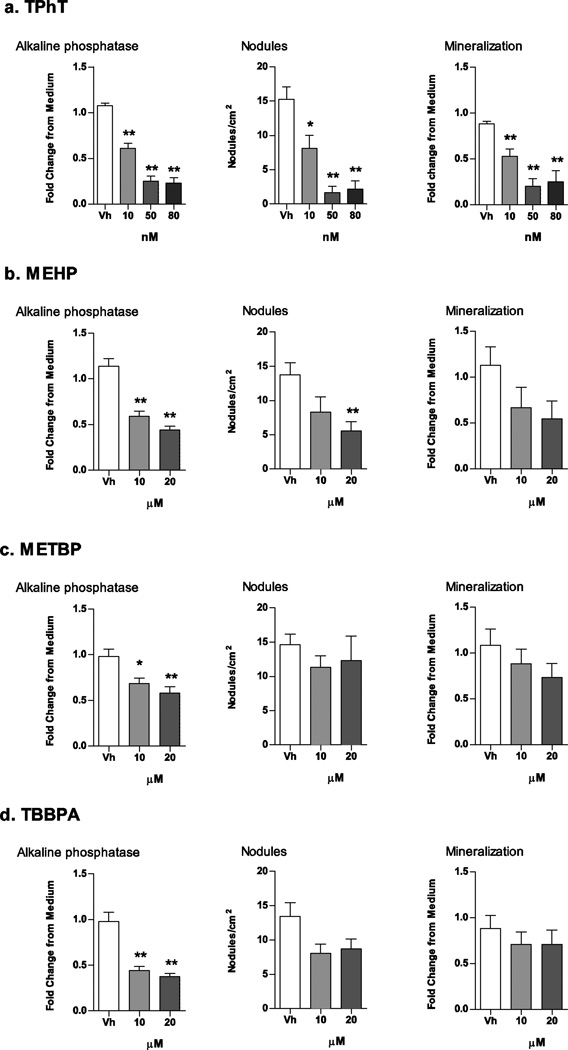
Next, we tested the ability of the environmental PPARγ ligands to suppress osteogenesis. Established bone marrow cultures were treated with Vh (DMSO, 0.1%), TPhT (10, 50, 80 nM), MEHP, TBBPA or METBP (10, 20 µM), in the presence of osteoinductive medium. A concentration of TPhT as low as 10 nM significantly decreased alkaline phosphatase activity, nodule number, and mineralization (Fig. 6A). The other ligands were less efficacious at suppressing osteogenesis. MEHP, METBP and TBBPA significantly suppressed alkaline phosphatase activity, but only MEHP significantly suppressed nodule number (Fig. 6B–D). The treatment regimen and concentrations did not reduce cellularity as measured by MTT labeling (Supplemental Fig. 1), indicating that the effect of the ligands was specific and not the result of overt toxicity.
Figure 6. Structurally distinct environmental PPARγ ligands suppress osteogenesis in mouse BM-MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with Vh (DMSO), TPhT (10–80 nM; A), MEHP (10–20 µM; B), METBP (10–20 µM; C), or TBBPA (10–20 µM; D), in the presence of osteoinductive media for 11 days. Osteogenesis was assessed by alkaline phosphatase activity, alizarin staining and bone nodule counting. Data are presented as means ± SE (n = 5–8 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s).
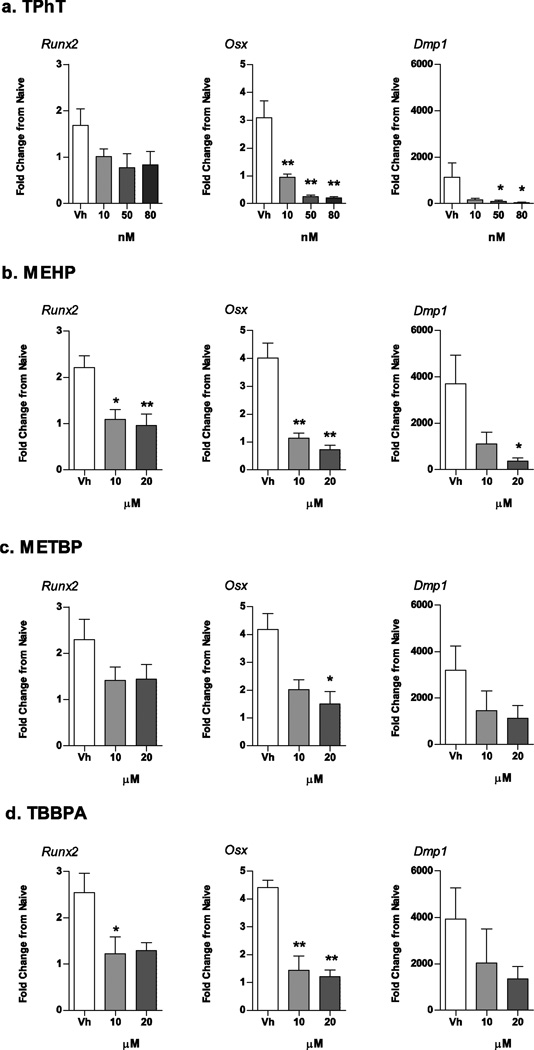
Decreases in the expression of osteoblast/osteocyte-related genes accompanied suppression of osteogenesis (Fig. 7). Runx2 expression was relatively resistant to toxicant exposure and was only reduced by treatment with MEHP and TBBPA (Fig. 7B and 7D). Osxexpression was the most sensitive, with all toxicants significantly reducing expression and TPhT, MEHP, and TBBPA reducing Osx expression at the lowest concentration (Fig. 7A, B and D). TPhT and MEHP also significantly reduced Dmp1 expression (Fig. 6A and 6B). As with the phenotypic assays, METBP had a limited capacity to suppress osteoblast-related gene expression (Fig. 6D).
Figure 7. Structurally distinct environmental PPARγ ligands suppress osteogenic gene expression in mouse BM-MSCs.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with Vh (DMSO), TPhT (10–80 nM; A), MEHP (10–20 µM; B), METBP (10–20 µM; C), TBBPA (10–20 µM; D) in the presence of osteoinductive media for 7 days. mRNA expression was quantified by RT-qPCR. Data are presented as means ± SD (n = 4–7 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s).
The data support the conclusion that suppression of osteogenesis is a common toxic effect of environmental toxicants that are capable of activating PPARγ.
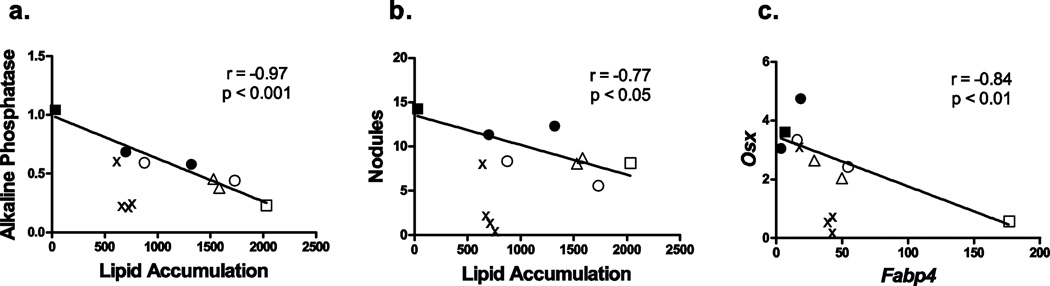
3.4 Effects on adipogenesis and osteogenesis are negatively correlated
A balance of transcriptional activity determines the fate of MSCs, with PPARγ promoting adipogenesis and negatively regulating Runx2, and Runx2 promoting osteogenesis following Wnt/β-catenin suppression of PPARγ (Kang et al., 2007). Thus, differentiation of MSCs has traditionally been viewed as either exclusively toward adipogenesis or exclusively toward osteogenesis. Despite the fact that the PPARγ ligands tested here vary widely in their structure, as well as their potency and efficacy for inducing adipogenesis, they demonstrate strong, significant relationships (p < 0.05, Pearson’s r, excluding organotins) between efficacy of induction of adipogenesis and efficacy of suppression of osteogenesis (Fig. 8 A–C). However, the organotins appear to be more efficacious at suppressing bone formation than the other toxicants. We hypothesize that this is because they are dual RXR and PPARγ ligands, thus they were removed from the correlation analysis, and this greatly improved the correlation. In general, these data support the conclusion that suppression of osteogenesis accompanies induction of adipogenesis by multiple environmental PPARγ ligands. However, the relationship of the efficacies of the effects may be ligand-specific.
Figure 8. Adipogenesis is inversely correlated with osteogenesis in PPARγ-ligand treated mouse BMMSCs.
Data points are representative of mean endpoint values for individual treatments and concentrations reported in Figs 2, 3, 5, 6, 7. (A) Fold change in alkaline phosphatase activity vs. lipid accumulation (Nile Red fluorescence) (All data Pearson’s r = −0.51). (B) Bone nodule number vs. lipid accumulation (All data Pearson’s r = −0.01). (C) Osx mRNA expression vs. Fabp4 mRNA expression (All data Pearson’s r = −0.57). ■ – Vh (DMSO); □ – Rosiglitazone (100 nM); ● – METBP; ○ – MEHP; Δ – TBBPA;×– TBT or TPhT. Linear fit excludes organotin data points (x). r = Pearson’s correlation coefficient (excluding organotins), number of pairs = 8.
3.5 The disproportionate effect of organotins is not mediated by Wnt10b
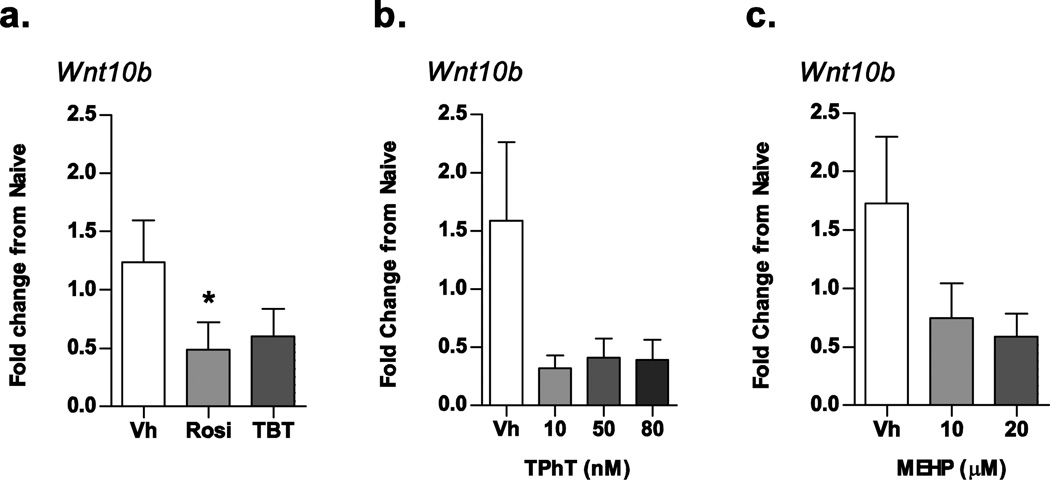
Recently, Rahman et al. (2012) found that the adipogenic effects of PPARγ activation are “sequestered” via β-catenin suppression, while the anti-osteogenic capacity of PPARγ is maintained through suppression of wingless-type MMTV integration site family, member 10b (Wnt10b), a secreted protein that activates the canonical Wnt-signaling pathway of osteogenesis. Stabilization of β-catenin suppressed PPARγ-mediated adipogenic effects, but PPARγ-dependent suppression of Wnt10b was maintained and resulted in an anti-osteogenic phenotype (Rahman et al., 2012). Given that our experiments suggest a disproportionate effect of osteogenic suppression by the organotins, we hypothesized that cells treated with organotins would show a disproportionate suppression of Wnt10b RNA expression compared to rosiglitazone and MEHP.
To test this hypothesis, we examined the expression of Wnt10b mRNA in osteogenic cultures treated with Vh (DMSO, 0.1%), rosiglitazone (100 nM), TBT (100 nM), TPhT (10, 50, and 80 nM) or MEHP (10 and 20 µM) for 7 days. Rosiglitazone was the only ligand that significantly suppressed the expression of Wnt10b mRNA (Fig. 9A), supporting the observation reported by Rahman et al. (2012) and Lecka-Czernik et al. (2002). However, neither TBT nor TPhT significantly reduced Wnt10b expression (Figs. 9A and B), and the Wnt10b expression level was not qualitatively different from the Wnt10b expression in MEHP-treated cultures (Fig 9C). Thus, the data do not support a role for disproportionate effects on Wnt10b in organotin-induced suppression of osteogenesis.
Figure 9. Differential suppression of osteogenic Wnt10b mRNA does not explain efficacious effect of organotins on osteogenesis.
Primary bone marrow cultures were established from male C57BL/6J mice and treated with Vh (DMSO), rosiglitazone (100 nM), TBT (100 nM), TPhT (10–80 nM), MEHP (10–20 µM) in the presence of osteoinductive media for 7 days. mRNA expression was quantified by RT-qPCR. Data are presented as means ± SE (n = 4–10 independent bone marrow preparations). *p < 0.05, **p < 0.01 compared to Vh-treated cultures (ANOVA, Dunnett’s).
4. Discussion
47 million individuals in the U.S. are estimated to be at risk for osteoporosis by 2020, representing a major public health concern among the aging U.S. population (USDHHS, 2004). Accordingly, it is important to understand the factors that contribute to low bone density, a significant risk factor for osteoporosis (USDHHS, 2004). Because precursor bone marrow cells are capable of differentiation into pre-osteocytes or pre-adipocytes, a shift towards adipocyte differentiation instead of osteocyte differentiation could have deleterious effects on bone health. In fact, increases in lipid accumulation are associated with greater fracture risk and the onset of osteoporosis (for review, see Rosen and Bouxsein (2006)), and treatment with therapeutic PPARγ ligands is associated with greater fracture risk among diabetics (Aubert et al., 2010). We hypothesized that environmental PPARγ ligands would promote differentiation of BM-MSCs into adipocytes at the expense of differentiation into osteocytes and bone formation.
A growing number of environmental toxicants have been identified as PPARγ ligands whose diversity of sources and high-volume production result in significant human exposures. Here, we examined the potency and efficacy of TBT, TPhT, MEHP, METBP, and TBBPA in activation of mouse PPARγ1 and PPARγ2 and then examined their pro-adipogenic and anti-osteogenic effects in mouse BM-MSCs. All of the toxicants tested activated both mPPARγ1 and γ2, with the rank potency of Rosiglitazone=TBT=TPhT>>TBBPA>MEHP>METBP. Their rank efficacy for PPARγ1 and γ2 activation was Rosiglitazone=TBT=TPhT>MEHP>TBBPA=METBP. MEHP, METBP and TBBPA were apparent partial agonists of PPARγ with, in the cases of MEHP and TBBPA, EC50’s similar to values previously reported (Hurst and Waxman, 2003; Riu et al., 2011). Similar to our studies, others have shown TBT and TPhT have similar potency inducing lipid accumulation in a multipotent MSC model and for activation of human PPARγ compared to rosiglitazone and troglitazone (Grun et al., 2006; Kanayama et al., 2005, Yanik et al., 2011).
The potency of PPARγ activation was largely reflected in each toxicant’s ability to stimulate adipocyte differentiation, as evidenced by lipid accumulation and PPARγ target gene expression. As expected, the potent therapeutic PPARγ ligand rosiglitazone was most efficacious at stimulating adipocyte differentiation in BM-MSCs, as has been observed in mouse and human models of preadipocytes and multipotent MSCs (Carfi et al., 2008; Grun et al., 2006; Kirchner et al., 2010; Yanik et al., 2011). The organotins, while apparent full agonists in terms of PPARγ transcriptional activation, were potent but not fully efficacious at stimulating lipid accumulation and PPARγ target gene expression in BM-MSCs. Our lipid accumulation and PPARγ activation results with TBT and TPhT confirm previous results showing sub-maximal efficacy in BMS2 cells (Yanik et al., 2011).
On the other hand, MEHP was an apparent partial agonist in terms of PPARγ transactivation, but efficaciously stimulated lipid accumulation and PPARγ target gene expression. The partial agonist nature of MEHP has been previously reported (Fiege et al 2007). In our study, MEHP (20 µM, 7–10 days, insulin and dexamethasone) induced lipid accumulation in mouse BM-MSCs to an extent comparable to the full agonist rosiglitazone, whereas Feige et al. (2007) found that NIH 3T3-L1 cells treated with MEHP (100 µM, 10 days, insulin) accumulated lipid (triglyceride content) to only 50% of rosiglitazone treatment. Both rosiglitazone and MEHP efficaciously induced genes important to adipogenesis in the BM-MSC and NIH 3T3 L1 models (e.g. Fabp4, Plin1 (BM-MSCs) and adiponectin (NIH 3T3-L1s)). However, other PPARγ gene targets (e.g. glycerol kinase, oxidized low density lipoprotein receptor 1, acyl-CoA synthetase Bubblegum 1) have been shown to only be efficaciously induced by rosiglitazone, mostly likely because MEHP selectively recruits coactivators to the PPARγ transcriptional complex (Feige et al., 2007).
METBP, the metabolite of an emerging environmental contaminant, di-(2-ethylhexyl) tetrabromophthalate, significantly stimulated lipid accumulation but was less efficacious at stimulating PPARγ-target gene expression, in line with its marginal ability to stimulate PPARγ transcriptional activity. These results are in accordance with a recent study showing that METBP induced adipocyte differentiation and PPARγ activation in NIH 3T3 L1 cells (Springer et al., 2012). That METBP should display an adipogenic profile similar to MEHP is not entirely surprising, given previous studies showing multiple phthalate monoester metabolites activating PPARγ with comparable potencies and efficacies (Hurst and Waxman, 2003).
Like MEHP, TBBPA appears to be a partial ligand of PPARγ with significant but not maximal efficacy in inducing adipocyte differentiation. The receptor activation data show that TBBPA is a low-potency PPARγ ligand with moderate efficacy. This is similar to studies conducted in human PPARγ-based reporter cells, where tri- and tetrabrominated BPA had the greatest potency and efficacy in activating PPARγ, with decreasing substitution significantly decreasing activity (Riu et al., 2011). Our results confirmed the ability of TBBPA to induce adipogenesis, accompanied by significant increases in Pparg1/2, Fabp4 and Plin1 expression.
This is the first report that TPhT, MEHP and TBBPA suppress osteogenesis. We expected to find that those environmental toxicants that induced adipogenesis would also suppress osteogenesis, owing to the inhibitory interactions between PPARγ and Runx2. Mechanistically, PPARγ directly interacts with Runx2 to prevent Runx2 transcriptional activity (Jeon et al., 2003). Additionally, PPARγ activation increases β-catenin degradation by the proteasome, removing β-catenin’s support of osteogenesis and β-catenin’s Wnt-mediated inhibition of PPARγ expression (Kang et al., 2007; Moldes et al., 2003). However, it is becoming clear that activation of adipogenesis and suppression of osteogenesis are distinct and separable activities of PPARγ (Choi et al., 2014), with ligands having selective abilities to impact adipogenesis and osteogenesis (Lecka-Czernik et al., 2002; Lazarenko et al., 2006; Kolli et al., 2014). Therefore, it is important to examine environmental PPARγ ligands for their effects on both adipogenesis and osteogenesis.
Interestingly, the effect of TBT and TPhT on osteogenesis was markedly more severe compared to the other ligands. The results with TBT are in line with studies demonstrating that TBT suppressed alkaline phosphatase activity in rat calvarial osteoblasts and reduced mineralization in adipose-derived stromal cells (Kirchner et al., 2010; Tsukamoto et al., 2004). The organotins were disproportionately efficacious in suppressing bone than inducing adipogenesis. One potential explanation for this dichotomy is that the organotins more readily activate the anti-osteogenic functions of PPARγ than its pro-adipogenic functions. These PPARγ functions were recently shown to be separable, and the anti-osteogenic effects of PPARγ were shown to be dependent upon Wnt10b (Rahman et al., 2012). However, while treatment with TBT, TPhT and MEHP appeared to suppress expression of Wnt10b, the effect was not statistically significant. These data suggest that unique modulation of a Wnt10b-mediated pathway by organotins does not appear to explain their selective effect on MSC differentiation.
The toxicants studied here join a growing list of environmental contaminants that compromise bone quality, an effect not exclusive to PPARγ ligands. Processes mediated by aryl hydrocarbon receptor ligands (e.g. 2,3,7,8-tetrachlorodibenzo-p-dioxin, benzo-a-pyrene) contribute to increased bone resorption in vivo (Iqbal et al., 2013) and impair MSC differentiation in vitro (Korkalainen et al., 2009). Additionally, co-exposure to dioxin and TBT appears to exacerbate the effects of exposure to each toxicant individually (Koskela et al., 2012). Heavy metals such as lead and arsenic are toxic to the skeleton of rats, causing decreased bone density, mineralization and strength (Beier, et al., 2013; Wu et al., 2014). It is largely unknown how complex exposures to multiple bone-suppressive toxicants may act cooperatively to impair bone quality.
Our results show that environmental PPARγ ligands are efficacious promoters of lipid accumulation and suppress osteogenesis in a mouse bone marrow-derived MSC model. While the efficacies varied by chemical and endpoint, it is important to note that effects were observed at environmentally-relevant concentrations. Additional concern should be directed towards identifying possible interactions and how multiple, simultaneous exposures could additively suppress bone formation in MSCs. While this study tested exposures on an individual toxicant basis, environmental exposures to these chemicals are likely to occur simultaneously. Further experiments should aim to assess chemical mixtures of ubiquitous and persistent compounds in order to more accurately characterize toxicant-induced bone loss.
Supplementary Material
Acknowledgements
This work was supported by the Superfund Research Program [P42ES007381] and the National Institute of Environmental Health Science at the National Institutes of Health [R21ES021136]. The authors would like to thank Ms. Faye Andrews for her superb technical assistance.
Abbreviations
- BM-MSC
bone marrow mesenchymal stromal cell
- DEHP
di-(2-ethylhexyl)phthalate
- DMP1
dentin matrix phosphoprotein
- MEHP
mono-(2-ethylhexyl)phthalate
- METBP
mono-(2-ethylhexyl)tetrabromophthalate
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- OSX
osterix
- PLIN1
perilipin
- pNPP
p-nitrophenyl phosphate
- PPARγ
peroxisome proliferator activated receptor γ
- RN18S
18s ribosomal RNA
- RUNX2
runt-related transcription factor 2
- TBBPA
tetrabromobisphenol-a
- TBT
tributyltin
- TPhT
triphenyltin
- WNT10b
wingless-type MMTV integration site family member 10b
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Harrad S, Goosey E, Neels H, Covaci A. "Novel" brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere. 2011;83:1360–1365. doi: 10.1016/j.chemosphere.2011.02.078. [DOI] [PubMed] [Google Scholar]
- Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ Int. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes, Obesity and Metabolism. 2010;12:716–721. doi: 10.1111/j.1463-1326.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, Puzas JE. Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect. 2013;121:97–104. doi: 10.1289/ehp.1205374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilik D, McEwen LN, Brown MB, Pomeroy NE, Kim C, Asao K, Crosson JC, Duru OK, Ferrara A, Hsiao VC, Karter AJ, Lee PG, Marrero DG, Selby JV, Subramanian U, Herman WH. Thiazolidinediones and fractures: evidence from translating research into action for diabetes. J Clin Endocrinol Metab. 2010;95:4560–4565. doi: 10.1210/jc.2009-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, et al. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82:170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfi M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, Gribaldo L. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology. 2008;249:11–18. doi: 10.1016/j.tox.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Choi SS, Kim ES, Koh M, Lee SJ, Lim D, Yang YR, Jang HJ, Seo KA, Min SH, Lee IH, Park SB, Suh PG, Choi JH. A novel non-agonist peroxisome proliferator-activated receptor gamma (PPARgamma) ligand UHC1 blocks PPARgamma phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity. J Biol Chem. 2014;289:26618–26629. doi: 10.1074/jbc.M114.566794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hollander W, Roosens L, Covaci A, Cornelis C, Reynders H, Campenhout KV, Voogt P, Bervoets L. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere. 2010;81:478–487. doi: 10.1016/j.chemosphere.2010.07.043. [DOI] [PubMed] [Google Scholar]
- de Wit CA, Herzke D, Vorkamp K. Brominated flame retardants in the Arctic environment--trends and new candidates. Sci Total Environ. 2010;408:2885–2918. doi: 10.1016/j.scitotenv.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- Grun F, Blumberg B. Environmental Obesogens: Organotins and Endocrine Disruption via Nuclear Receptor Signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- He S, Li M, Jin J, Wang Y, Bu Y, Xu M, Yang X, Liu A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ Toxicol Chem. 2013;32:1242–1247. doi: 10.1002/etc.2172. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARa and PPARg by environmental phthalate monoesters. Toxicol. Appl. Pharmacol. 2003 doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Sun L, Cao J, Yuen T, Lu P, Bab I, Leu NA, Srinivasan S, Wagage S, Hunter CA, Nebert DW, Zaidi M, Avadhani NG. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci U S A. 2013;110:11115–11120. doi: 10.1073/pnas.1220919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, Kim SY, Shin CS. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol. 2010;58:901–907. doi: 10.1007/s00244-010-9513-6. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health. 2003;206:77–83. doi: 10.1078/1438-4639-00205. [DOI] [PubMed] [Google Scholar]
- Kolli V, Stechschulte LA, Dowling AR, Rahman S, Czernik PJ, Lecka-Czernik B. Partial agonist, telmisartan, maintains PPARγ serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass. PLoS One. 2014;9:e96323. doi: 10.1371/journal.pone.0096323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkalainen M, Kallio E, Olkku A, Nelo K, Ilvesaro J, Tuukkanen J, Mahonen A, Viluksela M. Dioxins interfere with differentiation of osteoblasts and osteoclasts. Bone. 2009;44:1134–1142. doi: 10.1016/j.bone.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Koskela A, Viluksela M, Keinanen M, Tuukkanen J, Korkalainen M. Synergistic effects of tributyltin and 2,3,7,8-tetrachlorodibenzo-p-dioxin on differentiating osteoblasts and osteoclasts. Toxicol Appl Pharmacol. 2012;263:210–217. doi: 10.1016/j.taap.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPARgamma ligands with selective effects on bone and fat. Bone. 2006;38:74–84. doi: 10.1016/j.bone.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, Zhao LF, Liang Y, Zhang YH. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One. 2013;8:e62526. doi: 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Czernik PJ, Lu Y, Lecka-Czernik B. beta-catenin directly sequesters adipocytic and insulin sensitizing activities but not osteoblastic activity of PPARgamma2 in marrow mesenchymal stem cells. PLoS One. 2012;7:e51746. doi: 10.1371/journal.pone.0051746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome Proliferator-Activated Receptor gamma Is a Target for Halogenated Analogs of Bisphenol A. Environ Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer C, Dere E, Hall SJ, McDonnell EV, Roberts SC, Butt CM, Stapleton HM, Watkins DJ, McClean MD, Webster TF, Schlezinger JJ, Boekelheide K. Rodent thyroid, liver, and fetal testis toxicity of the monoester metabolite of bis-(2-ethylhexyl) tetrabromophthalate (TBPH), a novel brominated flame retardant present in indoor dust. Environ Health Perspect. 2012;120:1711–1719. doi: 10.1289/ehp.1204932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am. J. Ind. Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARg2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Ishihara Y, Miyagawa-Tomita S, Hagiwara H. Inhibition of ossification in vivo and differentiation of osteoblasts in vitro by tributyltin. Biochem Pharmacol. 2004;68:739–746. doi: 10.1016/j.bcp.2004.04.020. [DOI] [PubMed] [Google Scholar]
- USDHHS. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. Bone Health and Osteoporosis: A Report of the Surgeon General. [PubMed] [Google Scholar]
- Wu CT, Lu TY, Chan DC, Tsai KS, Yang RS, Liu SH. Effects of Arsenic on Osteoblast Differentiation and on Bone Mineral Density and Microstructure in Rats. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik SC, Baker AH, Mann KK, Schlezinger JJ. Organotins are potent activators of PPAR{gamma} and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol Sci. 2011;122:476–488. doi: 10.1093/toxsci/kfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.