Abstract
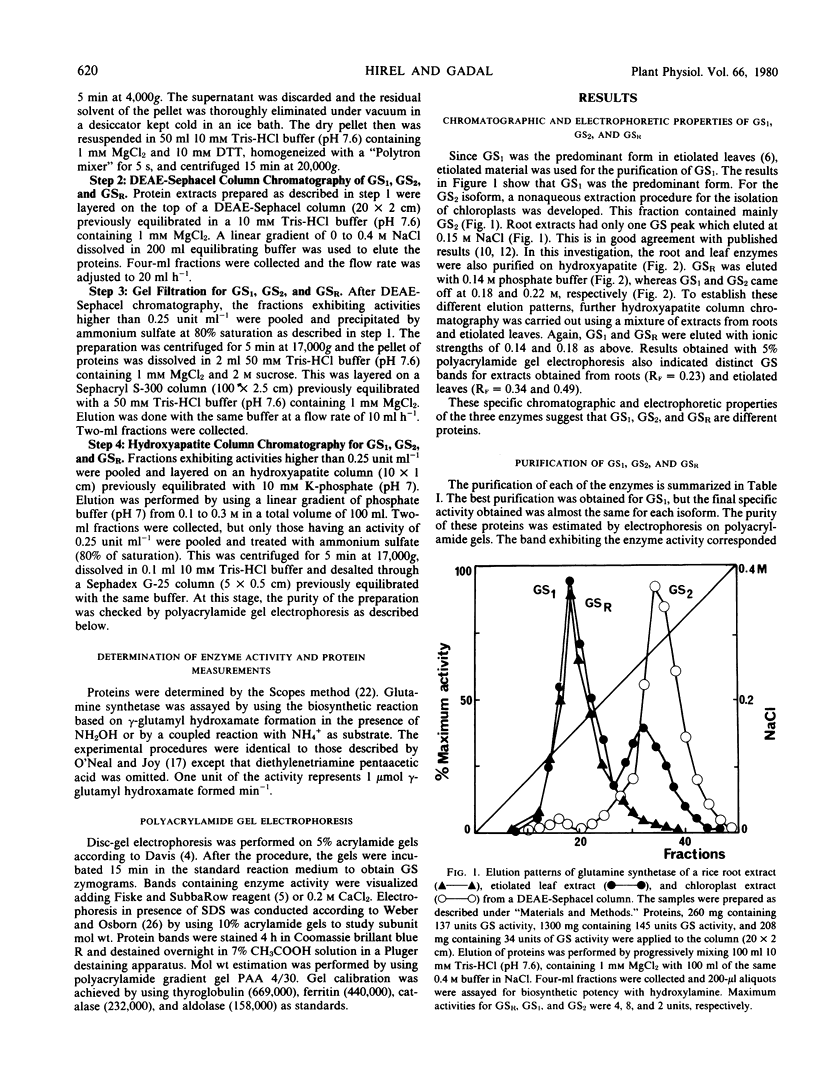
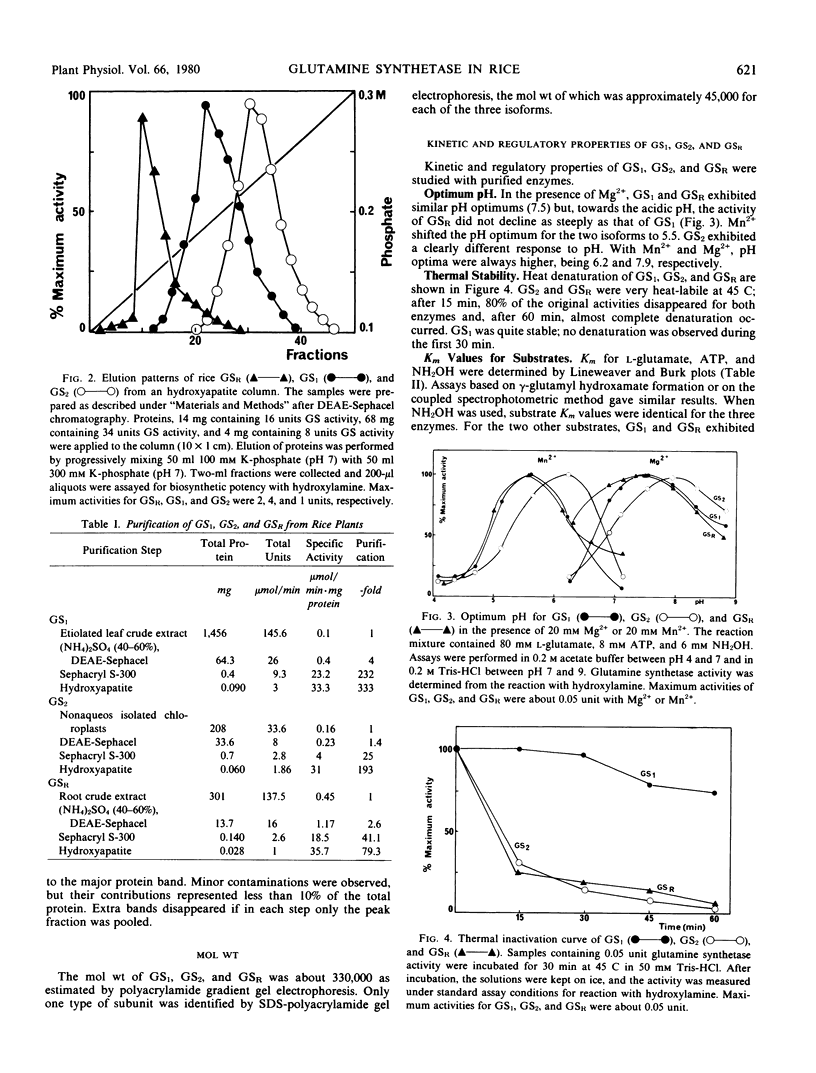
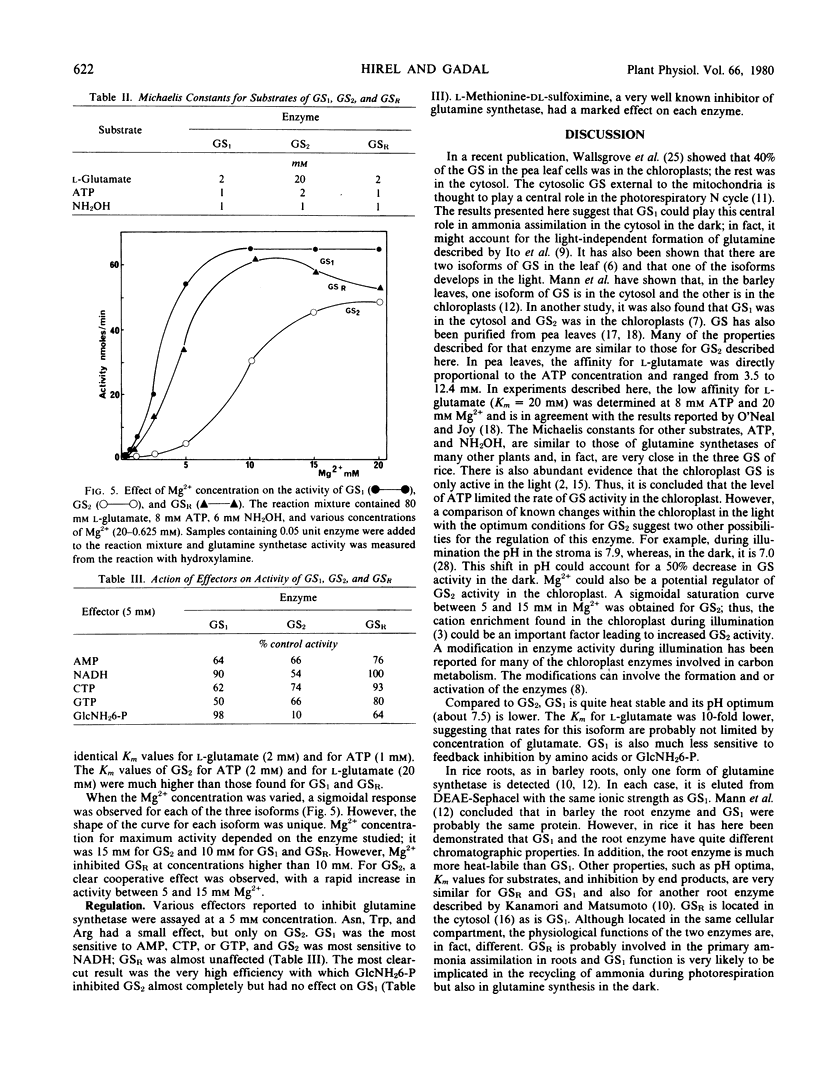
Chromatographic, kinetic, and regulatory properties of glutamine synthetase in rice were investigated. By DEAE-Sephacel column chromatography, two forms (glutamine synthetase 1 and glutamine synthetase 2) were identified in leaves and one form (glutamine synthetase R) was identified in roots. Purification on hydroxyapatite and gel electrophoresis showed that glutamine synthetase R was distinct from the leaf enzymes. The three isoforms were purified to similar specific activities and their properties were studied. Heat lability, pH optimum about 8, Km for l-glutamate of 20 millimolar, and inhibition by glucosamine 6-phosphate were the main characteristics of glutamine synthetase 2. Heat stability, pH optimum about 7.5, Km for l-glutamate of 2 millimolar, and no effect of glucosamine 6-phosphate differentiated glutamine synthetase 1 from glutamine synthetase 2. Glutamine synthetase R was also a labile protein but its kinetic and regulatory properties were quite similar to those of glutamine synthetase 1. These results clearly demonstrate the existence of three isoforms of glutamine synthetase in rice, two of which are located in the leaves and the third in the roots.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kanamori T., Matsuoto H. Glutamine synthetase from rice plant roots. Arch Biochem Biophys. 1972 Sep;152(1):404–412. doi: 10.1016/0003-9861(72)90230-5. [DOI] [PubMed] [Google Scholar]
- Mann A. F., Fentem P. A., Stewart G. R. Identification of two forms of glutamine synthetase in barley (Hordeum vulgare). Biochem Biophys Res Commun. 1979 May 28;88(2):515–521. doi: 10.1016/0006-291x(79)92078-3. [DOI] [PubMed] [Google Scholar]
- McParland R. H., Guevara J. G., Becker R. R., Evans H. J. The purification and properties of the glutamine synthetase from the cytosol of Soya-bean root nodules. Biochem J. 1976 Mar 1;153(3):597–606. doi: 10.1042/bj1530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov A. S., Evstigneeva Z. G., Kretovich V. L., Stel'mashchuk V. Ia, Samsonidze T. G., Kiselev N. A. Ochistka, svoistva i chetvertichnaia struktura glutaminsintetazy Khlorelly. Biokhimiia. 1977 Feb;42(2):350–358. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Siedel J., Shelton E. Purification and properties of Azotobacter vinelandii glutamine synthetase. Arch Biochem Biophys. 1979 Jan;192(1):214–224. doi: 10.1016/0003-9861(79)90086-9. [DOI] [PubMed] [Google Scholar]
- Stasiewicz S., Dunham V. L. Isolation and characterization of two forms of glutamine synthetsae from soybean hypocotyl. Biochem Biophys Res Commun. 1979 Mar 30;87(2):627–634. doi: 10.1016/0006-291x(79)91840-0. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wedler F. C., Hoffmann F. M. Glutamine synthetase of Bacillus stearothermophilus. I. Purification and basic properties. Biochemistry. 1974 Jul 30;13(16):3207–3214. doi: 10.1021/bi00713a002. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]



