Abstract
Background
The adenoma detection rate of the endoscopist has been related to the post-colonoscopy interval risk of colorectal cancer.
Objective
The objective of this article is to estimate the impact of adenoma detection rate on the long-term colorectal cancer prevention rate.
Methods
A Markov model was constructed to simulate the efficacy and cost of colonoscopy screening according to the adenoma detection rate of the endoscopist in 100,000 individuals. Post-colonoscopy interval colorectal cancer risk and the relative risk of interval cancer among endoscopists with different adenoma detection rates were extracted from the literature. A 1.5 relative risk was assumed between endoscopists with low and average adenoma detection rates, and a relative risk of 11 between those with average and high adenoma detection rates. Both efficacy and costs were projected over a steady-state American population.
Results
Screening colonoscopy performed by endoscopists with low adenoma detection rates resulted in a 7% absolute reduction in the long-term colorectal cancer incidence prevention rate as compared to the same procedure performed by those with an average adenoma detection rate (70% vs. 77%). This difference increased to 21% when comparing endoscopists with an average with those with a high adenoma detection rate. When projected on the US population, this reduced efficacy resulted in an additional 1728 and 16,123 colorectal cancer cases and the loss of $117 million and $906 million per year in the two scenarios, respectively. These estimates were sensitive to the risk of post-colonoscopy interval colorectal cancer.
Conclusions
A substantial reduction in long-term colorectal cancer prevention rate may be expected when screening colonoscopy is performed by endoscopists with a suboptimal adenoma detection rate. A substantial saving may be expected when implementing policies to improve endoscopist adenoma detection rate.
Keywords: Colorectal cancer screening, endoscopist, adenoma detection rate, interval cancer, colonoscopy, cost-effectiveness
Abbreviations
CRC: colorectal cancer; LOC: localized; REG: regional; DIS: distant; ICER: incremental cost-effectiveness ratio; ADR: adenoma detection rate; PDR: polyp detection rate
Introduction
The long-term efficacy of colonoscopy in preventing colorectal cancer (CRC) incidence and/or mortality has been addressed in cohort and case-control studies.1–4 Although the majority of these studies showed a very high CRC prevention rate, some studies showed a suboptimal CRC protection rate.2,3 This appeared to be related to an unexpectedly high risk of post-colonoscopy CRC in the early years after colonoscopy. In a large administrative cohort of largely symptomatic patients with negative colonoscopy, CRC prevention rate appeared to be markedly higher when assessed 10 years after colonoscopy rather than after five years—i.e. 72% vs. 41%—because of the unexpected occurrence of interval cancer in the early years following colonoscopy.3
Quality of endoscopy has been strictly related to the risk of post-colonoscopy CRC.5 In large administrative cohort or case-control studies, the risk of early post-colonoscopy cancer appeared to be independently predicted by a relatively low adenoma/polyp detection rate (ADR/PDR).6–8 In detail, such risk was higher when comparing the lowest quartile of endoscopists with those with a higher ADR/PDR. It was similarly lower when the ADR/PDR of the selected endoscopist was ranked as high (≥20%) as compared with those with a lower ADR.9 This has been recently confirmed in a randomized clinical trial (RCT) on sigmoidoscopy screening, in which the risk of distal interval cancer was significantly increased for patients of examiners with a low distal ADR.10
No study assessed the role of ADR in determining the long-term colonoscopy-related CRC prevention rate, causing uncertainty as to the potential benefit of any interventional policy on this issue. Additionally, it is unclear whether the main aim of such a policy would be either to simply focus on the (few) endoscopists with low ADR or to also include those with a medium ADR in order to achieve a uniformly high ADR.
The aim of this micro-modelling simulation was to calculate the potential impact of endoscopist ADR and related policies on the efficacy and costs of screening colonoscopy.
Methods
End-points of this analysis address the following:
What is the difference in long-term efficacy of colonoscopy between endoscopists with low ADR and those with average ADR, and between those with average and those with high ADR?
What is the projected impact on the United States (US) population of different degrees of long-term colonoscopy efficacy according to the ADR of the endoscopists?
What is the projected improvement in the efficacy of colonoscopy when implementing policies to increase the ADR of endoscopists with an initially low or average ADR?
To address these issues, we simulated primary prevention with colonoscopy in a theoretical cohort of 100,000 male and female American citizens generated by a Markov model. Briefly, endoscopic screening was simulated to be repeated every 10 years between 50 and 80 years, with post-polypectomy surveillance according to polyp size and histology. Age- and site-related CRC incidence and mortality were assumed from the Surveillance, Epidemiology, and End Results (SEER) database for the natural history cohort, in order to exclude any model-related uncertainty, due to the incomplete knowledge of the inter-polyp transition rates, as previously reported.11–13
Efficacy of colonoscopy screening
Long-term reduction of CRC incidence and mortality by colonoscopy screening was calculated in order to match with the risk of post-colonoscopy interval CRC available in the literature. In detail, we calibrated the overall efficacy of colonoscopy screening on the subsequent risk of post-colonoscopy interval CRC in order to match the 0.02% annual rate of interval CRC showed in the only available large series of screening colonoscopy, including 188,788 person-years of post-colonoscopy follow-up with a mean age of 55 years.9
Efficacy of colonoscopy screening according to ADR/PDR
In order to include the role of endoscopist ADR/PDR, we further calibrated the long-term efficacy of colonoscopy screening based on the available data on the association between the endoscopist ADR and the post-colonoscopy risk of interval cancer. In detail, we calibrated the long-term efficacy of colonoscopy screening for each ADR category in order to match with the relative risk (RR) of post-colonoscopy interval CRC among the different ADR categories shown in recent studies.6,8–10 The available series may be divided into two different scenarios (Table 1):
Those comparing the few (i.e. lowest quartile) endoscopists with low ADR with those with average (i.e. medium or high) ADR. For this scenario, we adopted a 1.5 RR of post-colonoscopy interval CRC between the endoscopists with low ADR and those with average ADR, as median of the RRs estimated by the available series (Table 1).6,8,10
Those comparing the few (i.e. 22%) endoscopists with high ADR with those with average (i.e. low or medium) ADR. For this scenario, we adopted a RR of 11 of post-colonoscopy interval CRC between the endoscopists with average ADR and those with high ADR.9
Table 1.
Relative risk (RR) for the incidence of post-colonoscopy interval colorectal cancer between the endoscopists below and above the ADR/PDR threshold adopted in the available studies
| Author | ADR/PDR | Threshold | Percentage of endoscopists above threshold | RR |
|---|---|---|---|---|
| Kaminski et al.9 | ADR | 20% | 22% | 11–12.5 |
| Rogal et al.10 | ADR | 8% | 75% | 1.4 |
| Cooper et al.6 | PDR | 25% | 75% | 1.2–1.4 |
| Baxter et al.8 | PDR | 14% | – | 1.3–1.9 |
ADR: adenoma detection rate; PDR: polyp detection rate.
Because of the lack of direct comparisons between the low and the high ADR endoscopists, we performed two different simulations (low vs. average ADR and high vs. average ADR) in order to base our model on the precise estimates of the original studies. Of note, the low- and the high-risk groups virtually correspond with the lowest and highest quartiles of the corresponding series, so that the first simulation actually compares the lowest quartile with the remaining three higher quartiles, while the second compares the highest with the remaining three lower quartiles.
Endoscopic training
In order to estimate the eventual cost of training for endoscopists in order to increase ADR, we assumed the 2010 estimate by the American Board of Internal Medicine of 12,907 board-certified gastroenterologists.14 Despite the fact that no recommendation on an eventual policy for improving the endoscopists ADR has been officially proposed, a two-session training course (one day) has actually been tested in two RCTs aiming at improving the ADR.15
Costs
Age-/size-/site-specific prevalence of non-advanced and advanced adenomas were matched with estimates from autopsy and endoscopic data in order to compute the costs related to polypectomy and follow-up (Supplementary material). Reimbursement data for direct costs of endoscopy and related complications, as well as for stage-specific CRC treatment, were based on Medicare data.16 According to a 2010 estimate by the Bureau of Labor Statistics, the mean annual wage for an American endoscopist was $200,000.17 In order to include in the simulation the potential higher costs of polypectomy and post-polypectomy surveillance, we assumed different sensitivities for adenomatous polyps, in order to match with the different prevalence of ADR available in the different series.9
Cost-effectiveness analysis
Future costs and life-years saved were discounted using an annual rate of 3%. The relative performance of the strategies was measured using the incremental cost-effectiveness ratio (ICER), defined as the additional cost of a specific strategy, divided by its additional clinical benefit, compared with the next least expensive strategy. An ICER of $50,000 per life-year gained was used as the willingness-to-pay threshold to differentiate between efficient and inefficient procedures.18
Simulation output
In the reference case scenario, we assumed a complete adherence, in order to simulate the efficacy and cost of colonoscopy screening in those actually screened (i.e. per protocol analysis), analogously to similar analysis.11,19 To obtain the national projection of the CRC prevention rate of a primary colonoscopy screening according to the ADR of the endoscopist, we corrected the initial simulation for the actual adherence rate (intention-to-treat analysis).11,19 In detail, adherence of the American population to CRC screening was estimated to be 65%.20 In order to project the outcomes of our simulation on the US population, we assumed a steady state for population size and age distribution, represented by the year 2009 US census data. No discounting was used in these national projections.19 The model was simulated by using Excel spreadsheets (Microsoft Corp., Redmond, WA).
Sensitivity analysis
A systematic sensitivity analysis was performed for all the variables of the model, with the most relevant results being reported.
Results
As shown in Table 2, in the no screening simulation, 5903 CRC cases and 2482 CRC-related deaths occurred in the simulated cohort of 100,000 Americans, resulting in the loss of 31,839 undiscounted life-years. Costs in the no screening simulation were purely related with the expenditure for CRC care, with an estimate of $2227 per person (Table 2).
Table 2.
Costs and efficacies for all the simulated strategies for a cohort of 100,000 individuals. The strategy indicated as “overall colonoscopy screening” represents the reference case scenario
| No screening | Overall colonoscopy screening | Low-ADR | Average ADRa | High ADR | |
|---|---|---|---|---|---|
| CRC cases, n | 5903 | 1460 | 1771 | 1353–1726 | 459 |
| CRC prevented, n | – | 4442 | 4132 | 4177–4549 | 5443 |
| CRC prevention rate, % | – | 75 | 70 | 71–77 | 92 |
| CRC deaths, n | 2482 | 681 | 808 | 637–790 | 273 |
| CRC death prevention rate, % | – | 73 | 67 | 68–74 | 89 |
| Life-years gained, n | – | 14,960 | 13,881 | 14,036–15,333 | 18,443 |
| Screening cost, $/person | – | 2387 | 2259 | 2387 | 2486 |
| Care for CRC, $/person | 2227 | 503 | 624 | 461–606 | 112 |
| Total, $/person | 2227 | 2890 | 2883 | 2848–2993 | 2598 |
| ICER vs no screening, $ per life-year gained | – | 4424 | 4781 | 3980–5451 | 2014 |
CRC: colorectal cancer; ICER: Incremental cost-effectiveness ratio. aThe upper and lower limits of each variable in this scenario represent the estimates for the average-risk endoscopists in the two different simulations. In detail, in the low- adenoma detection rate (ADR) scenarios, the average risk endoscopists were supposed to represent those in the upper 75% of the endoscopist population, whilst in the high-ADR scenario those in the lower 78%.
Reference case scenario
According to the model, the value of long-term CRC incidence prevention rate by screening colonoscopy corresponding to an age-adjusted 0.02% annual risk of post-colonoscopy interval CRC—as estimated in a previous study9—in the patients attending screening was equal to 75%. Colonoscopy screening also resulted in a substantial decrease in CRC treatment costs when compared with no screening ($503/person vs. $2227/person). This was offset by the cost of screening and follow-up testing ($2387/person), resulting in an overall discounted cost/person of $2890 (Table 2). Colonoscopy screening appeared to be a cost-effective alternative to no screening with an ICER of $4424 per life-year saved (Table 2).
Efficacy according to ADR-endoscopists
Low vs. average ADR
When assuming a 1.5 RR of interval CRC between endoscopists with low and average ADR and a 25%/75% distribution of the screening colonoscopies between the two groups, the corresponding values of long-term CRC incidence prevention rate by screening colonoscopy were 70% and 77%, respectively. Endoscopists with low ADR were associated with substantially higher CRC-related costs ($624/person vs. $461/person) that were only partially offset by the lower cost in polypectomy and post-polypectomy surveillance ($2259/person vs. $2387/person).
When assuming all the endoscopists with low ADR become average ADR endoscopists owing to the simulated training, the overall CRC incidence prevention rate increased from 75% (reference case scenario) to 77%. This training-related improvement was also associated with a further reduction of the CRC-related costs as compared with the reference case scenario ($461/person vs. $503/person) (Table 2). Being more effective and less costly, the reference case scenario appeared to be dominated by the post-training scenario with a discounted savings of $42 per person.
Average vs. high ADR
When assuming an RR of 11 of interval CRC between endoscopists with average and high ADR, as well as a 78%/22% distribution of the screening colonoscopies between the two groups, the corresponding values of long-term CRC incidence prevention rate were 71% and 92%, respectively. Endoscopists with high ADR were also associated with a substantial reduction of the CRC-related costs ($112/person vs. $606/person), while the additional cost for polypectomy and post-polypectomy surveillance was marginal ($2486/person vs. $2387/person), as shown in Table 2.
When assuming all the endoscopists with average ADR become high ADR endoscopists owing to the simulated training, the overall CRC incidence prevention rate increased from 75% (reference case scenario) to 92%. This training-related improvement resulted in a further reduction of the CRC-related costs as compared with the reference case scenario ($112/person vs. $503 person) (Table 2). Being more effective and less costly, the reference case scenarios appeared to be dominated by the high ADR endoscopist strategy, resulting in a discounted savings of $292 per person.
Projection on the US population
In the no screening scenario, the actual number of CRC cases and CRC deaths simulated for the entire US population were 144,424 and 58,311 per year, respectively, resulting in an annual undiscounted cost of $9.7 billion for CRC care (Table 3). When assuming a 65% adherence rate and the 75% CRC prevention rate shown in the reference case scenario, the absolute number of CRC and CRC deaths prevented per year by screening colonoscopy in the US population were 71,933 and 28,179, respectively (Table 3). The undiscounted annual cost of this strategy was equal to $10.9 billion. Of these, only $5.9 billion were due to CRC treatment cost, the remaining $5 billion being related to the costs of the screening program.
Table 3.
Projection of the model outputs on the American population. No discounting was applied because the model outputs reflected all persons aged 50–100 years of age at a given point in time in the steady state. The post-training estimates represent the potential improvement when applying endoscopist-training in the two scenarios of low- and high-ADR endoscopists (see text)
| No screening | Overall colonoscopy screening | Post-training low ADR scenario | Post-training high ADR scenario | |
|---|---|---|---|---|
| CRC cases, n | 144,424 | 72,491 | 70,763 | 56,368 |
| CRC prevented, n | – | 66,417 | 73,661 | 88,057 |
| CRC deaths, n | 58,311 | 30,132 | 29,449 | 23,754 |
| CRC deaths prevented, n | – | 28,179 | 28,862 | 34,557 |
| Total cost, $ billion | 9.7 | 10.9 | 10.8 | 10 |
ADR: adenoma detection rate; CRC: colorectal cancer.
When simulating the training-related improvement in the low and high ADR scenarios (see above), the additional reductions in CRC cases and CRC-related deaths (vs. the reference case scenario) were 1728 and 684 per year, and 16,123 and 6378, respectively (Table 3). Because of the additional reduction of CRC-related costs, the cost of the two post-training scenarios were $10.8 and $10 billion, respectively, corresponding to an annual difference of $117 million and $906 million. This gain appeared to be only marginally affected by the initial investment in endoscopist training ($770 for each endoscopist), resulting in a total of $2.4 and $8 million in the low- and high-ADR endoscopist scenarios, respectively.
Sensitivity analysis
Low vs. average ADR
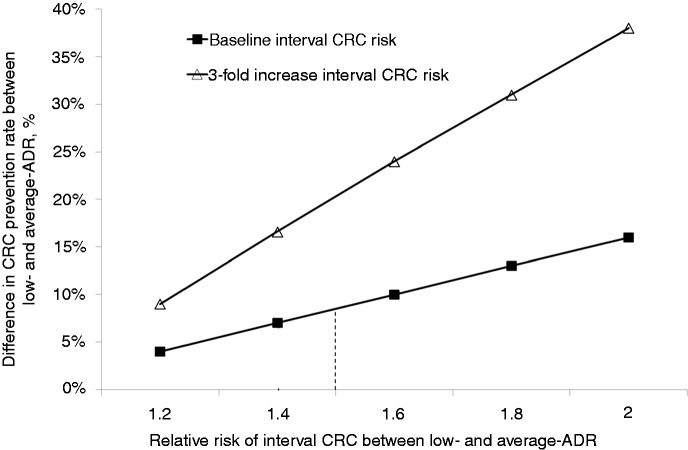
The difference in the CRC prevention rate according to the ADR of endoscopists was sensitive to the RR of post-colonoscopy interval cancer. When assuming a RR of 2 (as compared with the baseline 1.4), the values of long-term CRC incidence prevention rate for low and average ADR endoscopists appeared to be 64% and 79%, respectively, the absolute difference passing from 7% in the reference case scenario to 15% (Figure 1). In this scenario, the post-training-related improvement in the CRC prevention rate increased from the baseline 2% to 4%.
Figure 1.
Absolute difference in long-term CRC prevention rate between low and average ADR endoscopists according to the relative risk of post-colonoscopy interval CRC and the absolute risk of post-colonoscopy interval CRC (see text). CRC: colorectal cancer; ADR: adenoma detection rate.
The result of the analysis also varied according to the baseline risk of post-colonoscopy interval cancer. By increasing this risk from the baseline 0.02% to 0.06%—corresponding to a decrease of colonoscopy efficacy from 75% to 48%—the corresponding CRC prevention rates for low and average ADR endoscopists were 35% and 52%, respectively, the difference between the two groups increasing from the baseline 7% to 17% (Figure 1). By further increasing the risk of interval CRC to 0.1%—corresponding to a 30% CRC prevention rate—, the difference in long-term CRC prevention rate between the two groups would further increase to 23% (12% vs. 35%).
At two-way sensitivity analysis, when assuming a 0.06% risk of interval CRC and a RR of 2 between low and average ADR endoscopists, the difference in the CRC prevention rate between the two groups increased to 38% (19% vs. 57%) (Figure 1). In this case, the training-related improvement appeared to be associated with an absolute 7% increase in the CRC prevention rate (i.e. from 48% to 55%). The higher impact of the training in this scenario—in which a reduced overall efficacy of colonoscopy in preventing CRC was simulated—was related with the higher burden of CRC on which the improvement of quality could be applied.
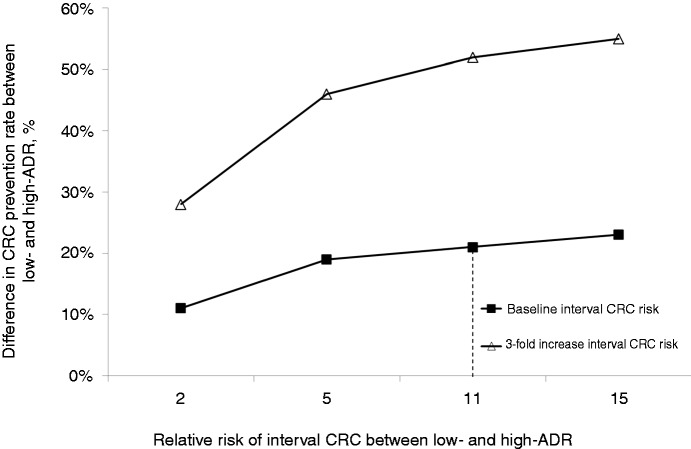
Average vs. high ADR
When assuming a 5 and 2 RR (as compared with the baseline 11) for interval CRC between the average and high ADR endoscopists, the absolute difference in CRC prevention rate between the two groups was reduced from 21% to 19% and 11%, respectively (Figure 2). In these cases, the post-training-related improvement in the CRC prevention rate was reduced from the baseline 17% to 15% and 9%, respectively.
Figure 2.
Absolute difference in long-term CRC prevention rate between average and high ADR endoscopists according to the relative risk of post-colonoscopy interval CRC and the absolute risk of post-colonoscopy interval CRC (see text). CRC: colorectal cancer; ADR: adenoma detection rate.
By increasing the baseline risk of post-colonoscopy interval cancer in the reference case scenario to 0.06%, the corresponding CRC prevention rates for average and high ADR endoscopists were 36% and 89% (Figure 2). At two-way sensitivity analysis, when assuming a 0.06% risk of interval CRC and a RR of 5 between the endoscopists with average and high ADR, the post-training improvement in the CRC prevention rate further decreased to 8%.
The main results of our analysis were robust to changes in overall adherence to colonoscopy screening.
Discussion
According to our simulation, there is a 7%–21% absolute difference in the long-term CRC prevention rate when subgrouping endoscopists with different ADR, accounting for a substantial loss of life-years due to unprevented CRC deaths and related costs in the US population. We also showed that there is a substantial difference when comparing two possible training policies for improving endoscopist ADR. In detail, the training-related improvement in long-term CRC prevention appeared to be three times higher when passing from an average to high ADR than when passing from low to average ADR. We also showed that the eventual cost of training for endoscopists only marginally affected the potential economic impact of any policy aiming at improving the endoscopist ADR.
According to our sensitivity analysis, the gradient between endoscopists with lower and higher ADR increased when assuming a suboptimal efficacy of colonoscopy in preventing CRC. When assuming a reduced protection by screening colonoscopy, the difference in CRC prevention rate between the endoscopists with lower and higher ADR substantially increased. When assuming the same 0.1% risk of interval CRC recently estimated in Canada,2 the difference in CRC prevention rate between the endoscopists with different ADR ranged from 23% to 52% according to the adopted classification.
The main limitation of our analysis is represented by the lack of studies showing a higher long-term CRC prevention rate when screening colonoscopy is performed by endoscopists with high or average ADR. We compensated for such a deficiency by exploiting a simulation model in order to convert the RRs of post-colonoscopy interval CRC to the long-term CRC prevention rate.
When subgrouping endoscopists with different ADR, a 7%–21% reduction in long-term CRC prevention rate may be estimated, resulting in a substantial loss of life-years and economic resources.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
DR has received research support from Olympus. The other authors have nothing to declare.
Guarantor of the article
CH, DR, CH, MK, GC: study concept and design; acquisition of data; drafting of the manuscript. CH: analysis and interpretation of data; statistical analysis; all authors approved the final version of the manuscript.
References
- 1.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150: 1–8. [DOI] [PubMed] [Google Scholar]
- 3.Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination: Evidence for a 10-year interval between colonoscopies. JAMA 2006; 295: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 4.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: RecommeCtions of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002; 97: 1296–1308. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GS, Xu F, Barnholtz Sloan JS, et al. Prevalence and predictors of interval colorectal cancers in Medicare beneficiaries. Cancer 2012; 118: 3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh H, Nugent Z, Demers AA, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: A population-based study. Am J Gastroenterol 2010; 105: 2588–2596. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140: 65–72. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 10.Rogal SS, Pinsky PF, Schoen RE. Relationship between detection of adenomas by flexible sigmoidoscopy and interval distal colorectal cancer. Clin Gastroenterol Hepatol 2013; 11: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan C, Rex DK, Cooper GS, et al. Primary prevention of colorectal cancer with low-dose aspirin in combination with endoscopy: A cost-effectiveness analysis. Gut 2012; 61: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 12.Hassan C, Rex DK, Zullo A, et al. Loss of efficacy and cost-effectiveness when screening colonoscopy is performed by nongastroenterologists. Cancer 2012; 118: 4404–4411. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg A, Delco F. Cost-effectiveness of a single colonoscopy in screening for colorectal cancer. Arch Intern Med 2002; 162: 163–168. [DOI] [PubMed] [Google Scholar]
- 14.CMS.gov. Centers for Medicare & Medicaid Services, http://www.cms.hhs.gov/pfslookup, www.cms.hhs.gov (accessed 15 September 2014).
- 15.Coe SG, Crook JE, Diehl NN, et al. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol 2013; 108: 219–226 quiz 227. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Labor. Bureau of Labor Statistics. Consumer price index for all urban consumers (CPI-U), U.S. city average, medical care, www.bls.gov/cpi/home.htm (accessed 14 February 2013).
- 17.U.S. Department of Labor. Bureau of Labor Statistics. Occupational Outlook Handbook, http://www.bls.gov/ooh/healthcare/physicians-and-surgeons.htm#tab-5 (accessed 14 February 2013).
- 18.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000; 38: 583–637. [DOI] [PubMed] [Google Scholar]
- 19.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology 2005; 129: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 20.Joseph DA, King JB, Miller JW, et al. Prevalence of colorectal cancer screening among adults—Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep 2012; 61(Suppl): 51–56. [PubMed] [Google Scholar]