Abstract
Purpose
IKKα has been recently identified as a key mediator of the inflammation and metastasis in prostate cancer. In the present study, we intend to silence the IKKα expression in prostate cancer cells using synthetic siRNAs and examine their biological effects on tumor cell invasiveness and growth.
Methods
Three synthetic siRNAs targeting different regions of the IKKα mRNA were designed, and the silencing effect was determined in PC-3 and DU145 cells. Numerous studies, including wound-healing assay, migration assay, invasion assay, cell attachment assay, cell proliferation, and cell cycle analysis, were conducted to investigate the biological effects of the IKKα siRNAs on prostate cancer cells.
Results
We have identified potent siRNAs that can silence the IKKα up to 74%. Inhibition of IKKα reduced the wound healing, migration, invasion and cell attachment capabilities of prostate cancer cells. Similar anti-invasive effects were also observed in the presence of RANKL. However, silencing of IKKα only showed a negligible effect on cell proliferation and cell cycle distribution.
Conclusion
This study presents compelling evidence that IKKα plays a major role in prostate cancer invasion and metastasis, but not in cell proliferation. Silencing of IKKα with siRNA may therefore provide a promising therapeutic strategy for prostate cancer patients.
Keywords: IKKα, invasiveness, prostate cancer, siRNA, RANKL
INTRODUCTION
Prostate cancer is the most common male malignancy and the second leading cause of cancer death in American men (1). It is characterized by an elevated Prostate Specific Antigen (PSA) level in the blood and urinary dysfunction, and it may also affect sexual function and performance. The current standard treatments include surgery, radiation, and adjuvant hormonal therapy. Although these therapies are relatively effective in the short-term treatment, a majority of patients initially diagnosed with localized prostate cancer ultimately relapse. Therefore, the major risk faced by prostate cancer patients is the development of metastasis, which may cause additional symptoms, including bone pain (often in the vertebrae, pelvis and ribs), weakness in legs, and urinary and fecal incontinence (1). Intensive efforts are underway to develop therapeutics for the treatment of prostate cancer metastasis. Among all approaches, gene modulation is one of the most promising strategies due to the fact that genomic instability is the hallmark of cancer development.
It is known that inflammation enhances tumor promotion through the NFκB-dependent pathway, and NFκB was found essential for epithelial-mesenchymal transition and metastasis (2,3). However, the molecular mechanisms linking inflammation and metastasis are not fully understood. Luo et al. recently found that IκB kinase alpha (IKKα) plays a key role at the crossroads of inflammation and metastasis (4,5). IKKα is a member of the IKK family which contains five distinct but closely related members, IKKα, IKKβ, IKKγ, TBK-1 and IKKε. IKKα is preferentially found as a heterodimer with IKKβ, but occasionally may also be found as a homodimer with IKKα. IKKα exerts its pro-metastatic effect by repressing the transcription of the Maspin gene, which is a putative suppressor of metastasis. The expression level of Maspin in normal epithelia cells is much higher than that in malignant cells (4). Infiltrating immune cells express RANKL (Receptor Activator of NFκB Ligand) that binds to its receptor, RANK, on prostate cancers and induces the activation and nuclear translocation of IKKα in prostate tumor cells. IKKα then interacts with the promoter of the Maspin gene and silences its expression, committing prostate cancer to a metastatic fate (4,5). Therefore, a selective inhibition of IKKα is expected to inhibit prostate cancer metastasis, which is a major risk for prostate cancer patients (5).
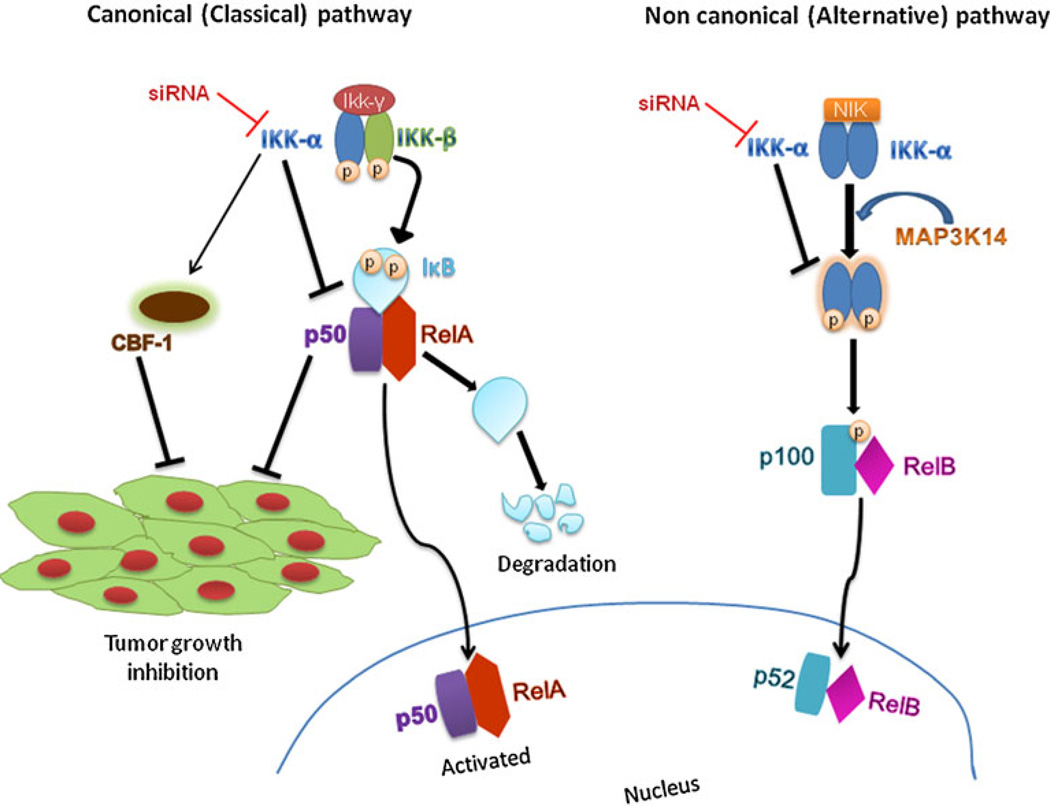
On the other hand, several lines of evidence have implicated that IKKα plays multiple roles in the regulation of NF-κB and various inflammatory and pathogenic conditions, such as invasion and metastasis of the cancer cells. NF-κB family is composed of RelA, RelB, c-Rel, p50/ p105, and p52/p100. In most resting cells, NF-κB is localized in the cytoplasm in an inactive form which is associated with the inhibitors of Kappa B (IκBs). Upon exposures to inflammatory cytokines, bacterial and viral toxins, and UV light, the IKK complex (IKKα/IKKβ/ NEMO) phosphorylates IκB and leads to dissociation of the IλB/NF-κB complex. This frees NF-κB and allows its translocation into the nucleus to regulate the related gene transcriptions. IKKα affects NF-κB in both of its two pathways (Fig. 1). As a part of the non-canonical pathway (alternative pathway) of the NF-κB activation, the MAP3K14-activated IKKα homodimer phosphorylates p100 that is associated with RelB, inducing its proteolytic processing to NF-κB2/p52, and formation of the RelB-p52 complexes (6). Also, it phosphorylates NCOA3 (Nuclear Receptor Coactivator 3) and ‘Ser-10’ of histone H3 at the NF-κB-regulated promoters during the inflammatory responses triggered by a cytokine (7–9). IKKα also activates the canonical pathway (classical pathway) of NF-κB and forms the IKK signalisome complexes with IKKβ and IKKγ to mediate the phosphorylation of IκBα (10,11). Knockdown of IKKα has been reported to strongly suppress the basal NF-κB reporter activity and stimulate the CBF-1 reporter activity, hence inhibiting the tumor growth (12). Nuclear IKK-α is reported to regulate not only NF-κB activity but also other transcription controllers. For example, IKKα activates the estrogen receptor-α downstream target such as cyclin D1 and promotes cell proliferation in breast cancer (13). It has also been reported that IKKα plays a role in cervical cancer in association with Notch-1, where the knockdown of IKKα by siRNA or its precipitation by antibodies sensitizes the cancer cells to cisplatin-induced apoptosis (12).
Fig. 1.
Roles of IKKκ in NF-αB canonical and non-canonical pathways. IKKa preferentially forms heterodimer with IKKβ, but occasionally forms homodimer with IKKa itself. Knockdown of KKa affects both canonical and non-canonical pathways. In the canonical pathway it stimulates CBF-1, leading to inhibition of tumor growth. Through the non-canonical pathway, knockdown of KKa prevents the MAP3K14-mediated phosphorylation of p100, thereby inhibiting the processing of p100 to p52.
Furthermore, it has been demonstrated that inhibition of IKKα was effective in preventing B-cell maturation and proliferation in mammary epithelial cells without disturbing innate and T-cell-mediated adaptive immune responses. It does not sensitize cells to TNFα-induced apoptosis (14). Taken together, IKKα is a promising target for prostate cancer therapy. The pharmaceutical industry has under-taken an enormous effort to identify inhibitors of IKKα using large compound libraries as well as combinational chemistry. However, no potent IKKα inhibitor has been identified so far (14).
Among various strategies to inhibit a gene expression, small interference RNA (siRNA) represents a considerable promise for cancer therapy due to its potent and specific knockdown effect (15,16). In the present study, we intend to silence the IKKα expression in prostate cancer cells using synthetic siRNA and evaluate its effects on tumor cell invasiveness and growth.
MATERIALS AND METHODS
Materials
Lipofectamine-2000 and TRIzol reagent were purchased from Invitrogen Corp. (Carlsbad, CA), and fetal bovine serum (FBS) was purchased from Atlanta Biologicals, Inc. (Lawrenceville, GA). Other cell culture products, including RPMI-1640 medium, Dulbecco’s Phosphate-Buffered Saline (DPBS), penicillin and streptomycin were obtained from Mediatech, Inc. (Manassas, VA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich Corporation (St. Louis, MO). SYBR Green-1 dye universal master mix and MultiScribe reverse transcriptase were purchased from Applied Biosystems, Inc. (Foster City, CA). 6.5 mm Transwell with 8.0 µm Pore Polycarbonate Membrane Insert was purchased from Corning Incorporated (Lowell, MA). BD Matrigel™ was obtained from BD Biosciences (San Jose, CA). CellTiter-Glo® Luminescent Cell Viability Assay Kit was purchased from Progema Corp (Madison, WI). β-actin antibody was purchased from Rockland Immunochemicals Inc. (Gilbertsville, PA), and the IKKα polyclone antibody (IKKα, H-116) was obtained from Cell Signaling Technology, Inc. (Danvers, MA). RNAKL was obtained from Shenandoah Biotechnology, Inc. (Warwick, PA).
Cell Culture
Human prostate cancer cell lines PC-3, DU-145 were purchased from American Type Culture Collection (Manassas, VA). PC-3 cells were maintained in RPMI 1640 with 10% FBS, penicillin (100unit/mL), and streptomycin (100 µg/mL), whereas DU-145 cells were maintained in DMEM with 10% FBS and same concentration of penicillin and streptomycin. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed every 2–3 days, and the cells were trypsinized when they reached 70∼80% confluence.
siRNA
Three predesigned siRNAs targeting human IKKα gene (Accession No. NM_001278.3) are listed in Table 1. All siRNAs (siRNA1, siRNA2 and siRNA3) were purchased from Ambion, Inc. (Austin, TX). These siRNAs are of 19 nucleotides with two thymidine deoxynucleotide (T) 3′ overhangs. All designed siRNA sequences were blasted against the human genome database to eliminate the cross-silencing phenomenon with non-target genes. Scrambled siRNA (purchased from Ambion, Inc.) that does not target any gene was used as the negative control (NC).
Table 1.
Sense Strand Sequences of the IKKα siRNAs (NM_001278.3)
| Number | Start site | Sequence |
|---|---|---|
| siRNA-1 | 356 | 5′-GGAGAUCUCCGAAAGCUGCtt-3′ |
| siRNA-2 | 202 | 5′-GCUAAGUACCAAAAACAGAtt-3′ |
| siRNA-3 | 2248 | 5′-GGGCAAUAGUAUGAUGAAUtt-3′ |
Transfection of siRNA
Cells were transfected with siRNA using Lipofectamine-2000 according to manufacturer’s instruction. Briefly, cells were seeded in 24-well plates at a density of 1.5×105 cells/ well in medium containing 10% FBS, penicillin (100unit/ mL), and streptomycin (100 µg/mL). After 12 h, cells were transfected with siRNA at a final concentration of 50nM using Lipofectamine-2000. One and a half microliters of siRNA (20 µM) were mixed with 1.2 µl lipofectamine-2000 in 200 µl of FBS free medium, and incubated at room temperature for 25 min to form a complex. The cell culture medium was then removed, and the siRNA/lipofectamine-2000 complex was added to the cells. Twenty-four hours after the transfection, medium was replaced with fresh RPMI-1640 medium containing 10% FBS, and penicillin-streptomycin. Forty-eight hours after the tansfection, cells were trypsinized for RNA and protein isolation.
Real-Time RT-PCR
Total RNA was isolated from cell pellets using TRIzol reagent (Invitrogen) as per the manufacturer’s protocol. RNA (200 ng) was converted to cDNA using random hexamer, primers, and MultiScribe Reverse Transcriptase Reagent. One hundred nanograms of cDNA were amplified by Real-Time PCR using the LightCycler 480 SYBR Green I Master on the LightCycler® 480 Real-Time PCR System (Roche Applied Science, Indianapolis, IN). The primers used for IKKα are 5′-TCTGGAACAGCGTGC CATTGATCT-3′ (forward) and 5′-ATTACTGAGGGC CACTTCCACCTT-3′ (reverse). Primers for Maspin are 5′-AGACA GACACCAAACCAGTGCAGA-3′ (forward) and 5′-ATGGTGCTGGGATTAGTCCACTGT-3′ (reverse). We used 18s ribosomal RNA as an internal control and the primers are 5′-GTCTGTGATGCCCT TAGATG-3′ (forward) and 5′-AGCTTATGACCCG CACTTAC-3′ (reverse). To confirm the PCR specificity, PCR products were subjected to a melting-curve analysis. Comparative threshold (Ct) method was used to calculate the relative amount of mRNA of treated sample in comparison to control samples. Each sample was performed in triplicate, and the mean value was calculated.
Western Blot Analysis
PC-3 cells were transfected with the IKKα siRNA for 24 h, and the protein was isolated using RIPA buffer containing protease and phosphtase inhibitor cocktails at 48 h post-transfection. Same amount of total protein (20 µg) was used for all the samples and was separated in a 10% SDS PAGE gel. The separated proteins were transferred to a nitrocellulose membrane, blocked, and probed with appropriate antibodies. The protein bands were then visualized using horseradish peroxidase-conjugated secondary antibodies and chemoluminescence detection kit.
Wound-Healing Assay
PC-3 cells were seeded in 12-well plates at a density of 3× 105 cells/well. After 12 h, the cells were transfected with 50nM siRNA as described above. Once the cells reached 90% confluence, a wound area was carefully created by scraping the cell monolayer with a sterile 200 µl pipette tip, from one end to the other end of the well. The detached cells were removed by washing with PBS. Then, the cells were incubated at 37°C in the incubator supplemented with 5% CO2. The wound-healing process was monitored microscopically over time as the cells moved into the wound area, and the images were taken from the same place at various intervals using an inverted microscope. Images taken immediately after creating the wound represent the time zero.
Cell Migration Assay and Invasion Assay
The effect of siRNA on invasiveness and metastatic potential of prostate cancer cells was evaluated using transwell migration and invasion assays as we described before (17). Forty-eight hours after the transfection, PC-3 cells were trypsinized and resuspended in FBS-free RPMI-1640 medium. A total of 6 × 104 cells in 200 µl volume were plated in the top chamber with a non-coated polycarbonate membrane (6.5 mm diameter insert, 8.0 µm pore size, Corning Incorporated) for migration assay. RPMI-1640 medium with 10% FBS was added in the lower chamber as a chemo-attractant. After incubation for 24 h, the cells migrated to the lower surface of the membrane were fixed with 10% buffered formalin for 10 min, followed by staining with 0.2% crystal violet for 20 min. The cells that did not migrate through the pores were mechanically removed with a cotton swab. The images of migrated cells were obtained by an inverted microscope at a magnification of 200×. The numbers of migrated cells were counted from four random fields.
The invasion ability of the PC-3 cells in vitro was examined by determining the number of cells that cross the matrigel-coated transwell inserts. The process was the same as the migration assay, except that the transwell was coated with 100 µl of matrigel (0.5 mg/ml) for 4 h at room temperature. The supernatant of the matrigel was removed, and then 1×105 cells were plated to the transwell.
Cell Attachment Assay
This assay was conducted as we reported before (17). Briefly, cells were trypsinized 48 h post-transfection and resuspended in FBS-free RPMI 1640 medium. Cells were plated in a 96-well plate which was pre-treated with 30 µg/ ml Type I Collagen (Becton Dickinson, Mountain View, CA) or 1% BSA for 1 h at 37°C, followed by blocking with 1% BSA at room temperature for 1 h. Cells were then allowed to attach to wells at 37°C for 1 h. The medium was removed, and attached cells were fixed with 10% buffered formalin for 10 min, followed by staining with 0.2% crystal violet for 20 min. The stained cells were then lysed in 100 µl of 1% SDS (sodium dodecyl sulfate) by shaking for 5 min. The absorbance, which is proportional to the number of attached cells, was quantitated by a spectrometer at the wavelength of 595 nm using a plate reader DTX 880 Multimode Detector (Beckman Coulter, Inc., Fullerton, CA). Cells placed in the 1% BSA-coated wells served as the control.
Cell Proliferation Assay
The effect of siRNA on cell proliferation was measured using CellTiter-Glo® Luminescent Cell Viability Assay Kit (Progema Corp. Madison, WI) as per the instructions. Briefly, PC-3 cells (15000cells/well) were seeded in a black 96-well plate. After 12 h, cells were transfected with 50nM siRNA, and the medium was changed at 24 h post-transfection. Ninety-six hours after the transfection, the culture medium was replaced with 50 µl of CellTiter-Glo® reagent plus 50 µl of RPMI medium. Cells were lysed by shaking on an orbital shaker for 2 min, followed by incubation at room temperature for 10 min to stabilize the luminescent signal. The luminescence was then detected using a DTX 880 Multimode Detector (Beckman Coulter, Inc., Fullerton, CA) with an integration time of 1 s.
Cell Cycle Assay
PC-3 cells were transfected with siRNA at 50nM for 24 h. Forty-eight hours after the transfection, cells were collected and fixed in ice-cold 90% ethanol for 15 min. After fixing, cells were washed with DPBS once and then stained with Propidium Iodide (PI)/RNase staining buffer for 30 min at room temperature. Cell cycle analysis was carried out with FACSCalibur Flow cytometer (BD Biosciences). The result was confirmed from three independent experiments; each group has three samples.
RANKL Treatment
In all RANKL-treated experiments, the cells were transfected with siRNA for 24 h, followed by incubation with 100 ng/ml human recombinant RANKL for 24 h.
Statistical Analysis
Data were expressed as the mean ±standard deviation (SD). Difference between any two groups was determined by ANOVA. P<0.05 was considered statistically significant.
RESULTS
Silencing of IKKα with Predesigned siRNAs
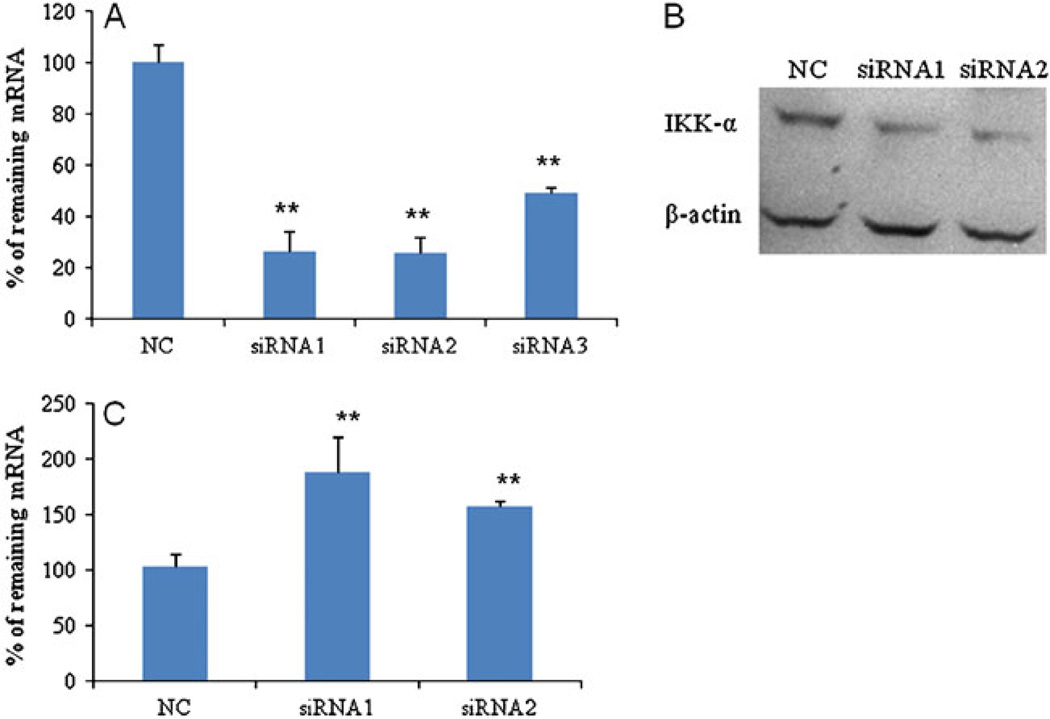
Three synthetic siRNAs (Table 1) were designed to target different regions of the human IKKα mRNA. Silencing effects of these IKKα siRNAs were examined in PC-3 cells at the mRNA level using real-time RT-PCR. A scrambled siRNA that does not target any specific gene was used as the negative control. All three siRNAs showed significant silencing effects (Fig. 2A). Compared to the scrambled siRNA, both siRNA1 and siRNA2 exhibited a potent silencing effect up to 74%. Considering the fact that siRNA transfection efficiency may vary in different cell lines, we also examined the silencing effect in another prostate cancer cell line, DU-145, and observed similar effects as that in PC-3 cells (data not shown). Furthermore, the silencing effect at the protein level was confirmed using western blot. As shown in Fig. 2B, both siRNA1 and siRNA2 dramatically suppressed the IKKα protein expression in PC-3 cells, which is consistent with the silencing effect at the mRNA level. Consequently, siRNA1 and siRNA2 were selected for following biological studies in prostate cancer cells.
Fig. 2.
Silencing effect of the predesigned IKKα siRNAs in PC-3 cells. PC-3 cells were transfected with the IKKα siRNAs and scrambled siRNA (NC) at a concentration of 50nM. Cells were harvested 48 h after the transfection, and the silencing effect of IKKα was determined at the mRNA level using RT real-time PCR (A). IKKα silencing was also estimated at protein level using western blot (B). The effect of IKKα silencing on the Maspin gene expression was determined using RT real-time PCR (C). (* p<0.05; ** p<0.01).
Recently, it has been shown that IKKα exerted a pro-metastatic effect in prostate cancer cells by repressing the transcription of the Maspin gene, which is a putative suppressor of metastasis (4). Therefore, we investigated the effect of IKKα siRNA on Maspin, a tumor suppressor gene, expression. There was an up-regulation of Maspin in the IKKα siRNA-treated groups compared to the scrambled siRNA-treated group (Fig. 2C).
Silencing of IKKα Inhibits Cell Motility
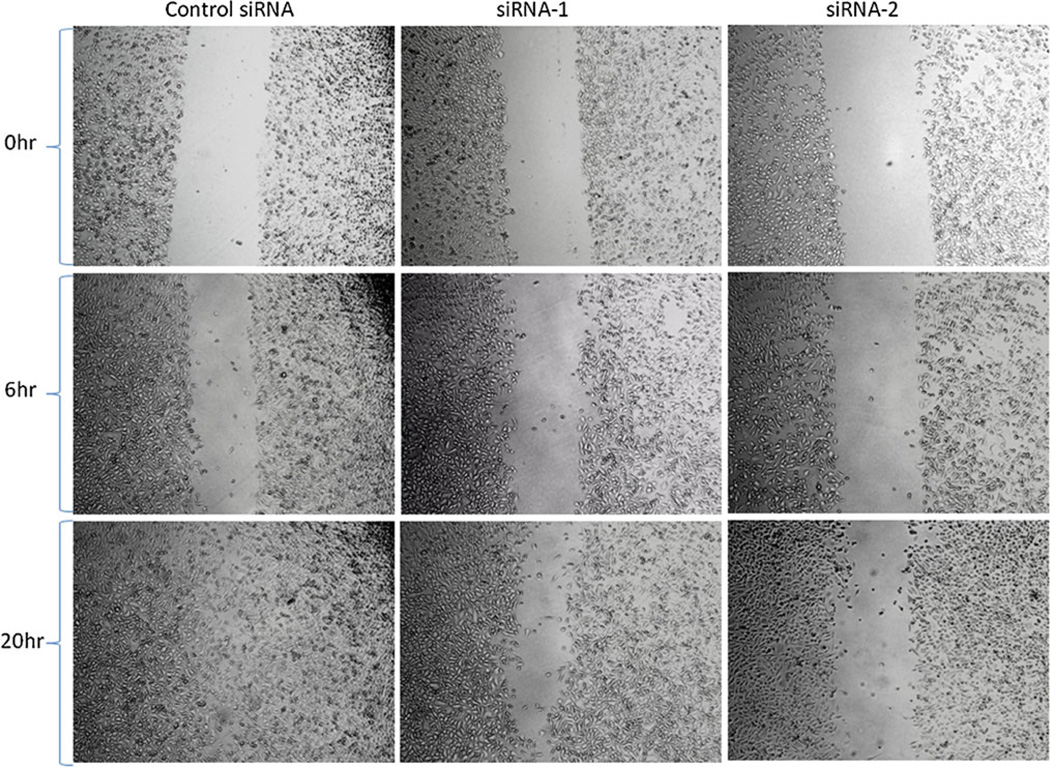
Given the result that IKKα siRNA up-regulates the expression of Maspin, a metastasis suppressor, we investigated the effect of IKK-α silencing on cell motility and invasiveness of prostate cancer cells. We first conducted a wound-healing assay, which is a classic in vitro assay to mimic the in vivo motility of cancer cells. Forty-eight hours after the transfection with siRNA, the cell monolayer was disrupted by scratching a line through the layer to simulate a wound in the cell monolayer. The time required to fill the gap (wound healing) is proportional to the cell migration rate during the in vivo wound-healing process. Cells transfected with siRNA1 and siRNA2 showed slower healing of the wound in comparison to the cells treated with scrambled siRNA (Fig. 3). After 20 h, cells transfected with the scrambled siRNA had completely filled the gap, while the cells treated with the IKKα siRNA still had an unhealed gap, indicating that IKKα siRNA can efficiently suppress the motility of prostate cancer cells.
Fig. 3.
Effect of IKKα siRNA on cell motility. A wound-healing assay was performed to evaluate the motility of PC-3 cells after silencing IKKα. The cells seeded in 12-well plate were transfected with IKKα siRNAs at 50nM. After 24 h, a line was drawn from one end of the well to the other end. The closing of the gap was monitored regularly at different time points by an inverted microscope with a magnification of 100x.
Depletion of IKKα inhibits cell migration and invasion
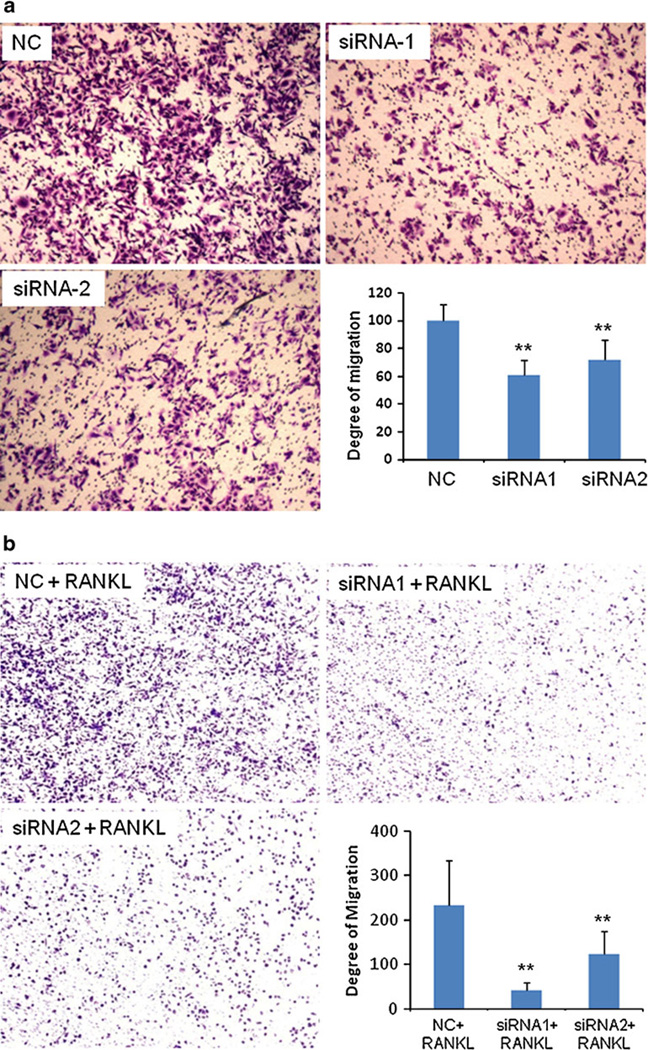
We next investigated the effects of IKKα siRNA on the migration and invasion abilities of prostate cancer cells. Migration towards a chemo-attractant is a distinct cellular phenotype of metastatic tumor cells, and it is an essential step for tumor invasion and metastasis. After transfection with IKKα siRNA, the migration ability of PC-3 cells was examined using an in vitro migration assay, which is a simplified model to simulate the in vivo metastatic process. As shown in Fig. 4A, there was a dramatic inhibition on cell migration after silencing the IKKα expression with siRNA1 and siRNA2. Compared to the scrambled siRNA, siRNA1 and siRNA2 reduced the migration ability of PC-3 cells up to 40% and 30%, respectively.
Fig. 4.
Silencing of IKKα inhibits the migration ability of PC-3 cells in the absence of RANKL (a) and in the presence of RANKL (b). (a) Cells were transfected with IKKα siRNAs (50nM) and scrambled siRNA (50nM) for 24 h. Forty-eight hours after the transfection, cells were trypsinized, re-suspended in a FBS free medium, and plated on the upper chamber of a trans-well. (b) Cells were first transfected with siRNA for 24 h, followed by RANK-L treatment for 24 h. Cells were then trypsinized, re-suspended, and plated on the upper chamber of trans-well. RPMI medium containing 10% FBS was added in the lower chamber as the chemo-attractant. After 24 h, the cells that migrated through pores to the bottom surface of the chamber were fixed, stained, and counted. Images of the migrated cells were obtained using an inverted microscope at a magnification of 100×. Four random microscopic fields were counted for each group to calculate the degree of migration. (* p<0.05; ** p<0.01).
The effect of IKKα silencing on the migration ability was also examined in the presence of RANKL, which can activate IKKα and enhance the migration of prostate cancer cells. Both siRNA1 and siRNA2 showed a more significant reduction in the number of migrated cells in the presence of RANKL (Fig. 4B). siRNA1 reduced the cell migration ability up to 82%, and siRNA2 reduced the migration up to 47%.
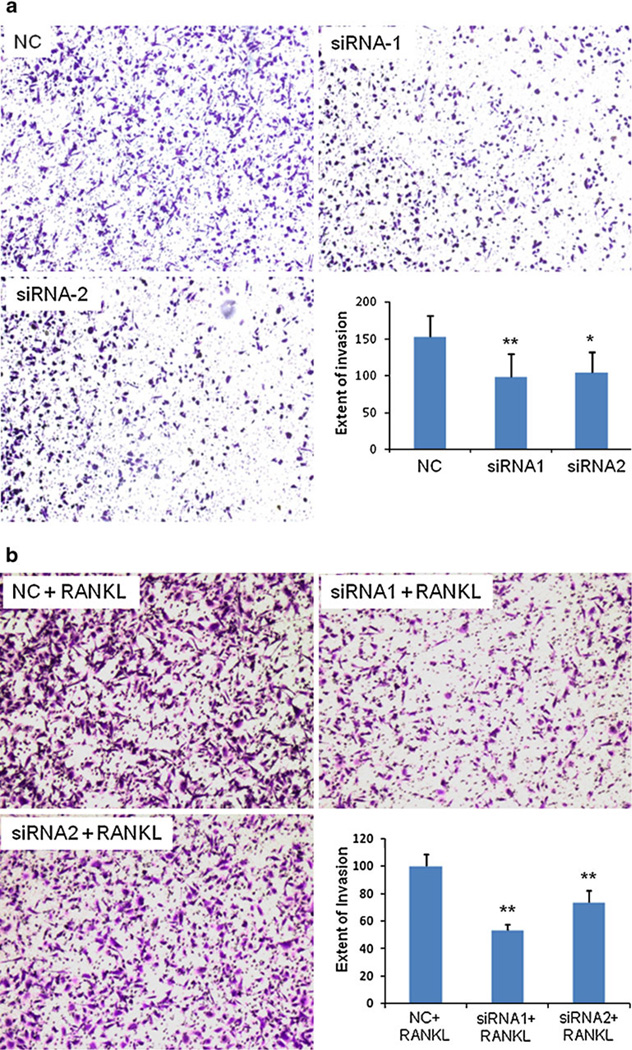
Additionally, matrigel-coated transwell chambers were used to investigate the invasive capacities of prostate cancer cells. Invasion assay is an in vitro assay which simulates the in vivo invasiveness and metastasis of cancer cells. As shown in Fig. 5A, PC-3 cells treated with siRNA1 and siRNA2 decreased cell invasion up to 35% and 32%, respectively. This result is in accordance with the finding in the migration assay (Fig. 4A). The same effect on the invasion capability was also observed in PC-3 cells in the presence of RANKL (Fig. 5B). siRNA1 and siRNA2 inhibited cell invasion up to 47% and 27%, respectively. We also performed the migration and invasion assays on DU-145 cells and found similar results (data not shown). These results indicate that IKKα siRNA can inhibit the invasiveness of prostate cancers, and the inhibition effect is more significant in the presence of RANKL.
Fig. 5.
Silencing of IKKα inhibits PC-3 cell invasion in the absence of RANKL (a) and in the presence of RANKL (b). The procedure for cell invasion assay is similar to migration assay except that the trans-well was pre-coated with 100 µl of Matrigel (0.5 mg/ml) for 4 h at room temperature. The cells that invaded to the other side of the membrane were fixed and stained, while un-invaded cells were mechanically removed. Images of invaded cells were obtained at 100× magnification using an inverted microscope. Number of cells invaded was counted from four random fields of the well to calculate the extent of invasion. (* p<0.05; ** p<0.01).
Effect of IKKα siRNA on Cell Attachment to ECM
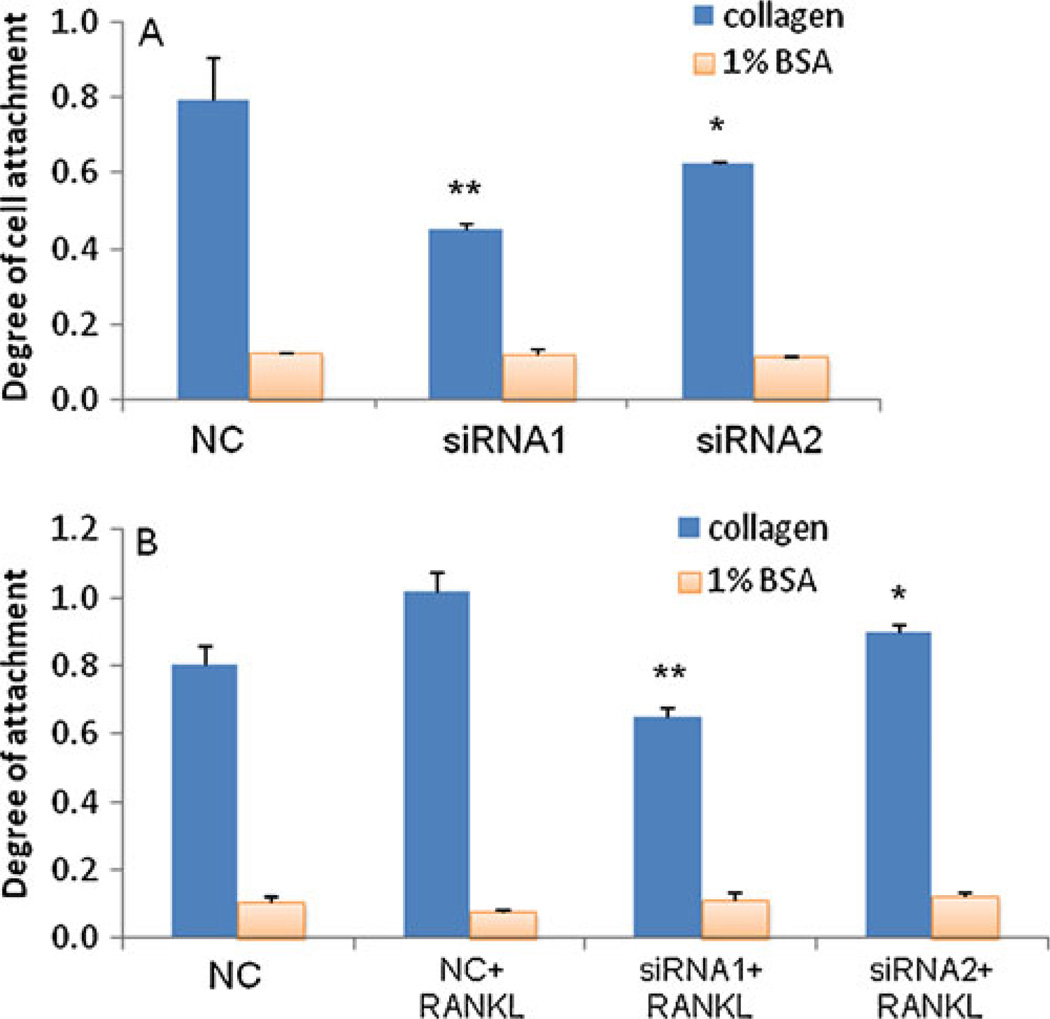
The importance of IKKα in cell migration and invasion suggests that it may regulate cell adhesion to ECM. Therefore, we examined the adhesion ability of prostate cancer cells to ECM, which is an important early step in the invasion and metastasis of cancer cells from its origin to the target tissue. This assay measures the interaction between tumor cells and extracellular matrix that is the major composition of cell membrane and tissue. Changes in the integrity of the basement membrane and the structure of the extracellular membrane favor the invasion and metastasis of cancer cells (18). PC-3 cells transfected with siRNA were incubated in a 96-well plate coated with type-I collagen protein, a major component of ECM. As Fig. 6A indicated, siRNA1 and siRNA2 dramatically inhibited the adhesion of PC-3 cells to type I collagen. Plates coated with BSA were used as the negative control, and all cells showed negligible adhesions. We also investigated the effect of RANKL on the cell adhesion ability. As shown in Fig. 6B, RANKL increased the adhesion of PC-3 cells to type I collagen. In the presence of RANKL, both siRNA1 and siRNA2 reduced the attachment of PC-3 cells to type I collagen in comparison to the scrambled siRNA. Similar results were found with DU-145 cells (data not shown). These results further demonstrated the inhibitory effect of IKKα siRNA on the metastatic properties of prostate cancer cells.
Fig. 6.
Silencing IKKα inhibits cell attachment. (A) Inhibition of cell attachment in the absence of RANKL. Cells were transfected with siRNA (50nM) using Lipofectamine-2000 for 24 h. Forty-eight hours after the transfection, the cells were trypsinized, re-suspended, and plated in a 96-well plate which was pre-treated with 50 µl of type I collagen (30 µg/ml) or 1% BSA. Cells were allowed to attach at 37°c for 1 hr. The attached cells were fixed and stained with crystal violet, followed by washing 4–5 times with DPBS. The attached cells were lysed with 100 µl of 1% SDS, followed by measuring the absorbance at 595 nm. (B) Inhibition of cell attachment in the presence of RANKL. Cells were transfected with siRNA for 24 h and then incubated with RANKL for another 24 h. Results are represented as mean ± SD (n=3). (* p<0.05; ** p<0.01).
Effect of IKKα siRNA on Cell Proliferation and Cell Cycle Distribution
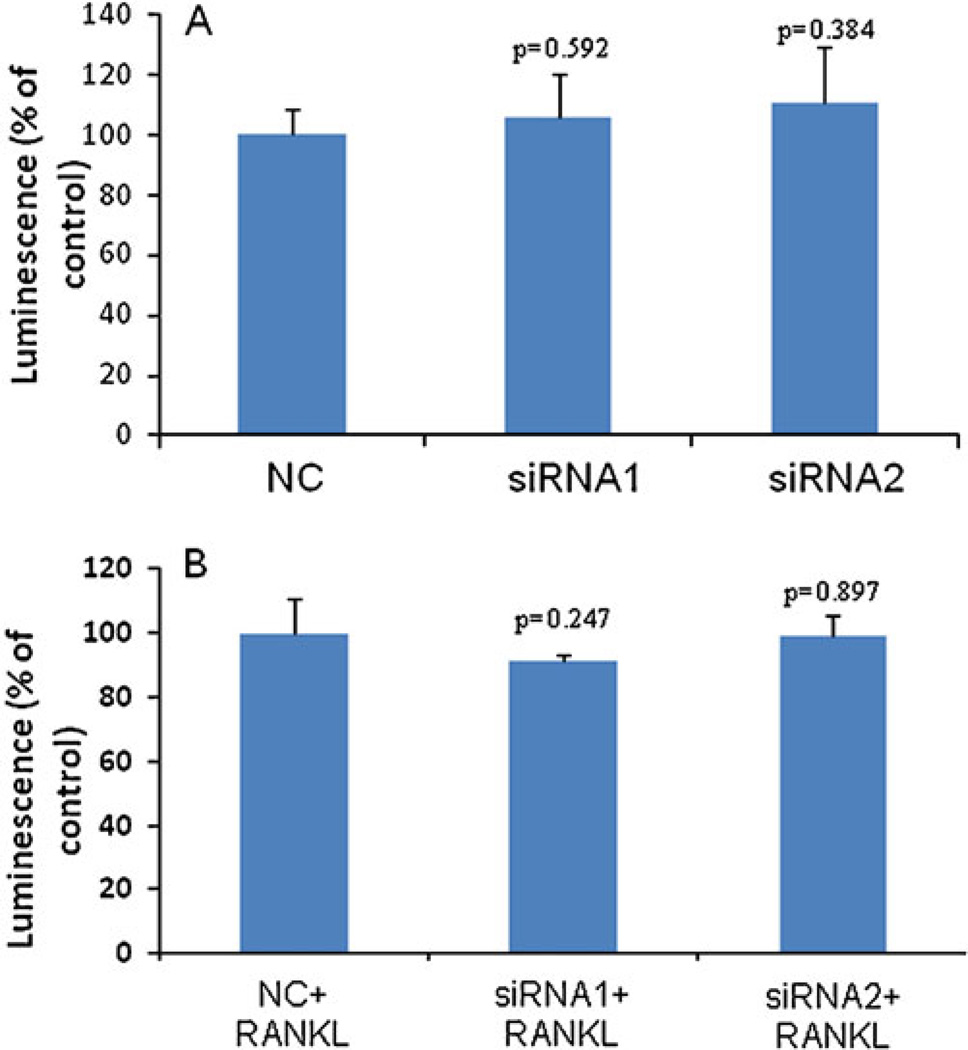
Having determined that silencing IKKα leads to inhibition of invasiveness, we next sought to determine whether the IKKα siRNAs suppress the proliferation of prostate cancer cells. PC-3 cells were treated with the IKKα siRNA, and the cell proliferation was measured by the CellTiter-Glo Luminescent Cell Viability Assay Kit at 96 h post-transfection. Compared to cells transfected with the scrambled siRNA, cells treated with IKKα siRNAs (siRNA-1 and siRNA-2) exhibited similar growth rates and viabilities (Fig. 7a). A similar result was also observed in PC-3 cells in the presence of RANKL (Fig. 7b). The experiment performed in DU-145 cells gave a similar result (data not shown).
Fig. 7.
Effect of IKKα siRNA on cell proliferation in the absence of RANKL (A) and in the presence of RANKL (B). PC-3 cells were plated in 96-well plates at a density of 10,000 cells/well and transfected with IKKα siRNAs for 24 h. Ninety-six hours after the transfection, cell proliferation was assayed using the CellTiter-Glo Luminescent cell viability kit. Results were represented as mean ± SD (n=3).
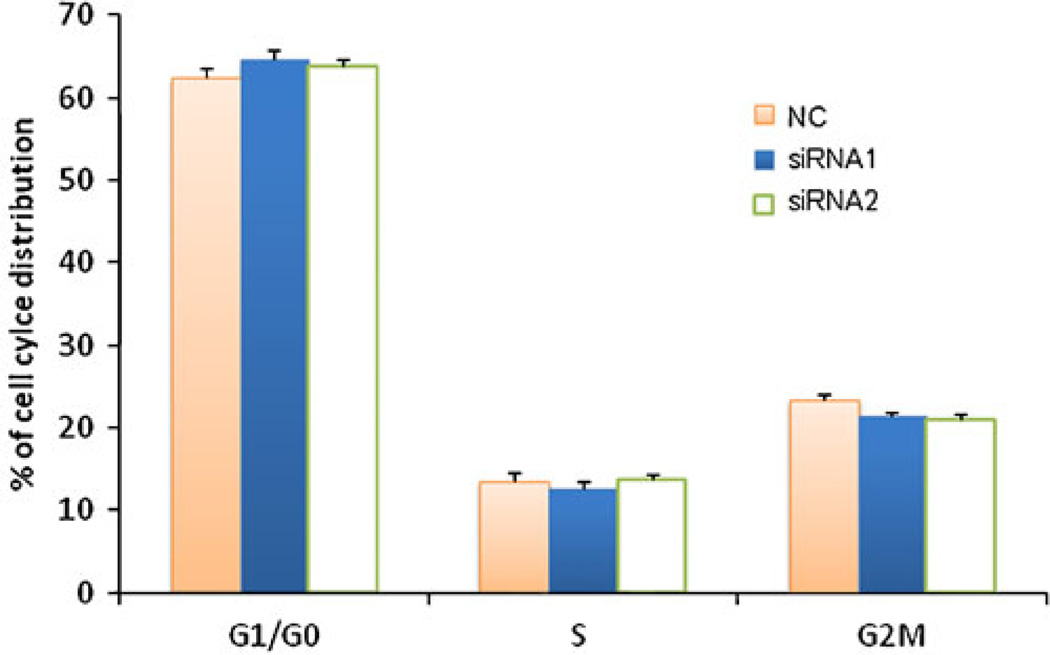
We further examined if knockdown of IKKα leads to changes in the cell cycle distribution of prostate cancer cells. As Fig. 8 showed, cells transfected with IKKα siRNAs induced a minor block in G1/G0 phase distribution compared to scrambled siRNA-treated cells. The percentages of cells in G1/G0 phase were 64.69% and 63.78% for cells treated with siRNA-1 and siRNA-2, respectively. In comparison, 62.39% of cells treated with scrambled siRNA were in G1/G0 phase. However, this difference in G1/G0 phase is not significant (p=0.09). Accordingly, there was a slight, but not significant, reduction in G2/M distribution in both siRNA-1- and siRNA-2-treated cells. This result is consistent with the cell proliferation data (Fig. 7). Taken together, these results demonstrated that IKKα silencing has a negligible effect on prostate cancer cell proliferation. This is in accordance with a recent finding that IKKα does not induce cell proliferation in breast cancer cells (19).
Fig. 8.
Effect of IKKa silencing on cell cycle distribution. Cell cycle distributions were determined by flow cytometry in PC-3 cells at 48 h post-transfection. The results were represented as mean ± SD (n=3).
DISCUSSION
NF-κB is an important transcriptional factor which controls a variety of biological processes including inflammation, cell survival or death, and cell cycle. It is notable that aberrant NF-κB activation can lead to tumorigenesis (20). For example, suppression of NF-κB by adenoviral vector that contains the IκB gene (an inhibitor of NF-κB) reduces the clonogenicity of prostate cancer cells (21). IKKα is an essential regulator of the NF-κB pathway, and it is reported to mediate NF-κB downstream gene expressions by modulating the promoter regions of NF-κB-regulated genes in the nucleus (8,22). Activation of the IKK complex is therefore a crucial step in the activation of NF-κB. As one of the catalytic kinases in the classic IKK complex, IKKα was recently identified as a key mediator in the inflammation and metastasis of prostate cancer via down-regulating the tumor suppressor gene Maspin that is a serpin peptidase inhibitor to block tumor metastasis (4,5,7). On the other hand, several lines of evidence implicate that NF-κB and NF-κB-related IκB kinases are involved in cell invasion and tumor metastasis (23,24). Therefore, inhibiting IKKα is a promising strategy to treat prostate cancer. In this study, we demonstrated for the first time that IKKα siRNA markedly decreases invasiveness of prostate cancer cells, even in the presence of RANKL, an activator of IKKα. Our findings further support the hypothesis that IKKα plays an important role in metastasis of prostate cancer.
First, we examined the silencing effect of three predesigned siRNAs in prostate cancer cells using real time RT-PCR and western blot. Two potent siRNAs, siRNA-1 and siRNA-2, were identified to investigate their biological effects on the tumor phenotypes of prostate cancer cells. It is believed that IKKα suppresses the promoter of the Maspin gene, and the expression level of Maspin in normal epithelia cells is much higher than that in malignant cells (4). We then analyzed the effect of the IKKα siRNA on the Maspin expression in prostate cancer cells. Significant up-regulation of the Maspin was observed in PC-3 cells treated with the IKKα siRNA (Fig. 2C), which is in agreement with the observation that IKKα inhibits Maspin (4).
We next conducted numerous experiments, including wound-healing assay, migration assay, and invasion assay, to access the effects of IKKα siRNA on the invasiveness properties of prostate cancer cells. Our studies showed that silencing of IKKα dramatically reduced the would-healing process of prostate cancer cells (Fig. 3). When wounded, cells respond to the disruption by healing the wound through a combination of migration and proliferation (25). The cells on the edge of the wound move toward the gap until new cell-cell contacts are reestablished (26). Therefore, the capability of cancer cells to heal an open wound predicts their migratory ability in metastasis.
We further evaluated the migratory abilities of prostate cancer cells using a Boyden chamber assay to quantify the migration and invasion potential of prostate cancer cells upon the silencing of IKKα. As Figs. 4 and 5 showed, IKKα siRNAs significantly inhibited the migration and invasion capabilities of PC-3 cells. These results are in full accordance with a recent finding that IKKα promotes prostate cancer metastasis (4). Using a transgenic mouse model of prostate cancer with an inactive IKKα, Luo and colleagues observed slow cell growth and inhibited metastatogenesis. IKKα exerted its pro-metastatic effect by suppressing the transcription of the Maspin gene, which is an NF-κB-independent regulation. In addition, they found that activated nuclear IKKα correlated with the metastatic progression of prostate cancer, and the expression of IKKα is the highest in stage 4 tumors which do not express Maspin (4). A similar anti-invasion effect was observed in breast cancer cells upon the knockdown of IKKα. Inhibition of IKKα also dramatically reduced the NF-κB activation (19).
Several million cancer cells per gram of the tumor tissue can be dissociated and released into the blood and lymphatic circulations on a daily basis (27). In order to metastasize to a new place, the disseminated cancer cells must re-establish an adhesive connection to the endothelium in the target tissues (28). Metastasis is mediated through the expression of numerous adhesion molecules, such as integrin, (29) ICAM-1, VCAM-1, and ELAM-1 (30), where the later ones are regulated by NF-κB. Therefore, we performed cell attachment assay and observed reduced attachment of PC-3 cells to ECM after the treatment with IKKα siRNA (Fig. 6a). A similar result was also observed in DU-145 cells (data not shown). The implication of integrin in cellular functions is based on the type of integrins. For example, α2β1 and α3β1 integrins mediated epithelial cell adhesion to basal lamina (29) and are down-regulated in carcinomas (31,32). On the contrary, αvβ3 (33) and α6β4 (34) integrins are up-regulated in carcinomas where they promote migration, invasion, and proliferation.
Since the infiltrating immune cells expressing RANKL induce activation and nuclear translocation of IKKα in prostate cancer cells to inhibit the tumor suppressor gene Maspin, we investigated whether IKKα siRNA can exert the same anti-invasive activity in the presence of RANKL. As shown in Figs. 4B, 5B and 6B, inhibiting IKKα expression reduced the stimulation effect of RANKL on cell migration, invasion and attachment to ECM. Expression of RANKL is low in normal cells, but significantly high in prostate cancers. It is also reported that androgen-independent prostate cancer cells exhibit a higher level of RANKL compared to androgen-dependent cells (35). In addition, most of the androgen-independent prostate cancers express IKKα. Consequently, silencing IKKα is a promising strategy to reverse the metastatic effect caused by RANKL, especially in the androgen-independent prostate cancer cells.
Tumor metastasis requires that the neoplastic cells have the capability to interconvert genetically between proliferative and invasive phenotypes, or just express both the phenotypes simultaneously (36,37). IKKα serves as a promoter for tumor metastasis in prostate cancer and regulates the inflammation through an independent pathway (38). Certain oncogenes, such as RAS, MAPK (mitogen-activated protein kinase) and MYC family members, cause remodeling of the tumor microenvironment through recruitment of leukocytes, expression of tumor-promoting chemokines and cytokines, or induction of an angiogenic switch (39,40). Invasive cells signal through the MAPK pathway, whereas highly proliferative cells use the Myc pathway. The Ras-MAPK and phosphatidylinositol 3-kinase-Akt (PI3K-Akt) pathways are associated with the phenotypes of invasive cells, but not the highly proliferative cells (36). Our results suggest that IKKα may regulate tumor invasion by the Ras-MAPK and PI3K-Akt pathways.
The effect of IKKα on tumor cell proliferation is contradictive (19,41). Karin and colleagues explored the role of IKKα in breast cancer and demonstrated that IKKα is a regulator of mammary epithelial proliferation (41). However, a contrary result was observed in breast cancer by Baldwin and his group (19). We performed the proliferation assay in PC-3 cells after the suppression of IKKα with siRNA. As Fig. 7 showed, there was no significant reduction in cell proliferation in the cells (in the absence or presence of RANKL) treated with IKKα siRNA in comparison to the control group. This result is in agreement with a recent report that knockdown of IKKα did not affect the proliferation of breast cancer cells, but caused a significant reduction in cancer cell invasion (19). To further confirm these results, we performed cell cycle analysis. Although we observed a slightly high distribution in G1/G0 phase in the cells treated with IKKα siRNA, the difference is not statistically different from the control group. These results suggested that IKKα siRNA has a negligible effect on cell proliferation of PC-3 cells.
In conclusion, results from this study provided significant evidence that IKKα plays a major role in the invasion and metastasis of prostate cancer cells; however, it poorly affects the prostate cancer cell proliferation. Considering the fact that the major risk faced by prostate cancer patients is the development of metastasis (1), the IKKα siRNA may provide a very promising approach for prostate cancer therapy.
ACKNOWLEDGMENTS
This project has been supported by a grant (1R21CA143683-01) from the National Cancer Institute at NIH. We also would like to express thanks for the financial support from a start-up package at the University of Missouri-Kansas City.
REFERENCES
- 1.Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9:115–139. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- 2.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 3.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 4.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 5.Affara NI, Coussens LM. IKKalpha at the crossroads of inflammation and metastasis. Cell. 2007;129:25–26. doi: 10.1016/j.cell.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Qing G, Xiao G. Essential role of IkappaB kinase alpha in the constitutive processing of NF-kappaB2 p100. J Biol Chem. 2005;280:9765–9768. doi: 10.1074/jbc.C400502200. [DOI] [PubMed] [Google Scholar]
- 7.Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14:5656–5662. doi: 10.1158/1078-0432.CCR-08-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 10.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 12.Song LL, Peng Y, Yun J, Rizzo P, Chaturvedi V, Weijzen S, et al. Notch-1 associates with IKKalpha and regulates IKK activity in cervical cancer cells. Oncogene. 2008;27:5833–5844. doi: 10.1038/onc.2008.190. [DOI] [PubMed] [Google Scholar]
- 13.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 15.Cheng K, Mahato RI. Gene modulation for treating liver fibrosis. Crit Rev Ther Drug Carrier Syst. 2007;24:93–146. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng K, Qin B. RNA Interference for cancer therapy. In: Lu Y, Mahato R, editors. Pharmaceutical perspectives of cancer therapeutics. AAPS-Springer publishing program; 2009. pp. 399–440. [Google Scholar]
- 17.Tai W, Qin B, Cheng K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm. 2010;7:543–556. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 18.Hagedorn HG, Bachmeier BE, Nerlich AG. Synthesis and degradation of basement membranes and extracellular matrix and their regulation by TGF-beta in invasive carcinomas (Review) Int J Oncol. 2001;18:669–681. doi: 10.3892/ijo.18.4.669. [DOI] [PubMed] [Google Scholar]
- 19.Merkhofer EC, Cogswell P, Baldwin AS. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–1248. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 21.Pajonk F, Pajonk K, McBride WH. Inhibition of NF-kappaB, clonogenicity, and radiosensitivity of human cancer cells. J Natl Cancer Inst. 1999;91:1956–1960. doi: 10.1093/jnci/91.22.1956. [DOI] [PubMed] [Google Scholar]
- 22.Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung B, Pandey MK, Nakajima Y, Nishida H, Konishi T, Chaturvedi MM, et al. Identification of a novel blocker of IkappaBalpha kinase activation that enhances apoptosis and inhibits proliferation and invasion by suppressing nuclear factor-kappaB. Mol Cancer Ther. 2008;7:191–201. doi: 10.1158/1535-7163.MCT-07-0406. [DOI] [PubMed] [Google Scholar]
- 24.Lee TK, Poon RT, Wo JY, Ma S, Guan XY, Myers JN, et al. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007;67:8800–8809. doi: 10.1158/0008-5472.CAN-07-0801. [DOI] [PubMed] [Google Scholar]
- 25.Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 27.Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–516. [PubMed] [Google Scholar]
- 28.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol. 2008;3:221–247. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- 30.van de Stolpe A, Caldenhoven E, Stade BG, Koenderman L, Raaijmakers JA, Johnson JP, et al. 12-O-tetradecanoylphorbol-13- acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269:6185–6192. [PubMed] [Google Scholar]
- 31.Ivaska J, Nissinen L, Immonen N, Eriksson JE, Kahari VM, Heino J. Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Mol Cell Biol. 2002;22:1352–1359. doi: 10.1128/mcb.22.5.1352-1359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens DM, Watt FM. Influence of beta1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: alpha3beta1, but not alpha2beta1, suppresses malignant conversion. Cancer Res. 2001;61:5248–5254. [PubMed] [Google Scholar]
- 33.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion—lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 36.Gao CF, Xie Q, Su YL, Koeman J, Khoo SK, Gustafson M, et al. Proliferation and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA. 2005;102:10528–10533. doi: 10.1073/pnas.0504367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence T, Bebien M. IKKalpha in the regulation of inflammation and adaptive immunity. Biochem Soc Trans. 2007;35:270–272. doi: 10.1042/BST0350270. [DOI] [PubMed] [Google Scholar]
- 39.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 40.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci USA. 2007;104:15852–15857. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]