Abstract
Background
Dental erosion is a complication of gastro-oesophageal reflux disease (GORD) according to the Montreal consensus statement. However, GORD has not been comprehensively characterized in patients with dental erosions and pH-impedance measures have not been reported.
Objectives
Characterize GORD in patients with dental erosions using 24-h multichannel intraluminal pH-impedance measurements (pH-MII) and endoscopy.
Methods
This single-centre study investigated reflux in successive patients presenting to dentists with dental erosion using pH-MII and endoscopy.
Results
Of the 374 patients, 298 (80%) reported GORD symptoms <2 per week, 72 (19%) had oesophagitis and 59 (16%) had a hiatal hernia. In the 349 with pH-MII the mean percentage time with a pH <4 (95% CI) was 11.0 (9.3–12.7), and 34.4% (31.9–36.9) for a pH <5.5, a critical threshold for dental tissue. The mean numbers of total, acidic and weakly acidic reflux episodes were 71 (63–79), 43 (38–49) and 31 (26–35), respectively. Of the reflux episodes, 19% (17–21) reached the proximal oesophagus. In 241 (69%) patients reflux was abnormal using published normal values for acid exposure time and reflux episodes. No significant associations between the severity of dental erosions and any reflux variables were found. The presence of GORD symptoms and of oesophagitis or a hiatal hernia was associated with greater reflux, but not with increased dental erosion scores.
Conclusions
Significant oligosymptomatic gastro-oesophageal reflux occurs in the majority of patients with dental erosion. The degree of dental erosion did not correlate with any of the accepted quantitative reflux indicators. Definition of clinically relevant reflux parameters by pH-MII for dental erosion and of treatment guidelines are outstanding. Gastroenterologists and dentists need to be aware of the widely prevalent association between dental erosion and atypical GORD.
Keywords: Tooth enamel, extra-oesophageal gastro-oesophageal reflux, pH-impedance, tooth pellicle, gastric acid
Introduction
Gastro-oesophageal reflux (GORD) is associated with extra-oesophageal pathology, as defined by the Montreal global consensus statement on GORD.1 Dental erosion, the chemical dissolution of enamel without bacterial involvement, is considered to be an established complication of GORD. Patients with dental erosion are divided into two groups: those with predominantly oesophageal reflux symptoms who are primarily consulting physicians, and those with dental or oral symptoms or signs presenting to dentists. It is unclear if these groups are comparable regarding their reflux characteristics, but it is likely that patients primarily presenting to dentists have more silent or oligosymptomatic reflux. The reported prevalence of dental erosion varies between 17–68% of patients with symptomatic GORD, and GORD has been demonstrated in 25–83% of patients presenting with dental erosion, many of whom are children.2–17 The prevalence of distinct erosive tooth wear in 3187 European adults aged 18–35 years was 29%.18 Median prevalence rates of 24% for dental erosion in GORD patients and of 32.5% for GORD in dental erosion patients have been suggested in an earlier review.19 The large prospective, longitudinal ProGERD study demonstrated extra-oesophageal reflux-associated disorders in 32.8% of 6215 patients with heartburn and in 34.9% of patients with erosive GORD.20 However, we are not aware of any comprehensive characterization of reflux with pH-impedance monitoring in oligosymptomatic patients with dental erosion.
GORD is confirmed using oesophageal pH-metry, and proximal oesophageal and weakly acidic reflux can now be quantified with 24-h multichannel intraluminal pH-impedance measurements (pH-MII). Normal values have been established for diagnostic and treatment purposes, which include the percentage of time with a pH <4, and the number and duration of acidic and weakly acidic reflux episodes in the distal and proximal oesophagus.21,22 Current guidelines relate primarily to oesophageal GORD and not to extra-oesophageal pathology, including dental erosion, where different tissue sensitivity applies and weakly or non-acidic and proximal reflux will be of greater importance. Consequently, the association between gastro-oesophageal reflux and dental erosion remains poorly defined and no diagnostic, prevention or treatment guidelines exist.
The development of dental erosion secondary to repeated or prolonged exposure to gastric secretions comprises three main phases: the loss of the protective tooth pellicle, the demineralization of enamel at a pH of 5.5 and below, dependent on the local availability of calcium and phosphate in saliva or in the solution surrounding the teeth (e.g. gastric juice), and the advanced structural matrix changes in the underlying dentine with open dentinal tubules23,24 (Figure 1). It should be noted that a minor degree of tooth wear is physiological with age and that a tool for quantifying pathological erosive tooth wear, the Basic Erosion Wear Examination (BEWE), has recently been validated.25 The consequences of dental erosion with permanent loss of tooth substance are hypersensitivity and functional and aesthetic impairment. Figure 2 shows the destruction of teeth due to nocturnal reflux. The costs of dental reconstructive work relating erosion can easily reach several €10,000s.
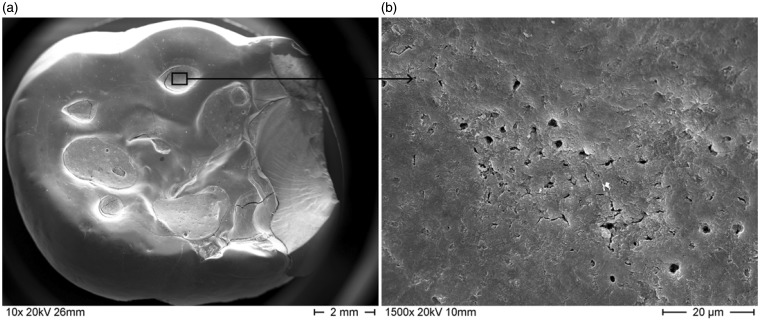
Figure 1.
Scanning electron microscope photographs of a human molar tooth with (a) dental erosion; (b) detail of the affected area clearly showing the exposed and open dentinal tubules.
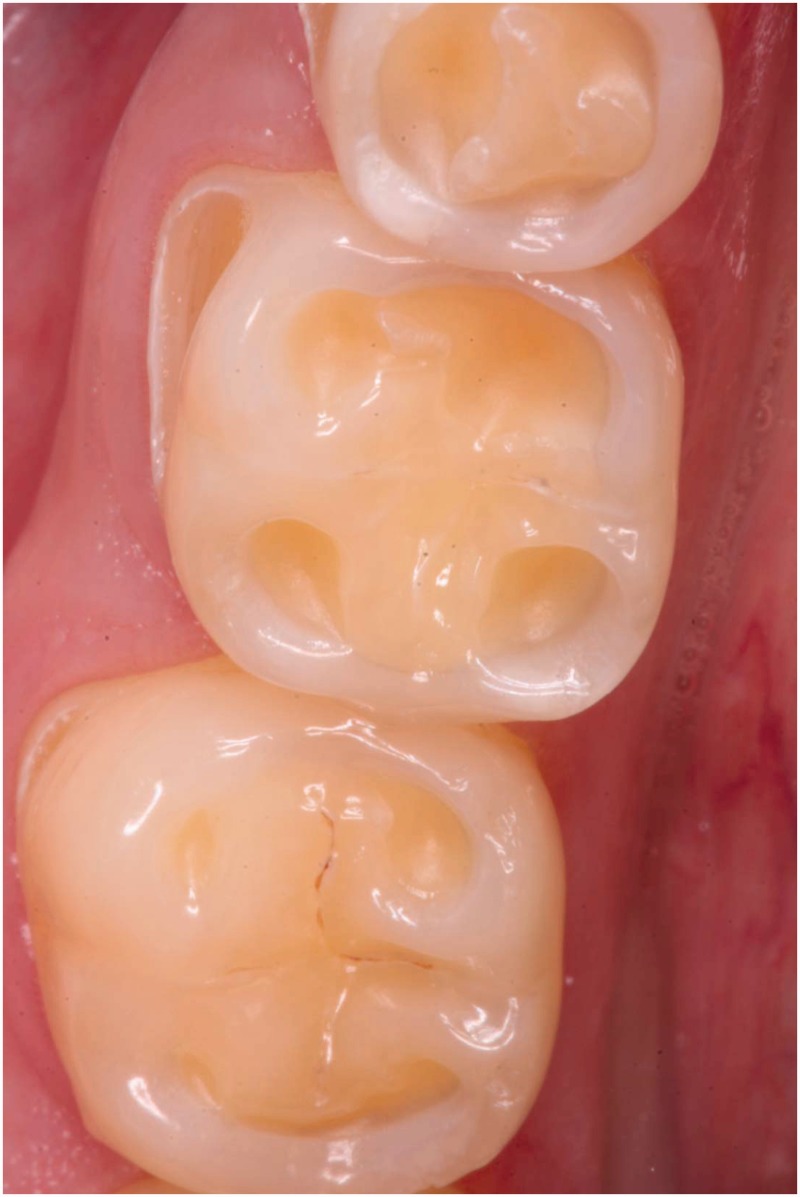
Figure 2.
Lower molar and premolar teeth of a patient with nocturnal reflux. The normal occlusal morphology is replaced by grooves. The exposed dentin (yellowish parts) caused hypersensitivity. The buccal aspects also show severe erosive tooth wear.
The aims of the current large, observational study in patients presenting to dentists with dental erosion were to characterize GORD using endoscopy and oesophageal pH-impedance testing and to investigate associated factors.
Methods
Consecutive patients presenting to the Department of Preventive, Restorative and Pediatric Dentistry, University of Bern, and affiliated dentists between 2009 and 2012 with dental erosion, defined by a Lussi score >1 or a BEWE >8, were referred to the Gastroenterology Group Practice for evaluation of GORD after exclusion of non-reflux causes of erosion by detailed medical history, dental examination, a standardized dietary diary, and measurement of salivary flow and buffering capacity using standard procedures.25,26 Patients with a history of bruxism, eating disorders, recurrent vomiting, severe obesity (BMI > 35 kg m−2) or past bariatric surgery (because of probably increased GORD prevalence), and dietary or abrasive causes for dental erosion were excluded. All patients were seen by a dedicated team of dentists experienced in the diagnosis of erosion, and the severity of erosion was graded using the BEWE once this was clinically implemented in 2012.25 Briefly, the BEWE is a simple scoring system based on the Lussi scoring and quantifies the size of a given lesion as a percentage of the surface affected. All teeth (vestibular, occlusal and palatal surfaces) except third molars are graded. The dentition is divided into sextants, the most severe score in a sextant is recorded, and a cumulative score from all sextants (maximum = 18) is calculated and represents the index value. Further, high-quality photographs of the teeth are recorded. Upon referral, every patient was examined by the same senior gastroenterologist (CWS), reflux symptoms were assessed by interview and the Reflux Disease Questionnaire and oesophago-gastro-duodenoscopy with gastric and, if clinically indicated, oesophageal biopsies and subsequent 24-h oesophageal pH-MII (Ohmega, MMS, Enschede, Holland) were performed.27 The single-use pH-MII catheter with impedance measurement sites at 3, 5, 7, 9, 15 and 17 cm and a distal pH sensor at 5 cm (pHersaflex, Sierra Scientific Instruments, Los Angeles, USA) above the lower oesophageal sphincter was introduced transnasally after an overnight fast and placed with the pH sensor at 5 cm proximal to the oesophago-gastric junction. Patients were instructed to perform their normal daily activities, eat and drink as usual, and document lying down, eating and drinking or reflux symptoms using the datalogger’s event markers. Any patients on antisecretory medication discontinued their dosing 10 days before the pH-MII recording. The numbers of all (pH <7), acidic (pH <4) and weakly acidic (pH > 4 and < 7) reflux episodes, the percentage time with pH <4 and <5.5, the percentage of proximal reflux episodes (reaching 15 cm above the gastro-oesophageal junction) in the total 24 h, the DeMeester score and the symptom associations by symptom association probability (SAP) >95% were analysed.21,28 Patients with a pH <4 for more than 5% of the measurement period or more than 75 total (acidic plus weakly acidic), 50 acidic and 33 weakly acidic reflux episodes at 5 cm and more than 30 total reflux episodes at 15 cm above the gastro-oesophageal junction were considered to have increased reflux.22 As this was a study analysing coded clinical data with no additional research-related procedures, no Institutional Ethics Board approval was required at the time. All authors had access to the study data and reviewed and approved the final manuscript.
Statistical analysis
Analyses were performed with the statistical Software R, Version 2.15.1 (http://www.R-project.org, Revolution Analytics, Vienna, Austria, 2008). The mean and 95% confidence intervals of normally distributed variables, and medians and interquartile ranges of non-normally distributed or non-continuous data are shown. The association between the percentage time with pH <4 and <5.5, age, the total, acid and weakly acid number of reflux episodes, the percentage of reflux episodes reaching 15 cm above the lower oesophageal sphincter (proximal reflux), the DeMeester score, and the BEWE score were assessed in those with complete data using the Spearman rank correlation test. The relationship between the presence of a hiatal hernia (yes/no), gender, the presence of GORD symptoms more than twice per week (yes/no) and BEWE scores were assessed using the exact Wilcoxon test for dichotomous variables. As this study was the first impedance study in dental patients, the analysis was explorative and correction for multiple testing was not performed. In case of significant associations of the above variables with erosion grades a multivariate analysis was planned. However, as none were found, this analysis was not performed.
Results
A total of 374 successive patients were accrued from January 2008 to December 2012 and endoscopy was performed in all. pH-impedance was evaluable in 349 patients, as 15 patients had poor tolerance of the catheter, but their endoscopic data is included in the analysis. Patient gastrointestinal and dental characteristics are shown in Table 1.
Table 1.
Baseline characteristics of 349 successive patients presenting with dental erosion and with 24-h intraluminal pH-impedance measurements
| Baseline characteristic | Value |
|---|---|
| Gender: male/female (n) | 222/127 |
| Age: years (mean, 95% CI) | 35.1 (33.9–36.3) |
| GORD symptoms >2 per week (n) | 76 (20%) |
| Reflux disease questionnaire score (median (interquartile range)) | 3 (1–5) |
| Previous PPI use | 0 |
| BEWE score (median and interquartile range)* | 13 (11–16) |
Available in last 162 patients. BEWE, basic erosive wear examination25 (values of 9–13 are considered medium, >13 as extensive erosive disease); GORD, gastro-oesophageal reflux disease; PPI, proton pump inhibitor.
Reflux symptoms
The median RDQ scores are shown in Table 1. Scores between 0 and 6 were reported in 275 patients (74%) and scores between 7 and 14 in 99 patients (26%). Of all patients, 274 (73%) had regular reflux symptoms less than once per week, 24 (6%) had symptoms between once and twice per week and 76 (20%) had reflux symptoms more than twice per week. None had previously received proton pump inhibitors.
Endoscopic findings
A hiatal hernia was found in 59 patients (16%), oesophagitis in 72 (19%) (grade A in 69, grade B in 3 according to Los Angeles classification), a gaping cardia in 48 (13%), gastritis in 19 (5%) and Helicobacter pylori in 4 (1%) patients.
pH-impedance results
Table 2 shows the group results of pH-impedance measurements in the 349 patients. The numbers of patients with abnormal results compared to published normal values in Western Europeans are shown in Table 3.22 Using the individual reflux criteria, 31–56% had abnormal reflux. If any of the classic published reflux criteria were applied, i.e. the percentage time with a pH <4 or the number of reflux episodes, 241 patients (69%) had abnormal reflux. Only three patients reported any GORD symptoms during pH-MII, obviating calculation of symptom associations.
Table 2.
pH-impedance results in patients presenting with dental erosion. Means and 95% confidence intervals are shown
| Result | (n = 349) |
|---|---|
| Time with pH <4 (%) | 11.0 (9.3–12.7) |
| Time with pH <5.5 (%) | 34.4 (31.9–36.9) |
| DeMeester score | 44.1 (25.5–62.7) |
| Number of all reflux episodes (pH <7) | 71 (63–79) |
| Number of acidic reflux episodes (pH <4) | 43 (38–49) |
| Number of weakly acidic reflux episodes (pH >4–pH <7) | 31 (26–35) |
| Percentage proximal reflux (15 cm above lower oesophageal sphincter) | 19 (17–21) |
Table 3.
Number and percentage of 349 patients with increased pH and impedance results compared to published normal values for oesophageal GORD.22
| Number (%) | |
|---|---|
| Increased percentage time with pH <4 | 193 (55) |
| Increased DeMeester score | 198 (56) |
| Increased number of all reflux episodes (pH <7) | 148 (42) |
| Increased number of acidic reflux episodes (pH <4) | 167 (48) |
| Increased number of weakly acidic reflux episodes (pH >4–pH <7) | 120 (35) |
| Increased number of proximal reflux episodes | 108 (31) |
| Any of the following increased: percentage time with pH <4, or number of all, acidic, or weakly acidic reflux episodes | 241 (69) |
Distal’ refers to 5 cm, and ‘proximal’ to 15 cm above the lower oesophageal sphincter. Any of the following increased: percentage time with pH <4, or number of all, acidic, or weakly acidic reflux episodes 241 (69%).
Associations between gastrointestinal variables and dental erosion
The median BEWE score (IQR) was 13 (11–16) in the 162 patients scored when the new score became available. There was no significant correlation between BEWE scores and the percentage time with pH <4 or with pH <5.5, and the total, acidic, weakly acidic and proximal numbers of reflux episodes (all r <0.12 and p >0.39) in these 162 patients. The post hoc dichotomous analysis showed corresponding results, as the median BEWE scores were not significantly different in patients with normal (<5%) or increased percentage time with pH <4 (12.0 (11–15) and 12.5 (11–15), respectively, p = 0.76), or with normal (<75) or increased total number of reflux episodes (13 (10–16) and 12 (10–14), respectively, p = 0.26). The only variable with any significant, albeit weak, correlation with the dental erosion grading was age (r = 0.17, p = 0.04).
The percentage time with pH <4 and with pH <5.5 were highly positively correlated (r = 0.76, p < 0.0001) in all 349 patients with pH-MII measurement, as the first variable is a subset of the second. There was a close correlation between the total number of reflux episodes and the numbers of acid (r = 0.82, p < 0.0001), weakly acid (r = 0.78, p < 0.0001) reflux episodes and a weak correlation with the percentage of proximal reflux (r = 0.17, p = 0.04), indicating a parallel increase of all types of reflux episodes.
Post hoc analysis showed that patients with GORD symptoms more than twice per week had a significantly greater time with a distal pH <4 and <5.5, as well as proximal reflux, but similar median BEWE scores (p = 0.26) to those with symptoms less than twice per week (Table 4). Patients with endoscopic demonstration of a hiatal hernia or oesophagitis had a significantly greater time with a distal pH <4 and <5.5 than those without, but their median BEWE scores were similar (p = 0.25) (Table 4). Gender did not have a significant association with BEWE scores (p = 0.29).
Table 4.
pH-impedance results and dental erosion grades (BEWE) in patients sub-grouped according to clinical presentation
| Frequency of GORD symptoms |
Presence of oesophagitis |
|||
|---|---|---|---|---|
| <2/week (n = 273) | >2/week (n = 76) | No (n = 277) | Yes (n = 72) | |
| Percentage time with pH <4* | 8.9 (7.0–10.8) | 16.8 (14.4–19.2)1 | 9.4 (10.7–8.1) | 13.4 (11.1–15.7)1 |
| Percentage time with pH <5.5* | 33.8 (31.5–36.1) | 38.5 (36.1–40.9)2 | 29.1 (25.8–32.4) | 40.8 (35.4–46.2)1 |
| All reflux episodes* | 71 (60–81) | 71 (51–91) | 71 (61–80) | 68 (52–83) |
| Acidic reflux episodes* | 43 (36–50) | 44 (30–58) | 43 (36–49) | 39 (29–48) |
| Weakly acidic reflux episodes* | 31 (25–37) | 27 (17–36) | 31 (26–36) | 29 (18–40) |
| Percentage proximal reflux episodes* | 17 (14–20) | 27 (25–29)2 | 18 (15–21) | 24 (20–28) |
| BEWE score# (n = 162) | 13 (10–16) (n = 130) | 12 (10–14) (n = 32) | 12 (10–16) (n = 132) | 14 (12–17) (n = 30) |
With versus without p < 0.001.
with versus without p < 0.05. *means and 95% confidence intervals. #medians and interquartile ranges.
Discussion
This large series of endoscopic and pH-MII examinations revealed significant, but largely silent gastro-oesophageal reflux in patients presenting to dentists with dental erosions. Only 20% of all patients reported minor GORD symptoms more than twice per week, 73% experienced GORD less than once per week and none reported previous PPI use. This low level of GORD symptoms was reflected in the very low median RDQ score of 3. Endoscopic findings of reflux were within the range of previous studies in healthy volunteers, except for a slightly higher rate of oesophagitis (19%).29,30 Distal and proximal, acidic and weakly-acidic reflux were increased in between 31–56% of patients with dental erosion (Table 3) using normal values established for oesophageal reflux disease in Western Europeans.22 If any of the classic published reflux criteria were applied, i.e. the percentage time with a pH <4 or the number of reflux episodes, 69% of patients had abnormal reflux. No significant associations between the severity of dental erosions and any reflux variables were found. Age correlated very weakly, albeit significantly with the BEWE scores.
It is evident that a substantial subgroup of patients presenting with dental erosions and after exclusion of other known causes of erosions have oligosymptomatic reflux potentially responsible for their dental tissue loss. However, none of the standard evaluation criteria for classic GORD by pH-MII or of predisposing anatomical factors correlated with the severity of the dental erosions. There are several possible underlying reasons, besides the uncertainties of cross-sectional sampling. The standard reporting criteria for GORD do not reflect the sensitivity threshold of dental tissue, which demineralizes at a pH of below 5.5 or 6 in an environment of decreased remineralization.23 Demineralization, therefore, occurs at a relatively high pH, but is also dependent on other factors in the oral milieu, such as mineral content of the saliva. The salivary flow rate and buffering capacity were normal in all our patients, but this does not exclude other salivary abnormalities. Additional factors in refluxate, such as proteases and bile, may well play a role in the induction of tissue damage. Pepsin shows proteolytic activity up to a pH of 6 and salivary pepsin tests have shown increased pepsin concentrations in patients with GORD and GORD-related supra-oesophageal pathologies.31–34 In a recent ex vivo study the additive erosive effects of pepsin and trypsin on teeth were shown.35 Further elaboration of the interaction of the erosive effects of acid with other salivary factors is needed. An additional factor contributing to the lack of association between the reflux characteristics measured by pH-MII and the degree of dental erosions is the difficulty of correlating acute measures of 24-h duration with effects of longstanding reflux. Loss of dental tissue occurs very gradually, with less than 15 µm per 6 months reflecting normal tooth wear and 30–40 µm loss annually constituting severe erosion.36 Clearly, macroscopically visible erosions are preceded by a protracted period of erosive and demineralizing forces. Furthermore, quantification of proximal oesophageal or even pharyngeal reflux is unlikely to linearly reflect surface dental pH. The most accurate assessment of the relationship between acidity and erosion would be pH measurements on the dental surface or in the dental acid pocket, however this is impractical to perform in vivo.37,38 Nonetheless, weakly significant correlations between distal oesophageal and oral pH, oral acid exposure and palatal dental erosions have been previously described, albeit using inadequately validated pH measures.7
Earlier studies have variably asserted a bidirectional association between dental erosion and reflux disease, but the link has remained tenuous for several reasons. These include the definition of GORD based solely on symptoms, on pH thresholds developed for oesophageal disease and unsuitable for dental tissue, on poorly validated proximal oesophageal pH-metric techniques and small study size. Weakly acidic reflux has until now not been assessed, nor has pH-MII been reported in dental erosions. A review by Pace et al. summarized available studies up to 2008 and nonetheless concluded that there is a strong association between GORD and dental erosion.19 In a pertinent dental review in 2012, Ranjitkar et al. stated that suspicion of an endogenous source of acid being associated with tooth erosion requires medical referral and management of the patient as the primary method of its prevention and control.39 Our data confirm the presence of ongoing GORD in a large subset of dental erosions patients, but also highlight the difficulty of applying classic GORD measures to extra-oesophageal manifestations. A clear limitation of the current study is the absence of a local control group without dental erosions, necessitating comparison with a published, albeit Northern European and very similarly aged control group.22 Potential differences in diet and BMI, amongst other factors, could therefore not be controlled for.
In summary, acidic and weakly acidic reflux was increased in 69% of oligosymptomatic patients with dental erosion using oesophageal GORD criteria. None of the classic measures of GORD by 24-h pH-MII correlated with the degree of dental erosion. Further definition of clinically relevant reflux parameters by pH-MII for dental erosion and of treatment guidelines are required. Gastroenterologists and dentists need to be aware of the widely prevalent association between oligosymptomatic dental erosion and GORD.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
None of the authors have a conflict of interest on disclosure.
References
- 1.Vakil N, van Zanten S, Kahrilas P, et al. The Montreal definition and classification of gastrooesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–1920. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JV, Herreros B, Sanchis V, et al. Dental and periodontal lesions in patients with gastro-oesophageal reflux disease. Dig Liv Dis 2003; 35: 461–467. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder PL, Filler SJ, Ramirez B, et al. Dental erosion and acid reflux disease. Ann Int Med 1995; 122: 809–815. [DOI] [PubMed] [Google Scholar]
- 4.Meurmann J, Toskala J, Nuutinien P, et al. Oral and dental manifestations in gastrooesophageal reflux disease. Oral Surg Oral Med Oral Pathol 1994; 78: 583–589. [DOI] [PubMed] [Google Scholar]
- 5.Gregory-Head B, Curtis DA. Erosion caused by gastrooesophageal reflux: diagnostic considerations. J Prosthodont 1997; 6: 278–285. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett DW, Evans DF, Smith BG. The relationship between gastro-oesphageal reflux disease and dental erosion. J Oral Rehabil 1996; 23: 289–297. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett DW, Evans DF, Anggiansah A, et al. A study of the association between gastro-oesophageal reflux and palatal dental erosion. Brit Dent J 1996; 181: 125–131. [DOI] [PubMed] [Google Scholar]
- 8.Pontefract HA. Erosive tooth wear in the elderly population. Gerodontology 2002; 19: 5–16. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan EA, Curzon ME, Roberts GJ, et al. Gastroesophageal reflux in children and its relationship to erosion of primary and permanent teeth. Eur J Oral Sci 1998; 106: 765–769. [DOI] [PubMed] [Google Scholar]
- 10.Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastro-oesophageal reflux disease and saliva: how are they related? J Dent 2004; 32: 489–494. [DOI] [PubMed] [Google Scholar]
- 11.Loffeld RJ. Incisor teeth status in patients with reflux oesophagitis. Digestion 1996; 57: 388–390. [DOI] [PubMed] [Google Scholar]
- 12.Jarvinen V, Meurman JH, Hyvarinen H, et al. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol 1988; 65: 298–303. [DOI] [PubMed] [Google Scholar]
- 13.Aine L, Baer M, Maki M. Dental erosions caused by gastroesophageal reflux disease in children. ASDC J Dent Child 1993; 60: 210–214. [PubMed] [Google Scholar]
- 14.Bohmer CJ, Klinkenberg-Knol EC, Niezen-de Boer MC, et al. Dental erosions and gastro-oesophageal reflux disease in institutionalized intellectually disabled individuals. Oral Dis 1997; 3: 272–275. [DOI] [PubMed] [Google Scholar]
- 15.Myklebust S, Espelid I, Svalestad S, et al. Dental health behavior, gastroesophageal disorders and dietary habits among Norwegian recruits in 1990 and 1999. Acta Odontol Scand 2003; 61: 100–104. [DOI] [PubMed] [Google Scholar]
- 16.Linnett V, Seow WK, Connor F, et al. Oral health of children with gastro-oesophageal reflux disease: a controlled study. Aust Dent J 2002; 47: 156–162. [DOI] [PubMed] [Google Scholar]
- 17.Dahshan A, Patel H, Delaney J, et al. Gastroesophageal reflux disease and dental erosion in children. J Pediatr 2002; 140: 474–478. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett DW, Lussi A, West NX, et al. Prevalence of tooth wear on buccal and lingual surfaces and possible risk factors in young European adults. J Dent 2013; 41: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 19.Pace F, Pallotta S, Tonini M, et al. Systematic review: gastro-oesophageal reflux disease and dental lesions. Aliment Pharmacol Ther 2008; 27: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 20.Jaspersen D, Kulig M, Labenz J, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther 2003; 17: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 21.Hirano I, Richter JE. ACG practice guidelines: oesophageal reflux testing. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 2007; 102: 668–685. [DOI] [PubMed] [Google Scholar]
- 22.Zerbib F, des Varannes SB, Roman S, et al. Normal values and day-to-day variability of 24-h ambulatory ooesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther 2005; 22: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 23.Ganss C, Lussi A, Schlueter N. Dental erosion as oral disease. Insights in etiological factors and pathomechanisms, and current strategies for prevention and therapy. Am J Dent 2012; 25: 351–364. [PubMed] [Google Scholar]
- 24.Magalhães AC, Wiegand A, Rios D, et al. Insights into preventive measures for dental erosion. J Appl Oral Sci 2009; 17: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett D, Ganss C, Lussi A. Basic erosive wear examination (BEWE): a new scoring system for scientific and clinical needs. Clin Oral Investig 2008; 12(Suppl 1): S65–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lussi A, Schaffner M, Hotz P, et al. Dental erosion in a population of Swiss adults. Community Dent Oral Epidemiol 1991; 19: 286–290. [DOI] [PubMed] [Google Scholar]
- 27.Aanen MC, Numans ME, Weusten BL, et al. Diagnostic value of the reflux disease questionnaire in general practice. Digestion 2006; 74: 162–168. [DOI] [PubMed] [Google Scholar]
- 28.Sifrim D, Castell D, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004; 53: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stål P, Lindberg G, Ost A, et al. Gastroesophageal reflux in healthy subjects. Significance of endoscopic findings, histology, age, and sex. Scand J Gastroenterol 1999; 34: 121–128. [DOI] [PubMed] [Google Scholar]
- 30.Dua KS, Surapaneni SN, Hafeezullah M, et al. Prevalence of abnormal upper GI findings in apparently healthy volunteers enrolled for research studies. Gastrointestinal Endoscopy 2009; 69: AB350–AB351. [Google Scholar]
- 31.Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol 2012; 2012: 646901–646901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potluri S, Friedenberg F, Parkman HP, et al. Comparison of a salivary/sputum pepsin assay with 24-hour oesophageal pH monitoring for detection of gastric reflux into the proximal oesophagus, oropharynx, and lung. Dig Dis Sci 2003; 48: 1813–1817. [DOI] [PubMed] [Google Scholar]
- 33.Saritas Yuksel E, Hong SK, Strugala V, et al. Rapid salivary pepsin test: blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope 2012; 122: 1312–1316. [DOI] [PubMed] [Google Scholar]
- 34.Samuels TL, Johnston N. Pepsin as a marker of extraoesophageal reflux. Ann Otol Rhinol Laryngol 2010; 119: 203–208. [DOI] [PubMed] [Google Scholar]
- 35.Schlueter N, Hardt M, Klimek J, et al. Influence of the digestive enzymes trypsin and pepsin in vitro on the progression of erosion in dentine. Arch Oral Biol 2010; 55: 294–299. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez JM, Austin RS, and Bartlett DW. In vivo measurements of tooth wear over 12 months. Caries Res 2012; 46: 9–15. [DOI] [PubMed]
- 37.Lussi A, Bossen A, Höschele C, et al. Effects of enamel abrasion, salivary pellicle, and measurement angle on the optical assessment of dental erosion. J Biomed Opt 2012; 17: 97009–1 DOI: 10.1117/1.JBO.17.9.097009. [DOI] [PubMed] [Google Scholar]
- 38.Lussi A, von Salis-Marincek M, Ganss C, et al. Clinical study monitoring the pH on tooth surfaces in patients with and without erosion. Caries Res 2012; 46: 507–512. [DOI] [PubMed] [Google Scholar]
- 39.Ranjitkar S, Kaidonis JA, Smales RJ. Gastroesophageal reflux disease and tooth erosion. Int J Dent 2012; 2012: Article ID 479850, DOI:10.1155/2012/479850. [DOI] [PMC free article] [PubMed]