Abstract
Systemic therapy is the standard care for patients with unresectable advanced colorectal cancer (CRC), but salvage surgery of metastatic disease should be considered in the case of adequate tumor shrinkage. Several drugs and combinations are now available for use in treating patients with advanced CRC, but the optimal sequence of therapy remains unknown. Moreover, the administration of antitumor therapy can be modulated by periods of maintenance or treatment breaks rather than delivered as full therapy until disease progression or unacceptable toxicity, followed by reintroduction of prior full therapy when required, before switching to other drugs. Consequently, randomized strategy trials are needed to define the optimal treatment sequences. Molecular testing for Kirsten rat sarcoma viral oncogene homolog (KRAS) and neuroblastoma RAS viral oncogene homolog (NRAS) is mandatory but not sufficient to select appropriate patients for epidermal growth factor receptor (EGFR) monoclonal antibody (MoAb) therapy.
Keywords: chemotherapy, colorectal cancer, maintenance, molecular targeted agents, strategy, treatment
Introduction
Colorectal cancer
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide but with a geographical variation in incidence and mortality. The highest mortality rates present in both sexes in Central and Eastern Europe [Globocan, 2012]. Metastatic disease can occur at the same time as the diagnosis of primary tumor (synchronous metastatic disease) or subsequently (metachronous metastatic disease) after surgery of primary tumor followed or not by adjuvant chemotherapy. The prognosis of patients with metachronous disease is usually more favorable.
Resectability of the metastatic disease should be assessed at the time of first diagnosis of involvement of one or several metastatic sites in a multidisciplinary approach with surgeons, medical oncologists, gastroenterologists, and radiologists. The theoretical definition of potentially resectable tumors and patient classification has been proposed, but mainly depends on the experience of each individual surgeon [Adams et al. 2013]. In the case of initially unresectable disease, systemic therapy is the standard care, but the evaluation for conversion to resectable disease should be considered at each tumor assessment.
Tumor biology
Half of the patients with advanced CRC harbor a Kirsten rat sarcoma viral oncogene homolog (KRAS) or neuroblastoma N-Ras (NRAS) tumor gene mutation, which is a negative predictive biomarker for anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAbs) therapy in these patients [Lièvre et al. 2006]. Thus, only patients with RAS wild-type metastatic CRC (mCRC) are eligible for MoAbs EGFR inhibitors therapy. When adding patients with serine/threonine-protein kinase B-Raf (BRAF) mutant tumors (10%), the RAS/RAF mutant population represents 60% of previously untreated mCRC patients (Figure 1A).
Figure 1.
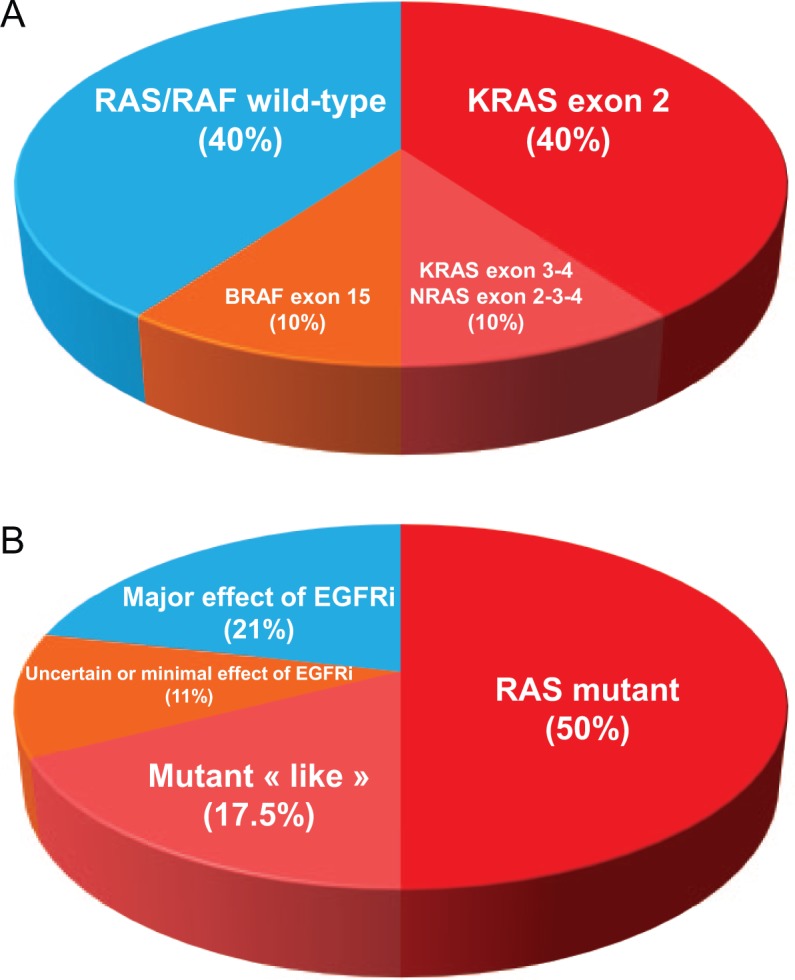
Colon cancer biology: focus on epidermal growth factor (EGF) pathway and sensitivity to epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAbs). (A) Frequency of KRAS, NRAS, and BRAF tumor genes mutations in patients with advanced colorectal cancer (CRC). (B) Role of tumor biology for the estimated sensitivity to EGFR MoAbs in metastatic CRC.
Drugs
Until the 2000s, 5-fluorouracil (5-FU) was the only approved drug for the treatment of advanced CRC, which administration was producing median overall survival (OS) of less than 1 year. Other orally active 5-FU prodrugs (capecitabine, tegafur-uracil, S1) are also available. Patient outcomes were improved with the use of irinotecan, an inhibitor of topoisomerase I, and oxaliplatin, which were approved by the United States (US) Food and Drug Administration (FDA) in 1998 and 2002, respectively. The combination of fluoropyrimidine with either irinotecan or oxaliplatin was associated with a near-doubling median survival, which surpassed 2 years with the addition of molecular-targeted agents. Vascular endothelial growth factor (VEGF) inhibition with MoAbs increased survival in combination with chemotherapy in first- or second-line treatment of patients with mCRC. Anti-EGFR MoAbs (i.e. cetuximab, panitumumab) also improved patient outcomes, but only in the absence of RAS tumor gene mutations. More recently, regorafenib, an orally active inhibitor of angiogenic, stromal and oncogenic kinases, improved survival in heavily pretreated CRC patients. But the optimal strategy or the best way to combine and sequence all of these drugs available in routine practice has not yet been established.
In the case of initially unresectable metastatic disease, the association of chemotherapy (single-agent to three-drug regimen) and a molecular targeted agent, either antiangiogenic (i.e. bevacizumab) or EGFR inhibitor monoclonal antibody (cetuximab, panitumumab), is the standard practice for first-line therapy. Of note, the combination of chemotherapy with both the EGFR and VEGF MoAbs inhibitors led to adverse outcomes in two large randomized phase III trials (PACCE, CAIRO2) [Hecht et al. 2009; Tol et al. 2009].
First-line therapy
Chemotherapy with antiangiogenic drugs
In the first-line setting, bevacizumab can be combined with an oxaliplatin-based (NO16966) [Saltz et al. 2008], an irinotecan-based (AVF2107g) [Hurwitz and Kabbinavar, 2005] chemotherapy doublet, chemotherapy triplet (GONO TRIBE) [Loupakis et al. 2014], or even with fluoropyrimidine monochemotherapy (MAX, AVF2192, AVEX) [Tebbutt et al. 2010; Kabbinavar et al. 2005; Cunningham et al. 2013]
Irinotecan-based chemotherapy with bevacizumab
In the AVF2107g study, the addition of bevacizumab to an irinotecan-based chemotherapy resulted in statistically significant improvement in OS (primary endpoint) among 813 patients with previously untreated mCRC [hazard ratio (HR)OS= 0.66; p < 0.001] [Hurwitz et al. 2004]. Secondary endpoints of progression-free survival (PFS; HRPFS = 0.54) and response rate (RR) were also improved.
Oxaliplatin-based chemotherapy with bevacizumab
The NO16966 study included 1401 patients with previously untreated mCRC with a median age of 60 years [Saltz et al. 2008]. The addition of bevacizumab to either FOLFOX (leucovorin/5-FU/oxaliplatin regimen) or XELOX (capecitabine plus oxaliplatin regimen) led to a 17% improvement of PFS (primary endpoint, HR = 0.83; p = 0.0023). This benefit was higher when censoring patients at the time of drug discontinuation (‘on-treatment PFS’, HR = 0.63). Unlike other trials, RR was similar with or without bevacizumab.
Triplet chemotherapy (irinotecan- and oxaliplatin-based) with bevacizumab
In the GONO TRIBE study, 508 patients with unresectable mCRC were randomized to receive 6 months of bevacizumab-based induction therapy with either FOLFIRI (regimen of leucovorin/5-FU/irinotecan) or FOLFOXIRI (regimen of leucovorin/5-FU/irinotecan/oxaliplatin), followed by maintenance therapy with fluoropyrimidine-bevacizumab [Loupakis et al. 2014]. The addition of oxaliplatin to FOLFIRI-bevacizumab significantly increased PFS (primary endpoint) from 9.7 to 12.1 months (HR = 0.75; p = 0.003) and RR from 53% to 65% (p = 0.006), but neither R0 salvage surgery rate (12% versus 15%, p = 0.33) nor OS were improved (HR = 0.79; p = 0.054).
Fluoropyrimidine-bevacizumab in elderly patients
The international Australasian Gastrointestinal Trials Group (AGITG) MAX study [Tebbutt et al. 2010] randomized 471 patients with unresectable mCRC considered suitable for initial monotherapy. Patients received low-intensity chemotherapy including capecitabine alone, capecitabine plus bevacizumab, or capecitabine-mitomycin C plus bevacizumab. The median age of patients was 68 years. After a median follow up of 31 months, median PFS (primary endpoint) was improved from 5.7 months in the capecitabine group to 8.5 months in the capecitabine–bevacizumab group (HR = 0.63, p < 0.001). Median survival was not statistically different between these patients (18.9 months in both groups). The triplet combination arm was not superior to the capecitabine–bevacizumab doublet arm neither for PFS nor OS. The MAX study results were consistent with an earlier phase II study (AVF2192) performed in patients over the age of 65 years and considered unfit for first-line irinotecan [Kabbinavar et al. 2005].
AVEX
In the AVEX randomized phase III trial, 280 patients with previously untreated, unresectable mCRC, and not eligible to oxaliplatin-based or irinotecan-based chemotherapy regimens were randomly assigned to receive the bevacizumab–capecitabine combination or capecitabine only [Cunningham et al. 2013]. The median age of patients was 76 years. PFS (primary endpoint) was significantly longer with bevacizumab and capecitabine than with capecitabine alone (9.1 versus 5.1 months, HR = 0.53; p < 0.0001). Thus, the combination of bevacizumab and fluoropyrimidine can be considered as the treatment of choice in elderly patients with mCRC.
Chemotherapy with anti-EGFR MoAbs
Anti-EGFR MoAbs can be combined with a doublet of chemotherapy that is either FOLFOX (PRIME) [Douillard et al. 2010, 2014a] or FOLFIRI (CRYSTAL) [Van Cutsem et al. 2009, 2011].
Anti-EGFR MoAbs with an oxaliplatin-based chemotherapy
The addition of panitumumab to FOLFOX has been evaluated in the PRIME study [Douillard et al. 2010, 2014a]. The primary endpoint was PFS. In the ‘all RAS wild-type’ population (N = 512), which represented 43% of the randomized population (N = 1183), the median PFS and OS were significantly higher (HRPFS = 0.70; p = 0.004 and HROS = 0.78; p = 0.04) in the combination arm [Douillard et al. 2013].
The addition of cetuximab to an oxaliplatin-based chemotherapy was evaluated in two randomized phase III studies [Maughan et al. 2011; Tveit et al. 2012]. In both studies, the addition of cetuximab led to a detrimental effect on survival, whatever KRAS mutational status. Of note, the chemotherapy regimen used was either FOLFOX or XELOX in the COIN study [Maughan et al. 2011], and FLOX (leucovorin/5-FU bolus/oxaliplatin regimen) in the NORDICVII study [Tveit et al. 2012].
Anti-EGFR MoAbs with an irinotecan-based chemotherapy
FOLFIRI in combination with cetuximab is a standard first-line regimen for patients with RAS wild-type tumors, based on a retrospective analysis of the prospective CRYSTAL study limited to patients with KRAS exon 2 wild-type tumors and including 89% of the overall population [Van Cutsem et al. 2011]. This combination yielded positive results in terms of RR (overall response (OR) = 2.07; p < 0.001), PFS (HR = 0.70; p = 0.001), and OS (HR = 0.80; p = 0.009) in favor of cetuximab containing arm [Van Cutsem et al. 2009, 2011].
Conclusions
Overall, if the preferred targeted agent is an anti-EGFR MoAb in first-line treatment of patients with RAS wild-type mCRC, two combinations can be recommended: either FOLFOX–panitumumab or FOLFIRI–cetuximab until progression or limiting toxicity. The combination of oral fluoropyrimidine (capecitabine or UFT) to oxaliplatin and cetuximab should definitely not be used [Maughan et al. 2011; Douillard et al. 2014b].
Dilemma for treatment-naïve mCRC patients: choice between antiangiogenics and anti-EGFR agents, two options
First-line irinotecan-based therapy
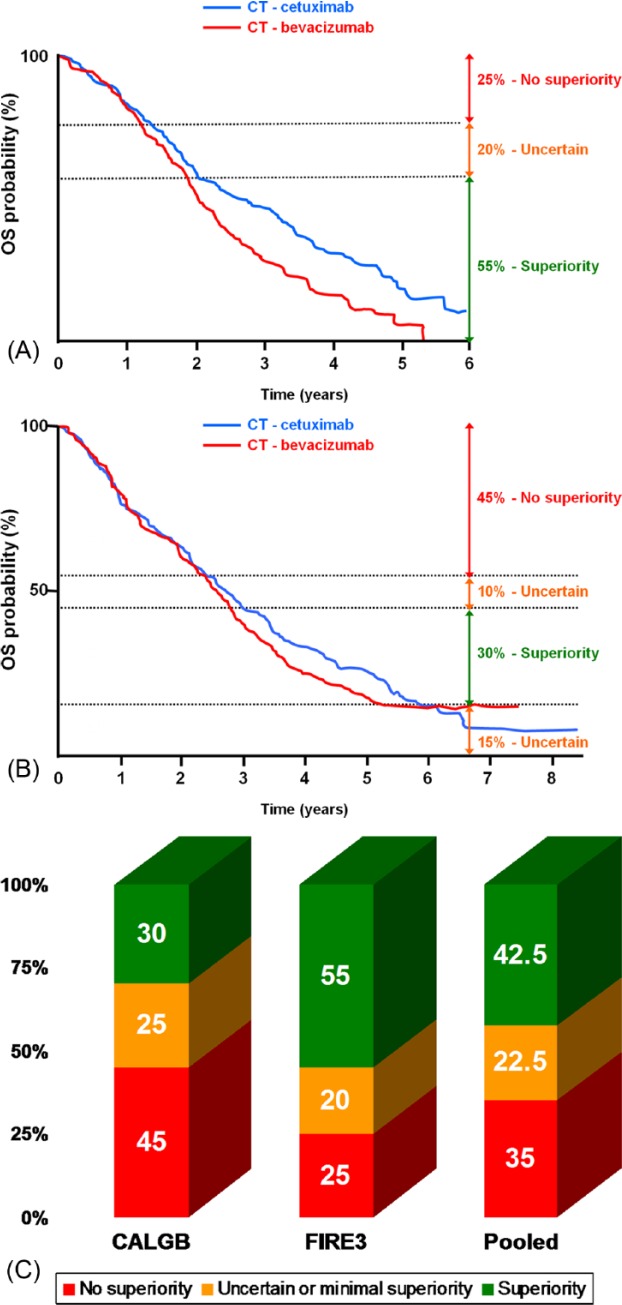
In the AIO FIRE-3 randomized study, 592 patients with KRAS exon 2 wild-type mCRC were treated with FOLFIRI in combination with either cetuximab (N = 297) or bevacizumab (N = 295) in 150 German or Austrian centers [Heinemann et al. 2014]. The RR (primary endpoint) and PFS were similar in the two treatment arms. Unexpectedly, a prolongation of OS (secondary endpoint), was observed in favor of the cetuximab arm from 25.0 to 28.7 months, corresponding to a HR of 0.77. In an exploratory analysis of RAS wild-type patients excluding 42% of the primary population (N = 342), there was still no difference in PFS (HR = 0.97; p = 0.770), but the benefit in OS was amplified with an absolute difference in median OS of 8.1 months (from 25.0 to 33.1 months, HR = 0.70; p = 0.006). This unexpected discrepancy between an OS advantage without any benefit during treatment period could be partly explained by a high number of active centers and the absence of predefined post-protocol treatment, leading to significant heterogeneity of post-progression therapy. Graphically, the OS Kaplan–Meier curves appear similar in 25% of patients, widely different in favor of cetuximab in 55% of patients, and slightly different in 20% in favor of cetuximab (Figure 2A).
Figure 2.
Proportion of patients (%) with graphical overall survival (OS) superiority (green), uncertain difference (orange) and absence of superiority (red) of cetuximab over bevacizumab in the AIO FIRE-3 study (A), in the CALGB/SWOG 80405 study (B), and estimation (mean) from both (C).
First-line oxaliplatin- or irinotecan-based therapy
The CALGB/SWOG 80405 study randomized patients with mCRC to receive chemotherapy with cetuximab, or bevacizumab, or both agents [Lenz et al. 2014]. The chemotherapy regimen (FOLFIRI or mFOLFOX6) was selected by physician. The main changes during the study included discontinuation of the study arm combining both targeted-MoAbs agents after the results of the PACCE and CAIRO2 trials [Hecht et al. 2009; Tol et al. 2009], and the limitation of eligibility to patients with KRAS exon 2 wild-type tumors. Of 1137 patients with KRAS exon 2 wild-type tumors, 526 (46.3%) were analyzed in an expanded RAS wild-type population (KRAS and NRAS exons 2, 3, and 4). No significant difference was observed between the cetuximab (N = 270) and the bevacizumab arms (N = 256) in combination with chemotherapy, both for OS (32.0 versus 31.2 months, HR 0.90; p = 0.40) and PFS (11.3 versus 11.4 months, HR = 1.10; p = 0.310). However, there was higher RR achieved in the cetuximab arm in the expanded RAS population than in the bevacizumab arm (68.6% versus 53.6%, p < 0.01). Graphically, the OS Kaplan–Meier curves appear similar in 45% of patients, widely different in favor of cetuximab in 30% of patients, and slightly different in 25% of patients (10% in favor of cetuximab and 15% in favor of bevacizumab) (Figure 2B). Subgroup analysis of patients treated with FOLFIRI did not confirm the results of the AIO FIRE-3 study. Neither PFS (HR = 1.10) nor OS (HR = 1.10) was different between the cetuximab and the bevacizumab arms when combined with FOLFIRI.
Based on a graphical analysis of both CALGB/SWOG 80405 and AIO FIRE-3 studies, the superiority of the MoAbs EGFR inhibitors over bevacizumab can be considered as probably unquestionable in 20% of all patients with previously untreated mCRC (40% of actual definition of ‘all RAS wild-type’ patients), and roughly 70% may not have any benefit of adding anti-EGFR MoAbs to chemotherapy. An uncertain or limited effect of anti-EGFR therapy is observed in further 10% of patients (Figures 1B and 2C).
Maintenance therapy
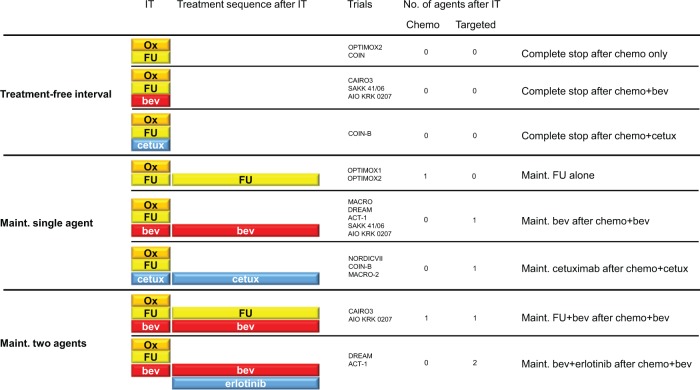
The aim of maintenance therapy (Table 1 and Figure 3) is to decrease the frequency and severity of adverse events induced by antitumor therapy, to improve health-related quality of life (HRQoL) while maintaining as long as possible the effects achieved with induction therapy, leading to lower treatments costs and decreased rates of drug resistance by stopping one or more drugs before disease progression.
Table 1.
PFS and OS in maintenance and TFI trials.
| Post-IT strategy | Strategy | Trials | Phase | N | IT duration (months) |
mPFS | PFS | mOS | OS | Trial result |
|---|---|---|---|---|---|---|---|---|---|---|
| Complete stop | TFI after chemo alone | COIN [Adams et al. 2011] | III | 815 | 3 | - | - | - | 14.4 | TFI < continuous |
| OPTIMOX2 [Chibaudel et al. 2009] | IIR | 104 | 3 | - | 6.6 | - | 19.5 | TFI < FU | ||
| TFI after chemo-bev | CAIRO3 [Koopman et al. 2013] | III | 279 | 4.5 | 4.1 | - | 18.2 | - | TFI < bev/FU | |
| AIO KRK 0207 [Arnold et al. 2014] | III | 158 | 6 | 3.6 | - | 23.3 | - | TFI < bev = bev/FU | ||
| SAKK 41/06 [Koeberle et al. 2013] | III | 123 | 4-6 | 3.1* | 8.5 | - | 22.8 | TFI < bev | ||
| TFI after chemo-cetux** | COIN-B [Wasan et al. 2014] | IIR | 64 | 3 | 3.1 | - | - | 16.8 | TFI < cetux | |
| Maint. 1 agent | FU maint. after chemo alone | OPTIMOX1 [Tournigand et al. 2006] | III | 309 | 3 | - | 8.7 | - | 21.2 | FU = continuous |
| OPTIMOX2 [Chibaudel et al. 2009] | IIR | 98 | 3 | - | 8.6 | - | 23.8 | FU > TFI | ||
| Bev maint. after chemo-bev | TTD MACRO [Diaz-Rubioet al. 2012] | III | 241 | 4.5 | - | 9.7 | - | 20.0 | bev = continuous | |
| AIO KRK 0207 [Arnold et al. 2014] | III | 156 | 6 | 4.6 | - | 22.6 | - | Bev > TFI | ||
| SAKK 41/06 [Koeberle et al. 2013] | III | 124 | 4-6 | 4.5* | 9.5 | - | 24.9 | Bev > TFI | ||
| DREAM [Chibaudel et al. 2014b] | III | 228 | 3-6 | 4.9 | 9.3 | 22.1 | 27.0 | Bev < bev/erlo | ||
| ACT-1 [Johnsson et al. 2013] | III | 79 | 4.5 | 4.2 | - | 22.8 | - | bev = bev/erlo | ||
| Cetux maint. after chemo-cetux** | NORDIC VII [Tveit et al. 2012]MACRO-2 [Alfonso et al. 2014] | IIIIIR | 109129 | 44 | − | 7.58.9 | − | 21.423.6 | cetux = continuouscetux < continuous | |
| COIN-B [Wasan et al. 2014] | IIR | 66 | 3 | 5.8 | - | - | 22.2 | cetux > TFI | ||
| Maint. 2 agents | Bev/FU maint after chemo-bev | CAIRO3 [Koopman et al. 2013]AIO KRK 0207 [Arnold et al. 2014] | IIIIII | 279158 | 4.56 | 8.56.2 | − | 21.723.4 | − | bev/FU > TFIbev/FU = bev |
| Bev/erlotinibmaint. after chemo-bev | DREAM [Chibaudel et al. 2014b]ACT-1 [Johnsson et al. 2013] | IIIIII | 224 80 | 3-64.5 | 5.95.7 | 10.0- | 24.921.5 | 30.0- | bev/erlo > bevbev/erlo = bev |
TTP; **KRAS wild-type population
Abbreviations: TTP, time to progression; PFS, progression-free survival; OS, overall survival; TFI, treatment-free interval; IT, induction therapy; m, median; chemo, chemotherapy; bev, bevacizumab; cetux, cetuximab; FU, fluoropyrimidine; erlo, erlotinib; maint., maintenance.
Figure 3.
Maintenance and treatment-free interval (TFI) trials with an oxaliplatin-based induction therapy.
Abbreviations: IT, induction therapy; chemo, chemotherapy; ox, oxaliplatin, FU, fluoropyrimidine; bev, bevacizumab; cetux, cetuximab; maint., maintenance.
OPTIMOX2 [Chibaudel et al. 2009]; COIN [Adams et al. 2011]; CAIRO3 [Koopman et al. 2013]; SAKK 41/06 [Koeberle et al. 2013]; AIO KRK 0207 [Arnold et al. 2014]; COIN-B [Wasan et al. 2014]; OPTIMOX1 [Tournigand et al. 2006]; DREAM [Chibaudel et al. 2014b]; ACT-1 [Johnsson et al. 2013]; NORDIC VII [Tveit et al. 2012]; MACRO-2 [Alfonso et al. 2014]; MACRO [Diaz-Rubio et al. 2012]
Several modalities have been evaluated in prospective trials, using chemotherapy alone (e.g. fluoropyrimidine), molecular-targeted agents only (e.g. antiangiogenics or EGFR inhibitors or both), or chemotherapy with targeted agents.
In addition to maintenance therapy drugs, the main differences between those trials are the induction therapy used (chemotherapy regimen, duration) and the post-progression strategy (reintroduction of induction therapy, switch to second line).
Single-agent maintenance
Maintenance with fluoropyrimidine alone: the oxaliplatin stop-and-go strategy.
The oxaliplatin stop-and-go strategy was validated in the OPTIMOX1 and OPTIMOX2 studies [Tournigand et al. 2006; Chibaudel et al. 2009]. The optimal duration of induction therapy is 3 months (6 fortnightly cycles), corresponding to a cumulative dose of oxaliplatin below or equal to 600 mg/m² which is a way to reach the maximal tumor response without severe sensory neuropathy. The oxaliplatin stop-and-go strategy has also shown the importance of reintroduction of induction therapy for improving survival [de Gramont et al. 2007]. The sensitivity to oxaliplatin reintroduction increases with the duration of oxaliplatin-free interval [Chibaudel et al. 2013].
Maintenance with antiangiogenic agent only
Maintenance with bevacizumab alone after a bevacizumab-based induction therapy is equivalent to maintenance with bevacizumab-fluoropyrimidine (AIO KRK 0207) [Hegewisch-Becker et al. 2014] or continuous therapy (TTD MACRO) [Diaz-Rubio et al. 2012], but is superior to a complete stop of therapy (AIO KRK 0207, SAKK 41/06) [Hegewisch-Becker et al. 2014; Koeberle et al. 2013].
MACRO: maintenance bevacizumab as efficient as continuous therapy
In the TTD MACRO trial, the bevacizumab maintenance therapy was initiated after an induction therapy with XELOX–bevacizumab, and compared with the continuation of this regimen until progression or severe toxicity [Diaz-Rubio et al. 2012]. The primary endpoint was PFS. This trial could not formally conclude to a statistical noninferiority of the maintenance arm over the continuous arm, but the absolute difference in median PFS was only 0.7 months (9.7 versus 10.4 months) and the HRPFS for the observed difference between the two arms was 1.10 [95% confidence interval (CI) 0.89–1.35]. Of note, the HR for OS was 1.05 (95% CI 0.85–1.29).
AIO KRK 0207: maintenance bevacizumab as efficient as bevacizumab–fluoropyrimidine
The AIO KRK 0207 study investigated whether a complete stop of treatment or maintenance with bevacizumab alone was noninferior to maintenance with fluoropyrimidine plus bevacizumab following 4-month oxaliplatin-based induction therapy in 852 patients with previously untreated mCRC [Hegewisch-Becker et al. 2014]. The primary endpoint was time to failure of strategy (TFS), defined as the time from randomization (starting maintenance) to progression after oxaliplatin reintroduction. The median age of patients was 65 years. The trial found bevacizumab maintenance until progression with or without fluoropyrimidine superior to stopping therapy in terms of PFS and TFS (maintenance fluoropyrimidine plus bevacizumab, HRPFS = 2.05, HRTFS = 1.27; maintenance with bevacizumab alone, HRPFS = 1.53, HRTFS not reported). The results in both maintenance arms were similar, despite a trend for PFS superiority in the fluoropyrimidine plus bevacizumab arm (HRPFS = 1.26, HRTFS = 1.03). Finally, the OS from maintenance was similar in all three arms, ranging from 22.6 to 23.4 months (p = 0.870). Of note, only 24% of patients received an oxaliplatin reintroduction in the maintenance arm with fluoropyrimidine plus bevacizumab, compared with 47% of patients in the other arms.
SAKK 41/06: maintenance bevacizumab better than treatment-free interval
The SAKK 41/06 phase III trial investigated a complete stop of treatment rather than maintaining bevacizumab until progression following 4–6 months bevacizumab-based induction therapy [Koeberle et al. 2013]. The primary objective was to demonstrate a noninferiority of treatment-free interval (TFI) over maintenance in terms of time to progression (TTP). The median TTP from randomization (i.e. starting maintenance or TFI) was 4.5 and 3.2 months in the maintenance and TFI arms, respectively (HR = 0.74). The noninferiority of TFI in terms of TTP could not be statistically demonstrated. The median OS from starting induction therapy was 25.1 and 22.8 months in the maintenance and TFI arms, respectively (HR = 0.83), without any statistical difference between the arms.
Maintenance with EGF receptor only
Maintenance cetuximab alone after a cetuximab-based induction therapy is equivalent to continuing chemotherapy until progression or limiting toxicity (NORDICVII, MACRO-2) [Tveit et al. 2012; Alfonso et al. 2014], but might be superior to a complete stop of therapy (COIN-B) [Wasan et al. 2014] Further randomized phase III studies are needed to evaluate the role of EGFR inhibitors MoAbs as maintenance therapy.
Double agents maintenance
Maintenance with antiangiogenic agent and fluoropyrimidine
In the AIO KRK 0207 study, maintenance therapy with bevacizumab and capecitabine prolonged PFS (HR = 2.05; p < 0.0001) but not OS (HR = 0.94) over a complete stop of therapy [Hegewisch-Becker et al. 2014]. However, there is no evidence of survival benefit over bevacizumab alone, both for PFS (HR = 1.26; p = 0.061) and OS (HR = 0.92)
In the CAIRO3 study, patients were randomly assigned to receive either maintenance therapy with capecitabine–bevacizumab or a complete stop of treatment after a 4.5-month induction therapy with XELOX–bevacizumab [Koopman et al. 2013]. The primary endpoint was PFS2, defined as the time interval from randomization (i.e. starting maintenance or TFI) to progression after reintroduction. Of the 635 patients treated with induction therapy, 558 (87%) were randomized. The median PFS1 was 8.5 and 4.1 months (HR = 0.44; p < 0.001), and the median PFS2 was 11.8 months and 10.5 months (HR = 0.81; p = 0.028) in favor of maintenance therapy. OS did not differ significantly between the two arms (HR = 0.87; p = 0.156). Of note, the oxaliplatin reintroduction rate was 47% after maintenance and 76% after TFI.
Maintenance with antiangiogenic agent and EGF receptor agent
Of the 701 patients registered in the GERCOR DREAM phase III trial across three countries (France, Canada, Austria), 452 were randomized to receive a maintenance therapy with bevacizumab (7.5 mg/kg, every 3 weeks) or bevacizumab (same dose) and erlotinib (150 mg/day continuously), an EGFR tyrosine kinase inhibitor [Chibaudel et al. 2014b]. After a median follow up of 50 months, the combination therapy led to an improvement of maintenance PFS (primary endpoint), maintenance OS, and RR. The maintenance PFS and OS were prolonged to 1 and 3 months, respectively, for a maintenance treatment duration lasting less than 4 months. This effect was observed whatever the KRAS mutational status and the subsequent therapy used.
This activity was previously suggested in the negative ACT-1 study [Johnsson et al. 2013] that reported similar HR for maintenance PFS or OS. The trial, however, was underpowered to demonstrate any statically significant benefit.
Conclusions
Bevacizumab with or without low-dose capecitabine is the standard maintenance therapy. However, the association of bevacizumab with a short period of erlotinib can be considered as a new treatment option after a bevacizumab-based induction first-line therapy in patients with unresectable mCRC.
A maintenance therapy with cetuximab only is still not recommended in routine practice.
Therapy-free intervals
Complete stop after induction therapy without targeted agents
The feasibility of a complete stop of chemotherapy was evaluated in the OPTIMOX2 and in the COIN trials [Chibaudel et al. 2009; Adams et al. 2011] (Table 1 and Figure 3).
In the OPTIMOX2 trial, patients were randomized to receive either FOLFOX induction therapy (3 months) followed by maintenance with fluoropyrimidine alone or the same induction therapy followed by a TFI [Chibaudel et al. 2009]. In the COIN trial, patients were randomized to receive either a continuous oxaliplatin-based chemotherapy until progression or an oxaliplatin-based induction therapy (3 months) followed by a TFI [Adams et al. 2011].
Complete stop after bevacizumab-based induction therapy
A TFI after a bevacizumab-based induction therapy was investigated in the CAIRO3, the AIO KRK 0207, and the SAKK 41/06 trials (see above) [Koopman et al., 2013; Arnold et al. 2014; Koeberle et al. 2013].
Complete stop after cetuximab-based induction therapy
COIN-B study was a randomized phase II trial in which patients with previously untreated mCRC were randomly assigned to receive either 3 months of induction FOLFOX-cetuximab therapy followed by TFI or to the same induction treatment followed by maintenance with cetuximab [Wasan et al. 2014]. FOLFOX reintroduction was recommended at progression in both arms. Ten-month failure-free survival (primary endpoint) was 50% in the intermittent group versus 52% in the maintenance arm, and median failure-free survival was 12.2 and 14.3 months, respectively.
Conclusions
TFI is inferior to continuous therapy until disease progression and to maintenance therapy (whatever the drug) in unselected mCRC patients. However, a detrimental effect in the median OS of more than 4 months has never yet been observed. Some patients could benefit of stopping therapy using predictive factors for prolonged TFI duration: pre-TFI chemotherapy duration of at least 6 months without any disease progression (i.e. controlled disease) and with normal carcinoembryonic antigen level during induction therapy and normal baseline platelet count (i.e. below 400,000/mm3) [Perez-Staub et al. 2008; Adams et al. 2011].
Second-line therapy
After progression on full first-line therapy, the standard practice is to change the chemotherapy regimen from 5-FU/oxaliplatin to 5-FU/irinotecan or the reverse sequence [Tournigand et al. 2004].
Failure of a first-line bevacizumab-based therapy
After failure of first-line therapy, the addition of an antiangiogenic agent to chemotherapy improved patient outcomes either in CRC patients previously treated with bevacizumab [Van Cutsem et al. 2012; Bennouna et al. 2013] or in bevacizumab-naïve patients [Giantonio et al. 2007; Van Cutsem et al. 2012]. The TML phase III study has shown that continuing bevacizumab beyond first progression and switching backbone chemotherapy regimen improves PFS and OS [Bennouna et al. 2013]. This treatment effect was observed whatever KRAS mutational status. In the VELOUR phase III study, patients were treated with FOLFIRI with either aflibercept or placebo [Van Cutsem et al. 2012]. All investigated patient outcomes (RR, PFS, and OS) were improved over chemotherapy alone, whatever the prior use of bevacizumab during first-line therapy. This study led to the approval of ziv-aflibercept in combination with FOLFIRI as second-line therapy. The RAISE phase III study compared ramucirumab plus FOLFIRI to placebo plus FOLFIRI as a second-line treatment in patients with mCRC after treatment with bevacizumab, oxaliplatin, and fluoropyrimidine in the first-line setting. Ramucirumab is a VEGF Receptor 2 antagonist that specifically binds VEGF Receptor 2 and blocks binding of VEGF receptor ligands VEGF-A, VEGF-C, and VEGF-D. The study showed a statistically significant improvement in OS (primary endpoint) and PFS. The most common severe toxicities increased when adding ramucirumab to FOLFIRI were neutropenia, fatigue, hypertension, and diarrhea [Tabernero et al, 2015].
The addition of panitumumab to FOLFIRI was superior to FOLFIRI alone in patients with KRAS wild-type mCRC after failure of an oxaliplatin-based therapy in terms of PFS (primary endpoint, HR = 0.82; p = 0.023) and RR (36% versus 10%; p < 0.001). The lack of OS superiority (HR = 0.92; p = 0.366) could be explained by the 34% of patients who received the MoAb EGFR inhibitor after progression in the FOLFIRI arm [Sobrero et al. 2012].
The SPIRITT randomized phase II study (study 20060141) investigated the safety and efficacy of FOLFIRI with either panitumumab or bevacizumab as second-line therapy in patients previously treated with an oxaliplatin-based first-line therapy with bevacizumab, without formal hypothesis [Hecht et al. 2013]. A total of 182 patients with KRAS wild-type mCRC were randomized. Neither PFS (primary endpoint, HR = 1.01) nor OS (HR = 1.06) seemed to be different between the two arms. Both antiangiogenics and anti-EGFR MoAbs in combination with chemotherapy are reasonable second-line therapeutic options in mCRC patients with RAS wild-type tumors. Further phase III studies comparing chemotherapy with either anti-EGFR MoAbs or antiangiogenics are needed in order to define the best treatment sequence after first-line in patients with wild-type RAS mCRC.
Failure of first-line anti-EGFR MoAb-based therapy
After failure of first-line therapy containing an EGFR inhibitor agent, second-line chemotherapy should be associated with an antiangiogenic agent, either FOLFOX-bevacizumab in the case of prior first-line irinotecan-based regimen [Giantonio et al. 2007] or FOLFIRI-aflibercept in case of prior first-line oxaliplatin-based regimen [Van Cutsem et al. 2012].
Subsequent lines of therapy
Anti-EGFR MoAbs (cetuximab, panitumumab) increase survival over best supportive care after failure of standard chemotherapy [Jonker et al. 2007; Van Cutsem et al. 2007]. A synergy between these drugs and irinotecan has been shown even in patients refractory to irinotecan [Cunningham et al. 2004].
Regorafenib, a multikinase inhibitor was approved in US (FDA) and Europe [European Medicines Agency (EMA)] for patients with mCRC refractory to all standard therapies with the Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1, based on the results of the CORRECT phase III study [Grothey et al. 2013]. An early stop in recruitment was decided after the results of a second interim analysis, showing that OS (primary endpoint) and PFS were improved with regorafenib versus placebo. Hazard ratios for death and progression were 0.77 and 0.49, respectively. The main adverse events were asthenia (63%), hand-and-foot syndrome (47%), and stomatitis (27%). Heath-related quality-of-life was not improved. There is no predictive biomarker for regorafenib efficacy.
TAS-102 is a new oral antitumor agent combining trifluridine (FTD, active component) and tipiracil hydrochloride (TPI, thymidine phosphorylase inhibitor), which prevents the degradation of FTD [Van Cutsem et al. 2014]. This drug has been evaluated in the RECOURSE phase III study against placebo in patients with heavily pretreated mCRC, in which TAS-102 prolonged OS (primary endpoint) from 5.3 to 7.1 months (HR = 0.68) and PFS (HR = 0.48) [Yoshino et al. 2014]. The survival benefit was observed whatever prior use of regorafenib (17%) and was consistent in all prespecified subgroups, except for patients who received only two lines of therapy prior to study entry. Hematological (neutropenia, anemia) and gastrointestinal (nausea, diarrhea) toxicities were the most frequent adverse events. The incidence of febrile neutropenia was 3.8% with TAS-102. One toxic related death was observed with TAS-102. There was not HRQoL evaluation, but the time to ECOG PS deterioration was prolonged with the use of TAS-102 (HR = 0.66). Those results are in line with previously published data from a randomized phase II study conducted in Japanese patients [Yoshino et al. 2012]. A high expression of TK1 could be associated with a higher sensitivity of tumor cells for TAS-102. The main differences and similarities between regorafenib and TAS-102 are shown in Table 2. With similar efficacy results, those oral drugs have two distinct safety profiles.
Table 2.
Differences and similarities between regorafenib and TAS-102 trials.
| Regorafenib | TAS-102 | |
|---|---|---|
| Drug | ||
| Pharmaceutical | Bayer | Taiho |
| Mechanism of action | Multi-TKI | Antimetabolic |
| Route | Oral | Oral |
| Dose | 160 mg daily, 3 weeks of each 4-week cycle | 35 mg/m² twice a day on days 1–5 and 8–12 every 4 weeks |
| Study | CORRECT | RECOURSE |
| Reference | [Grothey et al. 2013] | [Yoshino et al. 2014] |
| Trial phase | III | III |
| Primary objective (targeted HR) | OS (0.70) | OS (0.75) |
| Randomization ratio | 2:1 | 2:1 |
| Comparator | Placebo | Placebo |
| No of patients (investigational / placebo) | 505 / 255 | 534 / 266 |
| Recruitment | Early stop for positive results | Full |
| Prior antiangiogenic agent | 100% | 100% |
| Efficacy results | ||
| OS, HR (absolute median difference) | 0.77 (+1.4) | 0.68 (+1.8) |
| PFS, HR (absolute median difference) | 0.49 (+0.2) | 0.48 (+0.3) |
| DCR, % (absolute difference) | 41 (+26) | 44 (+28) |
| Safety profile | ||
| Neutropenia, any grade (grade ¾) | Not reported | 67 (38) |
| Anemia | 7 (3) | 76 (18) |
| Nausea | 14 (<1) | 48 (2) |
| Stomatitis | 27 (3) | 8 (<1) |
| Diarrhea | 34 (8) | 32 (3) |
| Hand-and-foot syndrome | 47 17) | 2 (0) |
| Asthenia | 63 (10) | 35 (4) |
| HRQoL | No change | Not done |
| Predictive biomarkers | None | TK1-high expression? |
| FDA/EMA approval | Yes | Not yet |
Abbreviations: TKI, tyrosine kinase inhibitor; RECOURSE, Refractory Colorectal Cancer Study; HR, hazard ratio; OS, overall survival ; PFS, progression-free survival ; DCR, disease control rate; HRQoL, heath-related quality of life; FDA, Food and Drug Administration; EMA, European Medical Agency.
Ongoing and future strategy trials
Multiline strategy
The GERCOR STRATEGIC-1 study [ClinicalTrials.gov identifier: NCT01910610; Chibaudel et al. 2014a] is an open-label, randomized, multicenter phase III trial designed to determine an optimally personalized treatment sequence in patients with unresectable RAS wild-type mCRC. Two standard treatment strategies are being compared: FOLFIRI-cetuximab, followed by an oxaliplatin-based chemotherapy with bevacizumab (arm 1) and OPTIMOX–bevacizumab, followed by an irinotecan-based chemotherapy with bevacizumab, and an anti-EGFR monoclonal antibody with or without irinotecan (arm 2). The primary endpoint is duration of disease control (DDC) that is defined as the sum of PFS of each active sequence. Main secondary endpoints are OS and HRQoL. Recruitment started in October 2013 and a total of 500 patients is expected to be randomized.
The Austrian PASSION study is a randomized phase II study evaluating first-line XELIRI–bevacizumab followed by XELOX–bevacizumab or the reverse sequence [ClinicalTrials.gov identifier: NCT02119026; Scheithauer, 2014]. The primary endpoint is DDC. The expected sample size is 120 patients with mCRC.
The GruppoItaliano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD) COMETS is a randomized phase III study comparing two different therapeutic sequences in patients with mCRC after failure of a first-line treatment with FOLFIRI-bevacizumab [ClinicalTrials.gov identifier: NCT01030042l GruppoItaliano per lo studio dei Carcinomi dell’ Apparato Digerente (GISCAD), 2014]. Patients are randomized to receive either second-line FOLFOX followed by cetuximab-irinotecan or the reverse sequence. The primary endpoint is OS. The recruitment of 350 patients is planned to be achieved in the second part of 2014.
Biomarker-driven trials
The FOCUS4 is a molecularly driven randomized study sponsored by Medical Research Council (MRC) in United Kingdom for patients with mCRC (EudraCT 2012-005111-12) [Pugh, 2014]. A biomarker panel analysis [BRAF, KRAS, NRAS, Phosphatase and tensin homolog (PTEN), Phosphoinositide-3-kinase (PI3KCA), and Mismatch Repair (MMR)] will be performed during the first 16 weeks of induction therapy, then patients with controlled disease will be randomized according to five molecular subtypes: BRAF mutant, PI3KCA subtype (mutation of PI3KCA gene or loss of PTEN protein), RAS mutant (KRAS or NRAS tumor gene mutation), ‘all wild-type’ subtype (no mutation), and nonclassified subtype. Each molecularly stratified trial aims to compare a novel intervention to placebo or standard care. The recruitment period is planned to be 4–5 years followed by 2-year follow up.
The MODUL trial [Schmoll et al. 2014] is a biomarker-driven maintenance treatment randomized study. All patients will receive 4 months of induction therapy with FOLFOX–bevacizumab. Subsequently, patients with controlled disease will be separated into two cohorts for maintenance therapy according to BRAF mutational status. In cohort one, BRAF mutant patients will be randomized to receive either fluoropyrimidine with cetuximab and vemurafenib (BRAF TKI) or fluoropyrimidine with bevacizumab. In cohort two, BRAF wild-type patients will be randomized to receive either fluoropyrimidine with bevacizumab or the same treatment with MPDL3280A (anti-PDL1 MoAb). The coprimary endpoints are tumor RR at 2 months and PFS. This study is based on an adaptive design with possibility to drop treatment comparisons upon sufficient data availability for the efficacy/safety results, and the possibility of adding future promising compounds into biomarker-driven maintenance phase.
Maintenance therapy
The AIO-KRK-0212 is a randomized phase II study investigating maintenance therapy with 5-FU with or without panitumumab in patients with RAS wild-type MCRC [ClinicalTrials.gov identifier: NCT01991873; Trarbach, 2014].
Immunomodulatory MGN1703 in Patients with Advanced Colorectal Carcinoma (IMPALA) is a randomized open-label phase III study that is investigating the role of the immunotherapy MGN 1703 (MOLOGEN AG) as maintenance therapy in patients with mCRC [ClinicalTrials.gov identifier: NCT02077868; Cunningham, 2014]. MGN1703 is a DNA-based TLR-9 agonist developed by MOLOGEN. This trial is designed to improve OS (primary endpoint) and requires 540 patients from more than 100 European centers.
Maintenance chemotherapy with low-dose metronomic regimen associating capecitabine, celecoxib, and methotrexate will be evaluated in a prospective phase II study (HaEmek Medical Center, Israel) in 80 mCRC patients having received FOLFIRI-bevacizumab induction therapy [ClinicalTrials.gov identifier: NCT01668680; Loven, 2014]. The primary endpoint is PFS.
The addition of ziv-aflibercept to the OPTIMOX strategy (oxaliplatin-based induction therapy followed maintenance therapy followed by oxaliplatin reintroduction) is being evaluated in the GERCOR VELVET phase II study [ClinicalTrials.gov identifier: NCT01802684; Chibaudel, 2014]. The recruitment is closed and the results will available in 2015.
Other drugs are currently being investigated as maintenance first-line therapy: axitinib (TTD-11-01) [ClinicalTrials.gov identifier: NCT01483638; Grávalos and Carrato, 2014] and simvastatin [ClinicalTrials.gov identifier: NCT01238094; Kang, 2014].
Conclusions
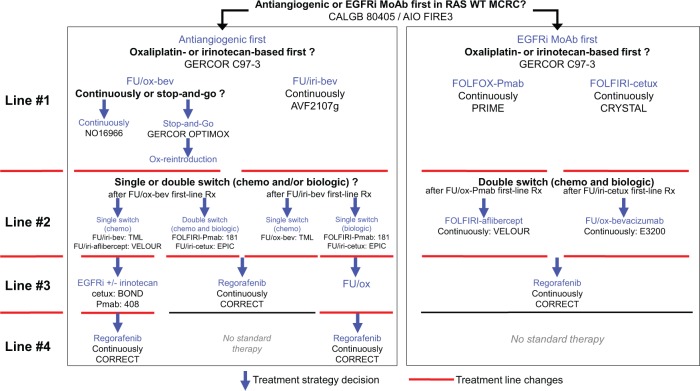
Several drugs and combinations are now available for the treatment of patients with advanced CRC, but the optimal sequence of therapy remains unknown (Figure 4). This is a step-by-step treatment decision level, from first-line with the choice of both biologic agent (antiangiogenic or EGFR) and chemotherapy regimen (oxaliplatin-based or irinotecan-based) to second and subsequent lines. Starting first-line with bevacizumab and a doublet of chemotherapy (either 5-FU/oxaliplatin or 5-FU/irinotecan) optimizes the availability of the number of lines with approved drugs. Moreover, the use of maintenance therapy during first-line and at push therapy-free intervals in selected patients can be used to limit severe toxicities and drug resistance. Finally, reintroduction of oxaliplatin after progression during first-line is an important prognostic factor for OS. Strategy trials are needed to better define an optimal treatment sequence in a given population.
Figure 4.
Multiline therapeutic strategies in wild-type RAS metastatic colorectal cancer (mCRC): treatment decisions from first-line to salvage treatment.
Abbreviations: chemo, chemotherapy; bev, bevacizumab; ox, oxaliplatin; cetux, cetuximab; iri, irinotecan; Pmab, panitumumab; FU, fluoropyrimidine, Rx, therapy.
GERCOR C97-3 [Tournigand et al. 2004]; NO16966 [Saltz et al. 2008]; OPTIMOX1 [Tournigand et al. 2006]; OPTIMOX2 [Chibaudel et al. 2009]; AVF2107g [Hurwitz et al. 2004]; PRIME [Douillard et al. 2010]; CRYSTAL [Van Cutsem et al. 2009]; TML [Bennouna et al. 2013]; 181 [Peeters et al. 2010]; EPIC [Sobrero et al. 2008]; ECOG 3200 [Giantonio et al. 2007]; VELOUR [Van Cutsem et al. 2011]; BOND [Cunningham et al. 2004]; 408 [Van Cutsem et al. 2007]; CORRECT [Grothey et al. 2013]
Molecular testing for KRAS and NRAS tumor genes (exons 2, 3, and 4) is mandatory but not sufficient to select appropriate patients for anti-EGFR MoAbs therapy (Figure 1B). An increase of selection level is critical to optimize treatment effect. Moreover, a period to acquire that information should also be shortened in order to give the best treatment from the diagnosis of advanced disease.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: BC has served as a consultant or in an advisory role for Roche and Sanofi. CT has served as a consultant or in an advisory role and received honoraria from Roche and Sanofi. FB has served as a consultant or in an advisory role for Roche, Nestlé, and Merck Serono, has received honoraria from Roche, Nestlé, Bristol-Myers Squibb, and Merck Serono, and has received research funding from Roche. TA has served as a consultant or in an advisory role for Amgen, Merck Serono, and Roche, and has received honoraria from Amgen, Merck Serono, and Roche. AdG has served as a consultant or in an advisory role for Roche, Sanofi, and PharmaEngine and received honoraria from Roche. MB and HR have no conflicts of interest to declare.
Contributor Information
Benoist Chibaudel, Department of Medical Oncology, Institut Hospitalier Franco-Britannique, 4, rue Kléber, 92300, Levallois-Perret, France.
Christophe Tournigand, Hôpitaux de Paris, Créteil, France.
Franck Bonnetain, Methodology and biostatistics Unit, Hôpital Besançon, Besançon, France.
Hubert Richa, Department of Surgery, Institut Hospitalier Franco-Britannique, Levallois-Perret, France.
Magdalena Benetkiewicz, GERCOR, Groupe Coopérateur Multidisciplinaire en Oncologie, Paris, France and; Fondation ARCAD, Aide et Recherche en Cancérologie Digestive, Paris, France.
Thierry André, Department of Medical Oncology, Hôpital Saint-Antoine, Assistance Publique – Hôpitaux de Paris, Paris, France.
Aimery de Gramont, Department of Medical Oncology, Institut Hospitalier Franco-Britannique, Levallois-Perret, France and GERCOR, Groupe Coopérateur Multidisciplinaire en Oncologie, Paris, France.
References
- Adams R., Meade A., Seymour M., Wilson R., Madi A., Fisher D., et al. (2011) Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 12: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R., Aloia T., Loyer E., Pawlik T., Taouli B., Vauthey J. (2013) Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 15: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso G., Benavides M., Sánchez Ruiz A., Guillen-Ponce C., Safont S., Alcaide J., et al. (2014) Phase II Study of first-line mFOLFOX plus cetuximab (C) for 8 cycles followed by mFOLFOX plus C or single agent (s/a) C as maintenance therapy in patients (p) with metastatic colorectal cancer (mCRC): the MACRO-2 trial (Spanish Cooperative Group for the Treatment of Digestive Tumors [TTD]). ESMO 2014, Abstract 4990. Ann Oncol 25 (Suppl. 4): iv167–iv209. [Google Scholar]
- Arnold D., Graeven U., Lerchenmuller C.A., Killing B., Depenbusch R., Steffens C-C., et al. (2014) Maintenance strategy with fluoropyrimidines (FP) plus Bevacizumab (Bev), Bev alone, or no treatment, following a standard combination of FP, oxaliplatin (Ox), and Bev as first-line treatment for patients with metastatic colorectal cancer (mCRC): A phase III non-inferiority trial (AIO KRK 0207). ASCO 2010. J Clin Oncol 28 [Google Scholar]
- Bennouna J., Sastre J., Arnold D., Osterlund P., Greil R., Van Cutsem E., et al. (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14: 29–37. [DOI] [PubMed] [Google Scholar]
- Chibaudel B. (2014) OPTIMOX-aflibercept as first-line therapy in patients with unresectable metastatic colorectal cancer (VELVET). http://clinicaltrials.gov/ct2/show/NCT01802684 (accessed 16 October 2014).
- Chibaudel B., Maindrault-Goebel F., Lledo G., Mineur L., Andre T., Bennamoun M., et al. (2009) Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 27: 5727–5733. [DOI] [PubMed] [Google Scholar]
- Chibaudel B., Tournigand C., Bonnetain F., Hug de, Larauze M., de Gramont A., Laurent-Puig P., et al. (2014a) STRATEGIC1-multi-line therapy trial in unresectable wild-type RAS metastatic colorectal cancer: A GERCOR randomized open-label phase III study. ASCO 2014, Abstract TPS3648. [Google Scholar]
- Chibaudel B., Tournigand C., Bonnetain F., Maindrault-Goebel F., Lledo G., Andre T., et al. (2013) Platinum-sensitivity in metastatic colorectal cancer: towards a definition. Eur J Cancer 49: 3813–3820. [DOI] [PubMed] [Google Scholar]
- Chibaudel B., Tournigand C., Samson B., Scheithauer W., Mesange P., Lledo G., et al. (2014b) Bevacizumab-erlotinib as maintenance therapy in metastatic colorectal cancer. Final results of the GERCOR DREAM study. ESMO 2014, Abstract 497O. Ann Oncol 25 (Suppl. 4):iv167–iv209. [Google Scholar]
- Cunningham D. (2014) Evaluation of MGN1703 Maintenance treatment in patients with mCRC with tumor reduction during induction treatment (IMPALA). http://clinicaltrials.gov/ct2/show/NCT02077868 (accessed 16 October 2014).
- Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., et al. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan refractory metastatic colorectal cancer. N Engl J Med 351: 337–345. [DOI] [PubMed] [Google Scholar]
- Cunningham D., Lang I., Marcuello E., Lorusso V., Ocvirk J., Shin D., et al. (2013) Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol 14: 1077–1085. [DOI] [PubMed] [Google Scholar]
- de Gramont A., Buyse M., Abrahantes J., Burzykowski T., Quinaux E., Cervantes A., et al. (2007) Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol 25: 3224–3229. [DOI] [PubMed] [Google Scholar]
- Diaz-Rubio E., Gomez-Espana A., Massuti B., Sastre J., Abad A., Valladares M., et al. (2012) First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 17: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard J., Oliner K., Siena S., Tabernero J., Burkes R., Barugel M., et al. (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Douillard J., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., et al. (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- Douillard J., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., et al. (2014a) Final Results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 25: 1346–1355. [DOI] [PubMed] [Google Scholar]
- Douillard J., Zemelka T., Fountzilas G., Barone C., Schlichting M., Heighway J., et al. (2014b) FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: the randomized phase II future study. Clin Colorectal Cancer 13: 14–26 e11. [DOI] [PubMed] [Google Scholar]
- Giantonio B., Catalano P., Meropol N., O’Dwyer P., Mitchell E., Alberts S., et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25: 1539–1544. [DOI] [PubMed] [Google Scholar]
- Globocan (2012) International Agency on Cancer Research. Cancer Incidence and Mortality Worldwide in 2012. Available at: http://globocan.iarc.fr/pages/fact_sheets_cancer.aspx (accessed 16 October 2014).
- Grávalos C., Carrato A. (2014) Axitinib as maintenance treatment in patients with metastatic CRC (TTD-11-01). http://clinicaltrials.gov/ct2/show/NCT01483638 (accessed 16 October 2014).
- Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 Trial. Lancet 381: 303–312. [DOI] [PubMed] [Google Scholar]
- Gruppo Italiano per lo studio dei Carcinomi dell’Apparato Digerente (GISCAD) (2014) Trial comparing two sequences of therapy in colorectal metastatic patients (COMETS). http://clinicaltrials.gov/ct2/show/NCT01030042 (accessed 16 October 2014).
- Hecht J., Cohn A., Dakhil S., Saleh M., Piperdi B., Cline-Burkhardt V., et al. (2013) SPIRITT (Study 20060141): A Randomized phase II study of FOLFIRI with either panitumumab or bevacizumab as second-line treatment in patients with wild-type KRAS metastatic colorectal cancer. ASCO GI 2013. J Clin Oncol 31(Suppl. 4): abstract 454. [DOI] [PubMed] [Google Scholar]
- Hecht J., Mitchell E., Chidiac T., Scroggin C., Hagenstad C., Spigel D., et al. (2009) A randomized phase IIIb trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 27: 672–680. [DOI] [PubMed] [Google Scholar]
- Hegewisch-Becker S., Graeven U., Lerchenmüller C., Killing B., Depenbusch R., Steffens C., et al. (2014) Maintenance strategy with fluoropyrimidine (FP) plus bevacizumab (Bev), Bev alone or no treatment, following a 24-week-first-line induction with FP, oxaliplatin (Ox) and Bev for patients with metastatic colorectal cancer: Mature data and subgroup analysis. ESMO 2014, abstract 498O. [Google Scholar]
- Heinemann V., von Weikersthal L., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S., et al. (2014) Lancet Oncol FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Kabbinavar F. (2005) Bevacizumab combined with standard fluoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology 69(Suppl. 3): 17–24. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Hagman H., Frodin J., Berglund A., Keldsen N., Fernebro E., et al. (2013) A randomized phase III trial on maintenance treatment with bevacizumab alone or in combination with erlotinib after chemotherapy and bevacizumab in metastatic colorectal cancer: The NORDIC ACT trial. Ann Oncol 24: 2335–2341. [DOI] [PubMed] [Google Scholar]
- Jonker D., O’Callaghan C., Karapetis C., Zalcberg J., Tu D., Au H., et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357: 2040–2048. [DOI] [PubMed] [Google Scholar]
- Kabbinavar F., Schulz J., McCleod M., Patel T., Hamm J., Hecht J., et al. (2005) Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II Trial. J Clin Oncol 23: 3697–3705. [DOI] [PubMed] [Google Scholar]
- Kang W. (2014) Trial of XELIRI/FOLFIRI + simvastatin followed by simvastatin maintenance in metastatic colorectal cancer. http://clinicaltrials.gov/ct2/show/NCT01238094 (accessed 16 October 2014).
- Koeberle D., Betticher D., Von Moos R., Dietrich D., Brauchli P., Baertschi D., et al. (2013) Bevacizumab continuation versus no continuation after first-line chemo-bevacizumab therapy in patients with metastatic colorectal cancer: A randomized phase III noninferiority trial (SAKK 41/06). ASCO 2013. J Clin Oncol 31(Suppl.): abstract 3503. [Google Scholar]
- Koopman M., Simkens Lieke H., Ten Tije A., Creemers G., Loosveld O., De Jongh F., et al. (2013) Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC): the phase III CAIRO3 study of the Dutch Colorectal Cancer Group (DCCG). ASCO 2013. J Clin Oncol 31(Suppl.): abstract 3502. [Google Scholar]
- Lenz H., Niedzwiecki D., Innocenti F., Blanke C., Mahony M., O’Neil B., et al. (2014) CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin(FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (Bv) or cetuximab (Cet) for patients (Pts) with expanded RAS analyses untreated metastatic adenocarcinoma of the colon Or rectum (mCRC). ESMO 2014, abstract A5010. [Google Scholar]
- Lièvre A., Bachet J., Le Corre D., Boige V., Landi B., Emile J., et al. (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995. [DOI] [PubMed] [Google Scholar]
- Loupakis F., Cremolini C., Masi G., Lonardi S., Zagonel V., Salvatore L., et al. (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014 371: 1609–1618. [DOI] [PubMed] [Google Scholar]
- Loven D. (2014) Maintenance metronomic chemotherapy for metastatic colorectal carcinoma. http://clinicaltrials.gov/ct2/show/NCT01668680 (accessed 16 October 2014).
- Maughan T., Adams R., Smith C., Meade A., Seymour M., Wilson R., et al. (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN Trial. Lancet 377: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Price T., Cervantes A., Sobrero A., Ducreux M., Hotko Y., et al. (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28: 4706–4713. [DOI] [PubMed] [Google Scholar]
- Perez-Staub N., Chibaudel B., Figer A., Cervantes A., Lledo G., Larsen A., et al. (2008) Who can benefit from chemotherapy holidays after first-line therapy for advanced colorectal cancer? A GERCOR study. ASCO 2008. J Clin Oncol 26(Suppl.): abstract 4037.18711196 [Google Scholar]
- Pugh C. (2014) FOCUS4: Molecular selection of therapy in colorectal cancer: a molecularly stratified randomised controlled trial programme. http://www.focus4trial.org/aboutfocus4/ (accessed 16 October 2014).
- Saltz L., Clarke S., Diaz-Rubio E., Scheithauer W., Figer A., Wong R., et al. (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III Study. J Clin Oncol 26: 2013–2019. [DOI] [PubMed] [Google Scholar]
- Scheithauer W. (2014) Efficacy and safety of XELIRI + Avastin followed by XELOX + Avastin or reverse sequence in metastatic colorectal cancer (PASSION). http://clinicaltrials.gov/ct2/show/NCT02119026 (accessed 16 October 2014).
- Schmoll H., Arnold D., de Gramont A., Ducreux M., Grothey A., O’Dwyer P., et al. (2014) MODUL–a multicentre randomised clinical trial of biomarker-driven therapy for the 1st-line maintenance treatment of metastatic colorectal cancer (mCRC): a signal-seeking approach. ESMO GI 2014, abstract 612. [Google Scholar]
- Sobrero A., Maurel J., Fehrenbacher L., Scheitauer W., Abubakr Y., Lutz M., et al. (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26: 2311–2319. [DOI] [PubMed] [Google Scholar]
- Sobrero A., Peeters M., Price T., Hotko Y., Cervantes-Ruiperez A., Ducreux M., et al. (2012) Final results from study 181: randomized phase III study of FOLFIRI with or without panitumumab (Pmab) for the treatment of second-line metastatic colorectal cancer (mCRC). ASCO GI 2012. J Clin Oncol 30(Suppl. 4): abstract 387.22215753 [Google Scholar]
- Tabernero J., Aranda E., Gomez A., Massuti B., Sastre J., Abad A., et al. (2010) Phase III study of first-line XELOX plus bevacizumab (Bev) for 6 Cycles followed by XELOX plus bev or single agent bev as maintenance therapy in patients with metastatic colorectal cancer: the MACRO trial (Spanish Cooperative Group for the Treatment of Digestive Tumors). ASCO 2010. J Clin Oncol 28(Suppl. 15): abstract 3501. [Google Scholar]
- Tabernero J., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.E., et al. (2015) RAISE: a randomized, double-blind, multicenter phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab (RAM) or placebo (PBO) in patients (pts) with metastatic colorectal carcinoma (CRC) progressive during or following first-line combination therapy with bevacizumab (bev), oxaliplatin (ox), and a fluoropyrimidine (fp). J Clin Oncol 33(Suppl. 3): abstract 512. [Google Scholar]
- Tebbutt N., Wilson K., Gebski V., Cummins M., Zannino D., Van Hazel G., et al. (2010) Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group randomized phase III MAX Study. J Clin Oncol 28: 3191–3198. [DOI] [PubMed] [Google Scholar]
- Tol J., Koopman M., Cats A., Rodenburg C., Creemers G., Schrama J., et al. (2009) Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360: 563–572. [DOI] [PubMed] [Google Scholar]
- Tournigand C., Andre T., Achille E., Lledo G., Flesh M., Mery-Mignard D., et al. (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22: 229–237. [DOI] [PubMed] [Google Scholar]
- Tournigand C., Cervantes A., Figer A., Lledo G., Flesch M., Buyse M., et al. (2006) OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer–a GERCOR Study. J Clin Oncol 24: 394–400. [DOI] [PubMed] [Google Scholar]
- Trarbach T. (2014) Maintenance therapy with 5-FU/FA plus panitumumab vs. 5-FU/FA alone after prior induction and re-induction after progress for 1st-line treatment of metastatic colorectal cancer (PanaMa). http://clinicaltrials.gov/show/NCT01991873 (accessed 16 October 2014).
- Tveit K., Guren T., Glimelius B., Pfeiffer P., Sorbye H., Pyrhonen S., et al. (2012) Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (NORDIC FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII Study. J Clin Oncol 30: 1755–1762. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Kohne C., Hitre E., Zaluski J., Chang Chien C., Makhson A., et al. (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Kohne C., Lang I., Folprecht G., Nowacki M., Cascinu S., et al. (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Ohtsu A., Falcone A., Yoshino T., Garcia-Carbonero R., Mizunuma N., et al. (2014) Phase III RECOURSE trial of TAS-102 vs. placebo, with best supportive care (BSC), in patients (pts) with metastatic colorectal cancer (mCRC) refractory to standard therapies. ESMO 2014, abstract LBA13. [Google Scholar]
- Van Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B., et al. (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25: 1658–1664. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Tabernero J., Lakomy R., Prenen H., Prausova J., Macarulla T., et al. (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- Wasan H., Meade A., Adams R., Wilson R., Pugh C., Fisher D., et al. (2014) Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol 15: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T., Mayer R., Falcone A., Ohtsu A., Garcia-Carbonero R., Mizunuma N., et al. (2014) Results of a multicenter, randomized, double-blind, phase III study of TAS-102 vs placebo, with best supportive care in patients with metastatic colorectal cancer refractory to standard therapies (RECOURSE). Ann Oncol 25: 114. [Google Scholar]
- Yoshino T., Mizunuma N., Yamazaki K., Nishina T., Komatsu Y., Baba H., et al. (2012) TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 13: 993–1001. [DOI] [PubMed] [Google Scholar]