Abstract
Background
Celiac disease has been linked to decreased quality of life and certain mood disorders. The effect of the gluten free diet on these psychological aspects of the disease is still unclear.
Objectives
The objective of this article is to review the literature on psychological morbidity of celiac disease.
Methods
We performed a PubMed search for the time period from 1900 until June 1, 2014, to identify papers on psychological aspects of celiac disease looking specifically at quality of life, anxiety, depression and fatigue.
Results
Anxiety, depression and fatigue are common complaints in patients with untreated celiac disease and contribute to lower quality of life. While aspects of these conditions may improve within a few months after starting a gluten-free diet, some patients continue to suffer from significant psychological morbidity. Psychological symptoms may affect the quality of life and the dietary adherence.
Conclusion
Health care professionals need to be aware of the ongoing psychological burden of celiac disease in order to support patients with this disease.
Keywords: Celiac disease, anxiety, depression, fatigue, quality of life
Introduction
Celiac disease (CD) is a chronic immune-mediated enteropathy1 characterized by a large spectrum of symptoms and signs that generally improve with good adherence to a gluten-free diet (GFD).2
In recent years, there has been an increased interest in how celiac patients perceive the impact of their disorder, how this perception relates to the clinical presentation of the disease and how their health is modified by treatment with a GFD. It has been recognized that the aspects of health that should be addressed go beyond the usual biological parameters and extend also to social functioning and psychological issues.3 Mood disorders such as anxiety, depression and fatigue are often linked to CD, before and after diagnosis, and therefore may influence the patient’s quality of life (QoL) and adherence to GFD.
Methods
This work is part of a project initiated by the Oslo group and British Society of Gastroenterology on the clinical management of CD.2 We examined the literature on the QoL of celiac patients and some psychological aspects associated with CD (anxiety, depression and fatigue), asking how treatment with a GFD may modify them. A PubMed search identified papers on QoL, anxiety, depression and fatigue published between 1900 and June 1, 2014. Four authors (FZ, GLS, TRC, JCB) carried out the literature searches and the data collection and took the main responsibility for the writing of the paper. DSS and JFL reviewed the paper, giving important feedback.
Results
QoL
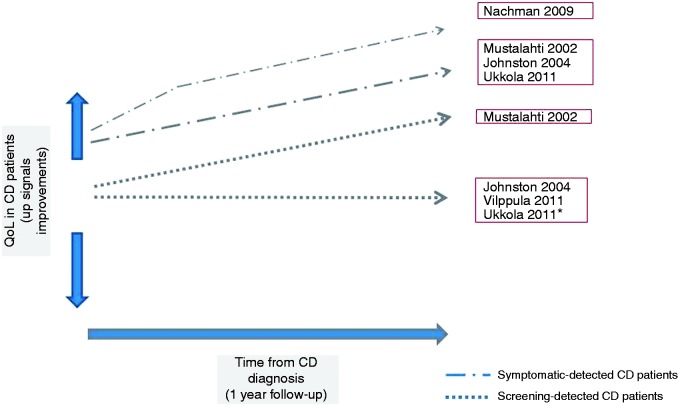
Several reports have described the difficulties of living with CD in adults, in particular as regards the impact of this condition on physical, social and emotional factors.4–23 Unfortunately, interest in the health perception of celiac patients has been affected by the lack of CD-specific QoL instruments allowing measurement of specific aspects of the disorder. Most studies exploring the QoL in CD patients used generic multi-item and multi-dimensional instruments developed for chronic disorders.24–28 The most widely used generic tools to estimate health-related QoL in CD were the Short Form Health Survey questionnaire26 and the Psychological General Well-being index.28 It is only recently that CD-specific questionnaires have been developed for pediatric29,30 and adult patients31,32 and translated into other languages.33–36 Although screen-detected37,38 and asymptomatic CD patients39 seem to have a better QoL than symptom-detected patients at the time of diagnosis, the effect of the GFD is still unclear. Mustalahti et al. reported a positive effect of the GFD both in symptomatic and screen-detected patients,37 Johnston et al. suggested the benefits were limited to those presenting with symptomatic disease,38 Vilppula et al.40 reported no change of the QoL in screen-detected patients on GFD and finally, Ukkola et al. described that the QoL improved in symptomatic and in screen-detected symptomatic patients but not in screen-detected asymptomatic patients41 (Figure 1). Interestingly, a time-course assessment of the effect of treatment showed that, in symptomatic patients, the most significant quantitative improvement of most items is seen in the first three months after starting the GFD, with some additional improvement up to one year39 (Figure 1). Compared to biological parameters, including serology, the time course for improvement of QoL measures seems to be earlier and faster.39 Paavola et al., analyzing CD patients on long-term GFD, reported that the QoL was unimpaired in screen-detected celiac patients and lower in symptom-detected patients, when compared to healthy controls.42 A recent randomized study showed that asymptomatic CD patients benefited from a GFD for anxiety and better health (based on the visual analog scale), but not for social function, when compared to similar patients following a gluten-containing diet.43 Finally, Roos et al. showed similar psychological well-being in long-treated celiac patients and healthy controls.16 Poor dietary adherence was associated with a poor QoL8,10 but whether one causes the other remains unknown, and consequently it is unclear which is the cause and which the effect. A recent long-term longitudinal study suggested that subsequent deterioration in QoL was associated with a lack of dietary adherence.44 However, other studies45,46 reported no differences in QoL scores between patients with full adherence and patients with partial/nonadherence to GFD. Several papers reported lower QoL in women with CD than in celiac men.9,11–13,18 Finally, Paarlahti et al.47 reported that a long duration of symptoms before diagnosis, psychiatric, neurologic or gastrointestinal comorbidities and persistent symptoms were predictors of a reduced QoL.
Figure 1.
Quality of life (QoL) in screen-detected and symptom-detected celiac disease (CD) patients after one year of gluten-free diet. *QoL improved in symptomatic screening-detected patients.
Anxiety
Anxiety has been widely described in CD patients, although a recent meta-analysis48 concluded that anxiety is neither more common nor more severe in adults with CD compared to healthy adults. However, large studies are lacking (Table 1). Levels of anxiety appear to increase prior to CD diagnosis, although a diagnosis may be associated with feelings of relief.5 Cannings-John et al.49 found that celiac patients had an increased number of general practice consultations compared with controls in the five years prior to diagnosis and in particular, three clinical features were independently associated with subsequent diagnosis: depression and/or anxiety, diarrhea and anemia. Addolorato et al. described an increased reactive “state” anxiety in CD patients at diagnosis,50,51 which decreased after one year of GFD.51 Conversely, among 441 German adult patients on a GFD, the levels of anxiety and risk of a probable anxiety disorder were found greater than the general population.52 Interestingly, anxiety levels were greater in female CD patients compared to male patients, and living alone was associated with a reduced risk of anxiety disorder. The authors speculated that problems with buying and preparing food, plus the associated expense, within a family group may contribute to anxiety. In another study, social phobia, assessed by the Liebowitz Social Anxiety Scale, was found to be significantly greater in 40 celiac patients.53 Among these patients, social phobia levels were similar in newly diagnosed and treated individuals.53 In a study of 68 patients treated for a mean of 10 years, Hallert et al.11 evaluated a nine-item Burden of illness protocol, assessing perceived worries, restrictions and subjective outcome. While the importance of dietary adherence was ranked similarly high by men and women with CD, 10 years after diagnosis women expressed more concerns about the impact of the disease on socializing with friends and having to abstain from important things in life. A recent study54 interviewed women with CD and looked at the impact of the condition on everyday living. They expressed a sense of loneliness and invisibility, especially when socializing with others. In another large qualitative study of nearly 6000 Canadians with CD,17 women reported significantly greater emotional responses to a GFD but, with time, were more accepting of it than men. Frustration and isolation were the most common negative emotions.
Table 1.
Previous literature on depression and anxiety in CD
| First author, year of publication | Study population | Outcomes measured | Conclusions |
|---|---|---|---|
| Addolorato, 199650 | 16 celiac patients 16 IBD 16 Healthy controls | STAI test Ipat Depression Scale Questionnaire | “State” anxiety in celiac patients and IBD patients was increased compared to healthy controls but no increase in personality ‘trait’ anxiety that was present in a similar percentage in all the participants evaluated. Depressive syndrome was more frequent in celiac patients than healthy control group. |
| Ciacci, 199871 | 92 Celiac patients 100 Healthy controls 48 Patients with CPH | MSDS | Depressive disorder was more frequent in celiac patients than in healthy controls and CPH patients. Age at CD diagnosis, duration of GFD and compliance with diet did not correlate with depression. |
| Addolorato, 200151 | 35 Celiac patients before (T0) and one year after GFD. 59 Healthy controls | STAI test MSDS | At T0 celiac patients showed high levels of state anxiety and depression compared to controls. After one year of GFD state anxiety decreased significantly but depression did not. |
| Hallert, 200211 | 68 Celiac patients on GFD Matched type-2 DM patients | Nine-item Burden of illness protocol (perceived worries, restrictions and subjective outcome) SF36 | Women with CD perceived the health burden to be worse than men and expressed more concern about the impact of socializing with friends. |
| Carta, 200267 | 36 Celiac patients 144 Healthy controls | International composite Diagnostic Interview for psychiatric diagnoses based on DSM-IV criteria. | CD patients tended to show higher prevalence of panic disorder and major depressive disorder. Thyroid disease appears to present a significant risk factor for these conditions. |
| Fera, 200360 | 100 Celiac patients on GFD 100 Healthy controls 100 DM patients | Professional semi-structured diagnostic interview based on DSM-IV criteria MSDS, STAI test, SF36, IBQ | Depression and anxiety were common features among celiac patients, these disorders tend to improve with the time and they did not depend on diet compliance or demographic variables; Depression and anxiety disorders are significantly higher in both celiac and diabetic patients than healthy controls. |
| Ciacci, 200313 | 581 Celiac patients on GFD | MSDS, VAS for the evaluation of anxiety, depression, and positive attitude | Most patients (83.6%) felt “very well” and “well.” Celiac women and patients diagnosed after 20 years of age have better dietary adherence, but more problems in their social life. Anxiety was related to feeling different from the general population and depression to an unsatisfactory sexual life. |
| Siniscalchi, 200558 | 71 Celiac patients at diagnosis 59 Celiac patients on GFD 80 Healthy controls | MSDS, CFS questionnaire, FSS, VAS for the evaluation of fatigue | Fatigue and depression symptoms were more frequent in celiac patients than controls. Patients on GFD showed more frequent and severe symptoms of depression than patients at diagnosis. |
| Ludvigsson, 200770 | 13,776 Celiac patients 66,815 Healthy controls | Celiac patients with mood disorders and bipolar disorders found through the Swedish National Inpatient Register | CD was associated with an increased risk of subsequent depression (Hazard ratio = 1.8) but it was not associated with subsequent bipolar disorders. Individuals with depression had an increased risk of CD. |
| Addolorato, 200853 | 40 Celiac patients on GFD 50 Healthy controls | Social Phobia assessed by Liebowitz Social Anxiety Scale MSDS | Social phobia and depression were significantly greater in CD patients than controls. |
| Garud, 200968 | 600 Celiac patients (mixed) 200 Healthy controls 200 IBS patients | The diagnosis of psychiatric disorders including depression is made from primary care provider notes and gastroenterologist notes. | The prevalence of depression in celiac patients was 17.2% and was similar to those in IBS patients (18.5%) and in controls (16.0%). Among CD patients, depression was more frequent in celiac patients with DM type 1. |
| Nachman, 201044 | 53 Celiac patients before (T0) and after one year and four years of GFD | SF36, BDI | At four years, the SF36 and BDI scores showed a significant deterioration compared with one year, but these scores remained significantly better than those at diagnosis. The significant deterioration of scores at four years was related to the lack of adherence to the GFD. |
| Zingone, 201057 | 30 Celiac patients at diagnosis 30 Celiac patients on GFD 30 Healthy controls | PSQI, MSDS, STAI test, SF36, VAS for the evaluation of fatigue | Sleep disorders were common in celiac patients at diagnosis and on GFD. Depression, fatigue and anxiety in celiac patients did not improve on GFD. Sleep disorders were directly correlated with the mood disorders. |
| Häuser, 201052 | 441 Celiac patients on GFD 235 IBD patients 441 Healthy controls | HADS | Levels of anxiety were greater in CD and IBD patients than controls. Levels were greater in females than males. Levels of depression did not differ among the three groups. |
| van Hees, 201361 | 2,265 Adult patients on GFD | HADS, Major Depression Questionnaire, self-report Leiden Index of Depression Sensitivity (short version) | Depression symptoms were present in 39% of CD patients. Long-term GFD (over five years) was associated with a reduced risk of depression. No association between compliance to GFD and depression. |
BDI: Beck Depression Inventory; CD: celiac disease; CFS: chronic fatigue syndrome; CPH: chronic persistent hepatitis; FSS: Fatigue Severity Scale; GP: general practice; GFD: gluten-free diet; HADS: Hospital Anxiety and Depression Scale; IBQ: Illness Behavior Questionnaire; IBD: inflammatory bowel disease; IBS: irritable bowel syndrome; MSDS: Modified Zung self-rating Depression Scale; PSQI: Pittsburgh Sleep Quality Index; SF36: Short Form 36 Health Survey; STAI: State & Trait Anxiety Inventory; DM: diabetes mellitus; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.
Depression
Depression has been associated with CD.48 Morris et al.55 and Hallert et al.56 were among the first to describe this association. Though numerous papers have followed the causes of this association and the effect of a GFD on depression, these are still poorly defined (Table 1).
Using the modified Self-Rating Depression Scale, Addolorato et al.51 described persistent depression after one year of GFD in celiac patients, Zingone et al.57 and Siniscalchi et al.58 showed that depression was present in CD at diagnosis, but that it persisted or even worsened in patients on a GFD. Nachman et al.,44 using the Beck Depression Inventory, showed that depressive symptoms were highly prevalent in untreated CD and there was a significant improvement in psychological symptoms after one year and four years of GFD.44 However, the Beck Depression Inventory score at four years showed a significant worsening compared to one year, though CD patients at the four-year visit still had less depression than at CD diagnosis. A low adherence to GFD might be considered either a cause or a consequence of the persistent depression on GFD. This has been suggested in a 2004 study by Addolorato et al., which showed a beneficial effect of psychological support for CD patients on a GFD both in relation to psychological disorders and to improved dietary adherence.59 Fera et al.,60 in a study of 100 patients treated for eight years, found a high rate of depression, detected by the modified Self-Rating Depression Scale, which tended to improve with time, but which was not correlated with dietary compliance. Similarly, a 2013 cross-sectional study,61 reporting a self-reported depression prevalence of 39% among 2265 adult CD patients (based on the Major Depression Questionnaire), described that the long-term adherence to the GFD (>5 years) was associated with a reduced risk of depression, but they found no association between insufficient adherence and current depression symptoms. Finally, Häuser et al.52 did not find any difference in depression between celiac patients on GFD and the general population. The authors reported no evidence that depression was predicted by diet adherence, years of GFD, presence of associated diseases, or delay in CD diagnosis. Finally, Barratt et al.62 described that patients on a GFD, at risk of anxiety and depression according to the Hospital Anxiety Depression Scale, reported more symptoms in response to occasional dietary gluten exposure.
Mechanisms explaining psychological morbidities
As Table 2 shows, a number of mechanisms may explain the relationship between CD and psychological morbidities such as anxiety and depression, either before or after CD diagnosis. Before diagnosis, they may be a consequence of the disease symptoms with a decreased sensation of general well-being.63 Equally, they may be due to cerebral hypoperfusion in some brain regions,64 be a consequence of vitamin deficiency due to malabsorption65 or of hyperhomocysteinemia, which might, by damaging the blood-brain barrier, expose neuronal tissue to neuro-irritative metabolites.66 On a GFD, they may be particularly sustained by dietary restrictions and by compromised daily social relationships.21 Independently of GFD, psychological morbidities in CD may also be secondary to CD associated with autoimmune diseases. For example, Carta et al. in 200267 showed that the association of CD with thyroid disease can represent a significant risk factor for depression and panic disorders. Some years later, Garud et al.68 described a similar risk of depression in CD when compared with the general population but a markedly elevated risk of depression in patients both with CD and type I diabetes. A possible explanation of this increased risk may be that the cytokines produced by immune reactions may exercise an effect on the brain circuits responsible for mood regulation.69 However, a large Swedish population-based study70 based on 13776 CD patients found that CD patients were at an 80% increased risk of depression compared to controls and the adjustment for type 1 diabetes or thyroid disease did not affect the risk estimates.70 Finally, these psychological morbidities could also be a consequence of a chronic condition: in fact while Ciacci et al. found a higher prevalence of symptoms of depression in CD patients than in patients with chronic hepatitis,71 others have found no difference between depression in CD compared to patients with irritable bowel syndrome (IBS),68 and depression and anxiety compared to patients with type 2 diabetes.60 Finally, Häuser et al.52 described levels of anxiety greater in CD and in patients with inflammatory bowel diseases compared to controls and similar levels of depression among the three groups.
Table 2.
Mechanisms potentially leading to psychological morbidity in celiac disease
Depression and anxiety may be associated with other factors including an unsatisfactory sexual life,13 fatigue58 and poor quality of sleep.57 Furthermore, functional disorders, such as IBS72–74 and functional dyspepsia74,75 are frequently diagnosed in CD patients at diagnosis and in those on a long-term GFD. Since these disorders are also associated with reduced QoL and increase the likelihood of anxiety and depression in CD,76,77 treating these comorbidities may improve QOL and mood disorders in CD.73
CD has been linked to a number of neurological disorders: ataxia,78 neuropathy,79 epilepsy,80 and headache81 while the evidence in multiple sclerosis has been mixed.79,82 Several of the above neurological disorders have been linked to poor QoL,83–85 and we cannot rule out that psychological dimensions of neurological comorbidity have thereby influenced QoL in CD patients. Neurological and psychological morbidities in CD patients might be caused by the mechanisms reported above, such as vitamin deficiency and hyperhomocysteinemia, as well as by the presence of antineuronal antibodies86 and brain dysfunctions.87 For example, a similar gray matter loss described in particular in the gyrus rectus and anterior cingulate gyrus of CD patients with neurological disturbances87 has also been reported in individuals with depression.88,89
Fatigue
There is now good evidence to show not only that fatigue can be a symptom of CD, but also that it is a common clinical presentation.90–93 Serological screening for CD is now recommended in the workup of chronic fatigue.94 Case finding studies suggest a CD prevalence of about 3% among those presenting with chronic fatigue, i.e. similar to that in patients with IBS.92 Less is known about the prevalence of fatigue among those already on a GFD, or whether treatment of CD with a GFD successfully treats this symptom. In the last decade a small number of studies have more directly addressed these issues. Siniscalchi et al. in 2005 demonstrated that celiac patients both at diagnosis and when on a GFD had higher levels of fatigue than healthy controls using a variety of validated scales.58 Perhaps more surprisingly, fatigue was not significantly different between newly diagnosed celiac patients and those on an established GFD. The same researchers from Naples have since shown that both treated and untreated celiac patients experience a worse quality of sleep than healthy volunteers57 perhaps offering an explanation of the mechanisms behind this condition. Again, treated celiac patients did not show significantly different characteristics compared with their untreated counterparts. More recently, a large questionnaire study of 5912 Canadian celiac patients has suggested that “extreme weakness/tiredness” is reported by patients to improve over a prolonged period on GFD, such that of those on GFD for more than five years 72.4% reported this symptom had recovered.95 Interpretation of these findings is made more difficult by the fact that data originate from cross-sectional studies, rather than from either randomized controlled trials of the effect of GFD, or cohort studies to permit examination of the alteration in state within individuals after establishment of a diet. It is therefore unwise to conclude too firmly that GFD either does or does not alleviate fatigue in CD from the available evidence. One other potentially relevant finding is the suggestion that dietary supplementation with L-carnitine may treat fatigue in CD.96 Since carnitine is absorbed in the small intestine,97 it is tempting to assume that resolution of malabsorption should facilitate the intestinal uptake of carnitine. However, the range of gastroenterological and non-gastrointestinal conditions in which similar effects have been suggested indicates that mechanisms other than the correction of a deficiency may be operating.98,99
Summary
Our search of the available literature suggests that CD has a considerable psychological impact. Some elements of this may relate to the disease and its biochemical effects, but other aspects relate to the patient’s subjective perception of the disorder and of the GFD used to treat it. The treatment of CD results in a significant improvement in QoL for symptomatic patients, but patients with subclinical CD often report no such effect. However, a proportion of subclinical patients may report improvement in QoL parameters after commencing treatment. Overall, levels of anxiety and depression are greater in patients with CD (Table 1). However, the causes of this may vary at different stages (Table 2). Prior to CD diagnosis, patients may express concerns about unexplained symptoms and may feel frustrated about repeated consultations that offer no adequate explanation of their problems. At the time of diagnosis, there may be concerns about investigations and a diagnosis of a long-term condition, although this may be accompanied by a feeling of relief that a diagnosis has finally been made. While some psychological problems may lessen with time as knowledge of the condition improves and perhaps biochemical abnormalities are corrected, it appears that many patients have ongoing concerns about coping with the diet and do not adhere to it, particularly when going out and in social interaction. Fatigue is sometimes the unique symptom at CD presentation. Conversely, 3% of patients with chronic fatigue may be found to have CD. The available studies have not been able to show a consistent positive effect of the GFD in diminishing perception of fatigue.
Conclusion
The literature on the effect of treatment in the outcome of depression, anxiety, fatigue and QoL in CD is not consistent. However, it is important to consider that ongoing problems with anxiety and depression in particular may affect dietary adherence and QoL. Thus, health care professionals need to be aware of the ongoing psychological burden of CD in order to support their patients. The lack of clear evidence of improved QoL in asymptomatic CD after treatment makes mass screening, where a majority of patients may be subclinical or asymptomatic, controversial if the aim of screening is to improve QoL. Further studies are required to better understand this specific aspect.
Clinical implications
Celiac disease (CD) has a considerable psychological impact.
Biochemical effects and the patient’s subjective perception of the disorder and of the gluten-free diet (GFD) may be causes of the psychological morbidities.
GFD improves quality of life (QoL) in symptomatic patients, but not always in asymptomatic patients.
Anxiety and depression may affect dietary adherence and QoL.
Fatigue is sometimes the unique symptom at CD presentation.
Health care professionals need to support CD patients on GFD.
There is no evidence to support mass screening as the GFD fails to improve the QoL in asymptomatic, screen-detected CD patients.
Funding
JFL was supported by grants from The Swedish Society of Medicine and the Swedish Research Council – Medicine (522-2A09-195). TC derives his salary from the University of Nottingham and the Nottingham University Hospitals NHS trust.
Conflict of interest
TC has received grant support from Coeliac UK: Crohn’s and Colitis UK. DSS has received an educational grant from Dr Schär (a gluten-free food manufacturer) to undertake an investigator-led research study on gluten sensitivity. He also has received an educational grant both from Biocard and Simtomax to undertake an investigator-led research study on point-of-care tests.
References
- 1.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut 2013; 62: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014; 63: 1210–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troncone R, Ivarsson A, Szajewska H, et al. Review article: Future research on coeliac disease—a position report from the European multistakeholder platform on coeliac disease (CDEUSSA). Aliment Pharmacol Ther 2008; 27: 1030–1043. [DOI] [PubMed] [Google Scholar]
- 4.Hallert C, Lohiniemi S. Quality of life of celiac patients living on a gluten-free diet. Nutrition 1999; 15: 795–795. [DOI] [PubMed] [Google Scholar]
- 5.Ciacci C, Iavarone A, Siniscalchi M, et al. Psychological dimensions of celiac disease: Toward an integrated approach. Dig Dis Sci 2002; 47: 2082–2087. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Newman JM. Celiac diet: Its impact on quality of life. J Am Diet Assoc 2003; 103: 1533–1535. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa A, Troncone A, Vacca M, et al. Characteristics and quality of illness behavior in celiac disease. Psychosomatics 2004; 45: 336–342. [DOI] [PubMed] [Google Scholar]
- 8.Häuser W, Stallmach A, Caspary W, et al. Predictors of reduced health-related quality of life in adults with coeliac disease. Aliment Pharmacol Ther 2007; 25: 569–578. [DOI] [PubMed] [Google Scholar]
- 9.Hallert C, Grännö C, Grant C, et al. Quality of life of adult coeliac patients treated for 10 years. Scand J Gastroenterol 1998; 33: 933–938. [DOI] [PubMed] [Google Scholar]
- 10.Usai P, Minerba L, Marini B, et al. Case control study on health-related quality of life in adult coeliac disease. Dig Liver Dis 2002; 34: 547–552. [DOI] [PubMed] [Google Scholar]
- 11.Hallert C, Grännö C, Hulten S, et al. Living with coeliac disease: Controlled study of the burden of illness. Scand J Gastroenterol 2002; 37: 39–42. [DOI] [PubMed] [Google Scholar]
- 12.Hallert C, Sandlund O, Broqvist M. Perceptions of health-related quality of life of men and women living with coeliac disease. Scand J Caring Sci 2003; 17: 301–307. [DOI] [PubMed] [Google Scholar]
- 13.Ciacci C, D’Agate C, De Rosa A, et al. Self-rated quality of life in celiac disease. Dig Dis Sci 2003; 48: 2216–2220. [DOI] [PubMed] [Google Scholar]
- 14.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: Results of a national survey. Am J Gastroenterol 2001; 96: 126–131. [DOI] [PubMed] [Google Scholar]
- 15.Häuser W, Gold J, Stein J, et al. Health-related quality of life in adult coeliac disease in Germany: Results of a national survey. Eur J Gastroenterol Hepatol 2006; 18: 747–754. [DOI] [PubMed] [Google Scholar]
- 16.Roos S, Kärner A, Hallert C. Psychological well-being of adult coeliac patients treated for 10 years. Dig Liver Dis 2006; 38: 177–180. [DOI] [PubMed] [Google Scholar]
- 17.Zarkadas M, Dubois S, MacIsaac K, et al. Living with coeliac disease and a gluten-free diet: A Canadian perspective. J Hum Nutr Diet 2013; 26: 10–23. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsson LR, Hallert C, Milberg A, et al. Coeliac disease—women’s experiences in everyday life. J Clin Nurs 2012; 21: 3442–3450. [DOI] [PubMed] [Google Scholar]
- 19.Byström IM, Hollén E, Fälth-Magnusson K, et al. Health-related quality of life in children and adolescents with celiac disease: From the perspectives of children and parents. Gastroenterol Res Pract 2012; 2012: 986475–986475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford S, Howard R, Oyebode J. Psychosocial aspects of coeliac disease: A cross-sectional survey of a UK population. Br J Health Psychol 2012; 17: 743–757. [DOI] [PubMed] [Google Scholar]
- 21.Lee A, Ng D, Diamond B, et al. Living with coeliac disease: Survey results from the USA. J Hum Nutr Diet 2012; 25: 233–238. [DOI] [PubMed] [Google Scholar]
- 22.Roos S, Hellström I, Hallert C, et al. Everyday life for women with celiac disease. Gastroenterol Nurs 2013; 36: 266–273. [DOI] [PubMed] [Google Scholar]
- 23.Sainsbury K, Mullan B, Sharpe L. Reduced quality of life in coeliac disease is more strongly associated with depression than gastrointestinal symptoms. J Psychosom Res 2013; 75: 135–141. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson N, Acquadro C, Alonso J, et al. International quality of life assessment (IQOLA) project. Qual Life Res 1992; 1: 349–351. [DOI] [PubMed] [Google Scholar]
- 25.Borgaonkar M, Irvine E. Quality of life measurement in gastrointestinal and liver disorders. Gut 2000; 47: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddle DL, Lee KT, Stratford PW. Use of SF-36 and SF-12 health status measures: A quantitative comparison for groups versus individual patients. Med Care 2001; 39: 867–878. [DOI] [PubMed] [Google Scholar]
- 27.Eypasch E, Williams J, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: Development, validation and application of a new instrument. Br J Surg 2005; 82: 216–222. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen NA, Norholm V, Bech P. The internal and external validity of the Psychological General Well-Being Schedule (PGWB). Quality of Life News Letter 1999; 22: 7–7. [Google Scholar]
- 29.van Doorn RK, Winkler LMF, Zwinderman KH, et al. CDDUX: A disease-specific health-related quality-of-life questionnaire for children with celiac disease. J Pediatr Gastroenterol Nutr 2008; 47: 147–152. [DOI] [PubMed] [Google Scholar]
- 30.Jordan NE, Li Y, Magrini D, et al. Development and validation of a celiac disease quality of life instrument for North American children. J Pediatr Gastroenterol Nutr 2013; 57: 477–486. [DOI] [PubMed] [Google Scholar]
- 31.Häuser W, Gold J, Stallmach A, et al. Development and validation of the Celiac Disease Questionnaire (CDQ), a disease-specific health-related quality of life measure for adult patients with celiac disease. J Clin Gastroenterol 2007; 41: 157–166. [DOI] [PubMed] [Google Scholar]
- 32.Dorn S, Hernandez L, Minaya M, et al. The development and validation of a new coeliac disease quality of life survey (CD-QOL). Aliment Pharmacol Ther 2010; 31: 666–675. [DOI] [PubMed] [Google Scholar]
- 33.Pouchot J, Despujol C, Malamut G, et al. Validation of a French version of the Quality of Life “Celiac Disease Questionnaire”. PloS One 2014; 9: e96346–e96346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zingone F, Iavarone A, Tortora R, et al. The Italian translation of the Celiac Disease-specific Quality of Life Scale in celiac patients on gluten free diet. Dig Liver Dis 2013; 45: 115–118. [DOI] [PubMed] [Google Scholar]
- 35.Casellas F, Rodrigo L, Molina-Infante J, et al. Transcultural adaptation and validation of the Celiac Disease Quality of Life (CD-QOL) survey, a specific questionnaire to measure quality of life in patients with celiac disease. Rev Esp Enferm Dig 2013; 2013: 585–593. [DOI] [PubMed] [Google Scholar]
- 36.Marchese A, Klersy C, Biagi F, et al. Quality of life in coeliac patients: Italian validation of a coeliac questionnaire. Eur J Intern Med 2013; 24: 87–91. [DOI] [PubMed] [Google Scholar]
- 37.Mustalahti K, Lohiniemi S, Collin P, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract 2002; 5: 105–113. [PubMed] [Google Scholar]
- 38.Johnston SD, Rodgers C, Watson RGP. Quality of life in screen-detected and typical coeliac disease and the effect of excluding dietary gluten. Eur J Gastroenterol Hepatol 2004; 16: 1281–1281. [DOI] [PubMed] [Google Scholar]
- 39.Nachman F, Maurino E, Vázquez H, et al. Quality of life in celiac disease patients: Prospective analysis on the importance of clinical severity at diagnosis and the impact of treatment. Dig Liver Dis 2009; 41: 15–25. [DOI] [PubMed] [Google Scholar]
- 40.Vilppula A, Kaukinen K, Luostarinen L, et al. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol 2011; 11: 136–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ukkola A, Mäki M, Kurppa K, et al. Diet improves perception of health and well-being in symptomatic, but not asymptomatic, patients with celiac disease. Clin Gastroenterol Hepatol 2011; 9: 118–123. [DOI] [PubMed] [Google Scholar]
- 42.Paavola A, Kurppa K, Ukkola A, et al. Gastrointestinal symptoms and quality of life in screen-detected celiac disease. Dig Liver Dis 2012; 44: 814–818. [DOI] [PubMed] [Google Scholar]
- 43.Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology 2014; 147: 610–617.e1. [DOI] [PubMed] [Google Scholar]
- 44.Nachman F, del Campo MP, González A, et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis 2010; 42: 685–691. [DOI] [PubMed] [Google Scholar]
- 45.Barratt SM, Leeds JS, Sanders DS. Quality of life in coeliac disease is determined by perceived degree of difficulty adhering to a gluten-free diet, not the level of dietary adherence ultimately achieved. J Gastrointestin Liver Dis 2011; 20: 241–245. [PubMed] [Google Scholar]
- 46.Hopman E, Koopman H, Wit J, et al. Dietary compliance and health-related quality of life in patients with coeliac disease. Eur J Gastroenterol Hepatol 2009; 21: 1056–1056. [DOI] [PubMed] [Google Scholar]
- 47.Paarlahti P, Kurppa K, Ukkola A, et al. Predictors of persistent symptoms and reduced quality of life in treated coeliac disease patients: A large cross-sectional study. BMC Gastroenterol 2013; 13: 75–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith D, Gerdes L. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr Scand 2012; 125: 183–193. [DOI] [PubMed] [Google Scholar]
- 49.Cannings-John R, Butler CC, Prout H, et al. A case-control study of presentations in general practice before diagnosis of coeliac disease. British J Gen Pract 2007; 57: 636–642. [PMC free article] [PubMed] [Google Scholar]
- 50.Addolorato G, Stefanini G, Capristo E, et al. Anxiety and depression in adult untreated celiac subjects and in patients affected by inflammatory bowel disease: A personality “trait” or a reactive illness? Hepatogastroenterology 1996; 43: 1513–1513. [PubMed] [Google Scholar]
- 51.Addolorato G, Capristo E, Ghittoni G, et al. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: A longitudinal study. Scand J Gastroenterol 2001; 36: 502–506. [DOI] [PubMed] [Google Scholar]
- 52.Häuser W, Janke KH, Klump B, et al. Anxiety and depression in adult patients with celiac disease on a gluten-free diet. World J Gastroenterol 2010; 16: 2780–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Addolorato G, Mirijello A, D’Angelo C, et al. Social phobia in coeliac disease. Scand J Gastroenterol 2008; 43: 410–415. [DOI] [PubMed] [Google Scholar]
- 54.Taylor E, Dickson-Swift V, Anderson K. Coeliac disease: The path to diagnosis and the reality of living with the disease. J Hum Nutr Diet 2013; 26: 340–348. [DOI] [PubMed] [Google Scholar]
- 55.Morris JS, Ajdukiewicz A and Read A. Neurological disorders and adult coeliac disease. Gut 1970; 11: 549–554. [DOI] [PMC free article] [PubMed]
- 56.Hallert C, Åstrøm J and Sedvall G. Psychic disturbances in adult coeliac disease. Scand J Gastroenterol 1982; 17: 25–28. [DOI] [PubMed]
- 57.Zingone F, Siniscalchi M, Capone P, et al. The quality of sleep in patients with coeliac disease. Aliment Pharmacol Ther 2010; 32: 1031–1036. [DOI] [PubMed]
- 58.Siniscalchi M, Iovino P, Tortora R, et al. Fatigue in adult coeliac disease. Aliment Pharmacol Ther 2005; 22: 489–494. [DOI] [PubMed]
- 59.Addolorato G, De Lorenzi G, Abenavoli L, et al. Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Aliment Pharmacol Ther 2004; 20: 777–782. [DOI] [PubMed]
- 60.Fera T, Cascio B, Angelini G, et al. Affective disorders and quality of life in adult coeliac disease patients on a gluten-free diet. Eur J Gastroenterol Hepatol 2003; 15: 1287–1292. [DOI] [PubMed]
- 61.Van Hees NJ, Van der Does W and Giltay EJ. Coeliac disease, diet adherence and depressive symptoms. J Psychosom Res 2013; 74: 155–160. [DOI] [PubMed]
- 62. Barratt SM, Leeds JS, Sanders DS. Factors influencing the type, timing and severity of symptomatic responses to dietary gluten in patients with biopsy-proven Coeliac disease. J Gastrointestin Liver Dis 2013; 22. [PubMed]
- 63.Kurppa K, Collin P, Mäki M, et al. Celiac disease and health-related quality of life. Expert Rev Gastroenterol Hepatol 2011; 5: 83–90. [DOI] [PubMed]
- 64.Addolorato G, Giuda DD, Rossi GD, et al. Regional cerebral hypoperfusion in patients with celiac disease. Am J Med 2004; 116: 312–317. [DOI] [PubMed]
- 65.Hallert C, Svensson M and Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment Pharmacol Ther 2009; 29: 811–816. [DOI] [PubMed]
- 66.Ferretti A, Parisi P and Villa MP. The role of hyperhomocysteinemia in neurological features associated with coeliac disease. Med Hypotheses 2013; 81: 524–531. [DOI] [PubMed]
- 67.Carta MG, Hardoy MC, Boi MF, et al. Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J Psychosom Res 2002; 53: 789–793. [DOI] [PubMed]
- 68.Garud S, Leffler D, Dennis M, et al. Interaction between psychiatric and autoimmune disorders in coeliac disease patients in the Northeastern United States. Aliment Pharmacol Ther 2009; 29: 898–905. [DOI] [PMC free article] [PubMed]
- 69.Tsigos C and Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002; 53: 865–871. [DOI] [PubMed]
- 70.Ludvigsson JF, Reutfors J, Ösby U, et al. Coeliac disease and risk of mood disorders: a general population-based cohort study. J Affect Disord 2007; 99: 117–126. [DOI] [PubMed]
- 71.Ciacci C, Iavarone A, Mazzacca G, et al. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol 1998; 33: 247–250. [DOI] [PubMed]
- 72.Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: A meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 359–365. [DOI] [PubMed] [Google Scholar]
- 73.Barratt SM, Leeds JS, Robinson K, et al. Reflux and irritable bowel syndrome are negative predictors of quality of life in coeliac disease and inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011; 23: 159–159. [DOI] [PubMed] [Google Scholar]
- 74.Turco R, Boccia G, Miele E, et al. The association of coeliac disease in childhood with functional gastrointestinal disorders: A prospective study in patients fulfilling Rome III criteria. Aliment Pharmacol Ther 2011; 34: 783–789. [DOI] [PubMed] [Google Scholar]
- 75.Giangreco E, D’agate C, Barbera C, et al. Prevalence of celiac disease in adult patients with refractory functional dyspepsia: Value of routine duodenal biopsy. World J Gastroenterol 2008; 14: 6948–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mönnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol 2011; 45: S98–S101. [DOI] [PubMed] [Google Scholar]
- 77.Filipović BF, Randjelovic T, Ille T, et al. Anxiety, personality traits and quality of life in functional dyspepsia-suffering patients. Eur J Intern Med 2013; 24: 83–86. [DOI] [PubMed] [Google Scholar]
- 78.Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: Epidemiology, genetic susceptibility and clinical characteristics. Brain 2003; 126: 685–691. [DOI] [PubMed] [Google Scholar]
- 79.Ludvigsson JF, Olsson T, Ekbom A, et al. A population-based study of coeliac disease, neurodegenerative and neuroinflammatory diseases. Aliment Pharmacol Ther 2007; 25: 1317–1327. [DOI] [PubMed] [Google Scholar]
- 80.Ludvigsson J, Zingone F, Tomson T, et al. Increased risk of epilepsy in biopsy-verified celiac disease: A population-based cohort study. Neurology 2012; 78: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 81.Hadjivassiliou M, Grünewald R, Lawden M, et al. Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology 2001; 56: 385–388. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigo L, Hernández-Lahoz C, Fuentes D, et al. Prevalence of celiac disease in multiple sclerosis. BMC Neurol 2011; 11: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferro MA. Risk factors for health-related quality of life in children with epilepsy: A meta-analysis. Epilepsia. Epub ahead of print 19 September 2014. [DOI] [PubMed]
- 84.Kim SY, Park SP. The role of headache chronicity among predictors contributing to quality of life in patients with migraine: A hospital-based study. J Headache Pain 2014; 15: 68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calvert M, Pall H, Hoppitt T, et al. Health-related quality of life and supportive care in patients with rare long-term neurological conditions. Qual Life Res 2013; 22: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 86.Briani C, Zara G, Alaedini A, et al. Neurological complications of celiac disease and autoimmune mechanisms: A prospective study. J Neuroimmunol 2008; 195: 171–175. [DOI] [PubMed] [Google Scholar]
- 87.Currie S, Hadjivassiliou M, Clark MJ, et al. Should we be ‘nervous’ about coeliac disease? Brain abnormalities in patients with coeliac disease referred for neurological opinion. J Neurol Neurosurg Psychiatry 2012; 83: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 88.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. Am J Psychiatry 2004; 161: 99–108. [DOI] [PubMed] [Google Scholar]
- 89.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 2002; 51: 273–279. [DOI] [PubMed] [Google Scholar]
- 90.Di Lazzaro V, Pilato F, Batocchi AP, et al. Tired legs—a gut diagnosis. Lancet 2010; 376: 1798–1798. [DOI] [PubMed] [Google Scholar]
- 91.Carnevale V, Filabozzi P, Cela P, et al. Tiredness: A feature of coeliac disease. Age Ageing 2000; 29: 462–463. [DOI] [PubMed] [Google Scholar]
- 92.Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol 2003; 15: 407–413. [DOI] [PubMed] [Google Scholar]
- 93.Skowera A, Peakman M, Cleare A, et al. High prevalence of serum markers of coeliac disease in patients with chronic fatigue syndrome. J Clin Pathol 2001; 54: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.National Collaborating Centre for Primary Care. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): Diagnosis and management of chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) in adults and children, London: National Institute for Health and Clinical Excellence, 2007. [Google Scholar]
- 95.Pulido O, Zarkadas M, Dubois S, et al. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can J Gastroenterol 2013; 27: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ciacci C, Peluso G, Iannoni E, et al. L-Carnitine in the treatment of fatigue in adult celiac disease patients: A pilot study. Dig Liver Dis 2007; 39: 922–928. [DOI] [PubMed] [Google Scholar]
- 97.Li B, Lloyd ML, Gudjonsson H, et al. The effect of enteral carnitine administration in humans. Am J Clin Nutr 1992; 55: 838–845. [DOI] [PubMed] [Google Scholar]
- 98.Anty R, Marjoux S, Bekri S, et al. Plasma carnitine is associated with fatigue in chronic hepatitis C but not in the irritable bowel syndrome. Aliment Pharmacol Ther 2011; 33: 961–968. [DOI] [PubMed] [Google Scholar]
- 99.Pistone G, Marino AD, Leotta C, et al. Levocarnitine administration in elderly subjects with rapid muscle fatigue: Effect on body composition, lipid profile and fatigue. Drugs Aging 2003; 20: 761–767. [DOI] [PubMed] [Google Scholar]