Abstract
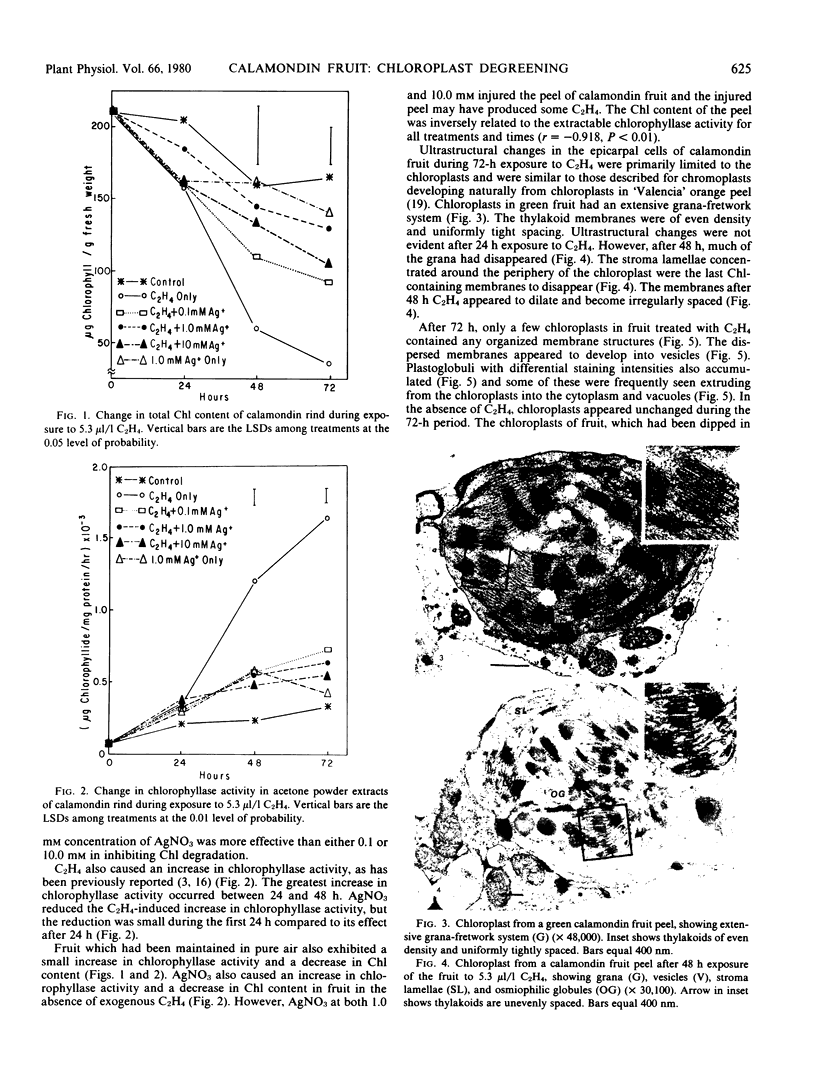
C2H4 disrupts the internal membranes of the chloroplast and induces an increase in chlorophyllase activity in degreening calamondin [x Citrofortunella mitis (Blanco) Ingram and Moore] fruit. Whether the loss of chlorophyll in the peel is causally related to breakdown of the chloroplast and/or chlorophyllase activity is not readily apparent. Chlorophyllase levels were inversely related to chlorophyll content, but electron micrographs also showed that internal membranes of the chloroplasts were disrupted simultaneously with the decrease in chlorophyll content. Silver, a potent inhibitor of C2H4-mediated effects, retarded the loss of chlorophyll in calamondin rind, reduced the C2H4-induced increase in chlorophyllase level, and prevented the disruption of the chloroplast membranes. The results do not permit the proposal of a mechanism of C2H4 metabolism in the degreening of calamondin fruit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Goldschmidt E. E., Ben-Yehoshua S. Involvement of endogenous ethylene in the induction of color change in shamouti oranges. Plant Physiol. 1976 May;57(5):836–838. doi: 10.1104/pp.57.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]