Abstract
Hypoxia is an important factor in regulation of cell behavior both under physiological and pathological conditions. The mechanisms of hypoxia-induced cell death have not been completely elucidated yet. It is well known that Ca2+ is critically related to cell survival. Hypoxia-inducible factor-1α (HIF-1α) is a core regulatory factor during hypoxia, and L-type voltage-dependent Ca2+ channels (L-VDCCs) have been reported to play a critical role in cell survival. This study was conducted to explore the relationship between L-VDCC expression and HIF-1α regulation in PC12 cells under hypoxia. PC12 cells were treated at 20 or 3 % O2 to observe its proliferation and the intracellular calcium concentration. Then, we detected the protein expression of HIF-1α and L-VDCCs subtypes, Cav1.2 and Cav1.3. At last, to verify the relationship between HIF-1α and Cav1.2 and Cav1.3, we got the expression of Cav1.2 and Cav1.3 with Western blot and luciferase report gene assays after PC12 cells were treated by echinomycin, which is an HIF-1α inhibitor. Compared with 20 % O2 (normoxia), 3 % O2 (hypoxia) inhibited cell proliferation, increased the intracellular calcium concentration, and induced protein expression of HIF-1α. The protein expression of two L-VDCCs subtypes expressed in the nervous system, Cav1.2 and Cav1.3, was upregulated by hypoxia and reduced dose dependently by treatment with echinomycin, a HIF-1α inhibitor. Luciferase report gene assays showed that the expression of Cav1.2 and Cav1.3 genes was augmented under 3 % O2. However, echinomycin only slightly and dose dependently decreased expression of the Cav1.2 gene, but not that of the Cav1.3 gene. These data indicated that Cav1.2 might be regulated by HIF-1α as one of its downstream target genes and involved in regulation of PC12 cells death under hypoxia.
Keywords: L-type voltage-dependent Ca2+ channels, Hypoxia, Cell proliferation, PC12 cells
Introduction
Reduced O2 tension, or “hypoxia,” is an event that cells experience under both physiological and pathological conditions (Patel and Simon 2008). Physiological hypoxia stimulates rapid cell proliferation during early embryonic development including the development of hematopoietic and circulatory systems (Patel and Simon 2008). On the other hand, severe hypoxia results in cell death under pathological conditions. Tissue injury resulting from deprivation of oxygen is involved in the development of various diseases such as stroke and myocardial infarction. It is believed that apoptotic cell death plays a significant role in ischemia-induced tissue injury (Guo et al. 2001). It has been reported that the infarct center area after stroke is in a state of hypoxia, which facilitates passing of Ca2+ through the tight junctions into endothelial cells and causes cell death (Brown and Davis 2002). In vitro experiments have also shown that <1 % O2 induces the death of different type of cells including human neural stem cells(Santilli et al. 2010a).
Hypoxia-inducible factors (HIFs) are the principal transcription factors that regulate gene expression in response to hypoxia and mediate the adaptive response of cells to hypoxia (Patel and Simon 2008). They form a heterodimer composed of α and β subunits. Expression of the β subunit is independent of O2 concentration, whereas the protein stability of the α subunit is regulated according to cellular O2 levels. Under normoxic conditions, the α subunit is degraded through a process involving hydroxylation. During hypoxia, HIF-1α accumulates and binds to the specific hypoxia response element (HRE) of target gene promoters to initiate gene transcription (Loor and Schumacker 2008). In response to hypoxia, HIFs upregulate the expression of various genes such as vascular endothelial growth factor (VEGF), erythropoietin, and transferring (Wenger 2002).
The mechanisms of hypoxia-induced cell death have not been completely elucidated yet. It is well known that Ca2+ is critically related to cell survival. An increase in Ca2+ may promote cellular proliferation, whereas intracellular calcium overload will induce a severe oxidative stress reaction causing cell death. Electrophysiological data show that hypoxia promotes the closure of O2-sensitive K+ channels (Youngson et al. 1993), which leads to cell depolarization followed by activation and opening of voltage-dependent Ca2+ channels (VDCCs). In addition, hypoxia upregulates the expression of these ion channels (Del Toro et al. 2003; Alberdi et al. 2010). The inflow of excessive Ca2+ through these VDCCs results in the inhibition of cell proliferation. Among VDCCs, L-type VDCCs (L-VDCCs) are closely related to neuron survival or death (West et al. 2001; Veng et al. 2003). Moreover, L-VDCCs are classified into four subtypes, Cav1.1, Cav1.2, Cav1.3, and Cav1.4. Only Cav1.2 and Cav1.3 are expressed in the nervous system (Dolphin 2009). However, it is unknown whether hypoxia induces inhibition of cell proliferation by influencing the expression of L-VDCCs in PC12 cell.
Some studies have reported that HIF regulates the expression of VDCCs. In smooth muscle cells of mouse pulmonary arteries, chronic hypoxia upregulates the expression of store-operated Ca2+ channels (SOCCs). HIF-1α overexpression in these cells also causes an increase in SOCCs, even under normoxia (Wang et al. 2006). In PC12 cells, the expression of T-type voltage-dependent Ca2+ channels increases under chronic hypoxia but decreases with expression of anti-sense HIF-1α messenger RNA (mRNA). These data suggest that the level of VDCCs may be regulated by HIF-1α (Del Toro et al. 2003). However, neither relationship of HIF-1α and L-VDCC expression nor the mechanisms of HIF-1α regulating the expression L-VDCCs have been addressed clearly.
So now, we know that severe hypoxia results in cell death, intracellular calcium overload will induce cell death, but it is still not clear about the relationship between hypoxia and calcium channel. In the present study, we used PC12 cells as a cell model. The PC12 cell line is an O2-sensitive noradrenergic clonal line of rat adrenal pheochromocytoma cells, which is a useful model system for neurobiological and neurochemical studies (Conrad et al. 2001; Greene and Tischler 1976). We evaluated the effects of hypoxia on the cell viability (Spicer and Millhorn 2003), HIF-1α expression, and intracellular Ca2+ concentration ([Ca2+]i) of PC12 cells. To explore the regulation of L-VDCC expression by HIF-1α, the HIF-1α inhibitor echinomycin was used to block the function of HIF-1α (Kong et al. 2005). Protein expression of two neuron-specific L-VDCCs, Cav1.2 and Cav1.3, was examined under normoxia and hypoxia with or without echinomycin treatment. Luciferase reporter assays were also performed to test the effects of HIF-1α on the expression of Cav1.2 and Cav1.3.
Materials and methods
PC12 cell culture
PC12 cells were cultured in RPM 1640 medium (Hyclone, Utah, USA) supplemented with 10 % fetal bovine serum (Hyclone, Utah, USA). The cells were incubated in a humidified atmosphere with 5 % CO2 at 37 °C.
Cell viability assay under hypoxia
Cell counting and a cell viability assay were used to evaluate cell proliferation under hypoxia (Wang et al. 2013; Shi et al. 2011). Cells were seeded in 12-well plates (Corning, New York, USA) at a density of 1.5 × 105 cells/ml for cell counting or 96-well plates (Corning, New York, USA) at a density of 1.5 × 105 cells/ml for the viability assay. After incubation for 24 h in 20 % O2 to stabilize the cell status, the cells were placed in a hypoxic incubator with 3 % O2/5 % CO2/92 % N2 or maintained in the normoxic incubator for another 24 h. Cells for counting were harvested and stained with trypan blue, and the cell number was determined using a hemocytometer. During the viability assay, MTS (tetrazolium compound,3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt) (Promega, Wisconsin, USA) was added to each well followed by incubation for 4 h at 37 °C. Absorbances at 490 nm were then quantified in a microplate reader (Bio-Rad, California, USA).
Ca2+ imaging by cofocal laser scanning microscopy
Methods for [Ca2+]i (intracellular [Ca2+]) assay were modified from Guo et al. (2010a). Cells were cultured on coverslips for 24 h to stabilize in 20 % O2 and then incubated in 20 % or 3 % O2 for another 24 h. A Ca2+-specific fluorescent dye, Fluo4-AM (Dojindo Laboratories, Japan) at 2 μM was loaded to the cells followed by incubation at 37 °C for 30 min. After washing with PBS to remove excessive extracellular dye, the cells were maintained in 37 °C for 20 min to completely convert intracellular Fluo4-AM to Fluo4 through esterolysis. The fluorescence intensity of three randomly selected areas under normoxia or hypoxia was then measured by cofocal laser scanning microscopy (CLSM) (Zeiss, Germany). In each area, approximately 25–30 cells were randomly selected for analysis. The mean intensity was calculated and analyzed as the [Ca2+]i.
Echinomycin concentration screening
Cells were seeded in 96-well plates at a density of 1.5 × 105 cells/ml and incubated for 24 h to stabilize under normoxia. The HIF inhibitor echinomycin at 0.2, 1, 5, and 25 nM was added to the cultures. The cells were then placed in a hypoxic incubator or maintained in the normoxic incubator for another 24 h. Then, MTS (10 μl/well) was added to the cells, followed by incubation at 37 °C for 4 h. Absorbances at 490 nm were quantified in the microplate reader. Echinomycin concentrations with minimum cytotoxicity were selected as the treatment doses in the following experiments that tested the effects of HIF-1α inhibition on the expression of L-VDCCs.
Western blot analysis
Cells were plated in 60-mm dishes (Corning, New York, USA) at a density of 1.5 × 105 cells/ml and incubated for 24 h to stabilize under normoxia. Echinomycin at 1 or 5 nM was then added to the dishes. The cells were then placed in a hypoxic incubator or maintained in the normoxic incubator for another 24 h. Then, the cells were harvested, and their proteins were extracted with RIPA Buffer (50 mM Tris–HCl, pH 8.0, with 150 mM sodium chloride, 1.0 % Igepal CA-630 (NP-40), 0.5 % sodium deoxycholate, and 0.1 % sodium dodecyl sulfate) (Sigma, New Jersey, USA). About 80 μg of the each protein extract was separated by SDS-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (Millipore, Boston, USA) in a wet-blotting system (Bio-Rad, California, USA) for 120 min. Membranes were blocked for 2 h at room temperature in a solution containing 5 % milk powder and then incubated overnight with anti-Cav1.2 (rabbit polyclonal; Alomone, Israel), anti-Cav1.3 (rabbit polyclonal; Alomone, Israel), anti-α-tubulin (mouse monoclonal; Sigma, New Jersey, USA), anti-HIF-1α (mouse monoclonal; Novus, Colorado, USA), or anti-β-actin (mouse monoclonal; Sigma, New Jersey, USA) primary antibodies. The membranes were then incubated with goat anti-mouse or goat anti-rabbit secondary antibodies coupled to horseradish peroxidase for 2 h at room temperature. The antigen–antibody complexes were visualized using an ECL kit (Thermo Fisher, Massachusetts, USA) followed by exposure to photographic film (Kodak, Rochester, New York, USA).
Real-time RT-PCR
Cells were plated in 60-mm dishes at a density of 1.5 × 105/ml and then incubated for 24 h to stabilize under normoxia. Echinomycin at 1 or 5 nM was added to the dishes. The cells were then placed in a hypoxic incubator or maintained in the normoxic incubator for another 24 h. Total mRNA was extracted from the cells and cDNA was synthesized by reverse transcription. The primer was Oligo-dT. The enzyme used was MLV reverse transcription kit (Promega, Wisconsin, USA). We used the SYBR system and the comparative Ct method. The following primers were used for real-time PCR: VEGF F, 5′-GCAACGTCACTATGCAGATCATG-3′; VEGF R, 5′-GCTATGCTGCAGGA AGCTCAT-3′; Cav1.2 F, 5′- ACGCCCAGCTCATGCCAACA-3′; Cav1.2 R, 5′-ATACTGCTGCCGCTTCCGCT-3′; Cav1.3 F, 5′-CTGCGCAGGCAAAACAGCCA -3′; Cav1.3 R, 5′-AGGCCTGCAACGGCCATGAT-3′; β-actin F, 5′-CCAGTTCGCCATGGATGAC -3′; and β-actin R, 5′-ATGCCGGAGCCGTTGTC-3′. β-Actin was used as the internal control. The real-time PCR assay kit was SYBRR Premix Ex TaqTM (Takara, Japan). PCR conditions were as follows: 95 °C for, 5 min; 95 °C for 30 s, 56 °C for 20 s, and 72 °C 20 s for 40 cycles; and 95 °C for 1 min.
Luciferase reporter assays
The gene sequences of Cav1.2 and Cav1.3 were obtained from the NCBI database, and then primers were designed to amplify target fragments (Cav1.2 F, 5′-CCGCTCGAGCCAGTGCAGGTTAACA CAGTTA-3′ and Cav1.2 R, 5′-CCCAAGCTTTGTGACTCCAGTTGGGGACCAA-3′; Cav1.3 F, 5′-CCGCTCGAGAGTATTCATTCCTAAACGAGTC-3′ and Cav1.3 R, 5′-CCCAAGCTTCACG AACATTTATTGAGCGCCTACT-3′). Endonucleases XhoI and HindIII were used to digest the PCR products and pGL3 plasmids (Promega, Wisconsin, USA). T4 ligase was used to clone the specific fragments into the plasmids. Escherichia coli were transformed with the plasmids containing target fragments for amplification. The control plasmid was pRL-SV40 (Promega, Wisconsin, USA). PC12 cells were seeded in 24-well plates and cultured overnight. The cells were transfected with the reporter gene plasmids pGL3-Cav1.2/pGL3-Cav1.3 or the pRL-SV40 control plasmid. Echinomycin at 1 or 5 nM was added to the cells after 24 h. The cells were then placed in a hypoxic incubator or maintained in the normoxic incubator for another 24 h. Then, the cells were harvested and applied to a Dual-Luciferase Reporter Assay (Promega, Wisconsin, USA). Firefly luciferase activity and Renilla luciferase activity were detected with a Fluoroskan Ascent FL (Thermo Scientific, Massachusetts, USA).
Statistical analysis
All data are presented as the means ± SEM. Statistical significance was assessed using the Student’s t test for unpaired samples or one-way analysis of variance followed by the Student–Newman–Keuls post hoc test for data from more than two groups apart from the results of cell counting, which was assessed with Welch’s test. P < 0.05 was considered to be statistically significant.
Results
Hypoxia inhibits the proliferation of PC12 cells
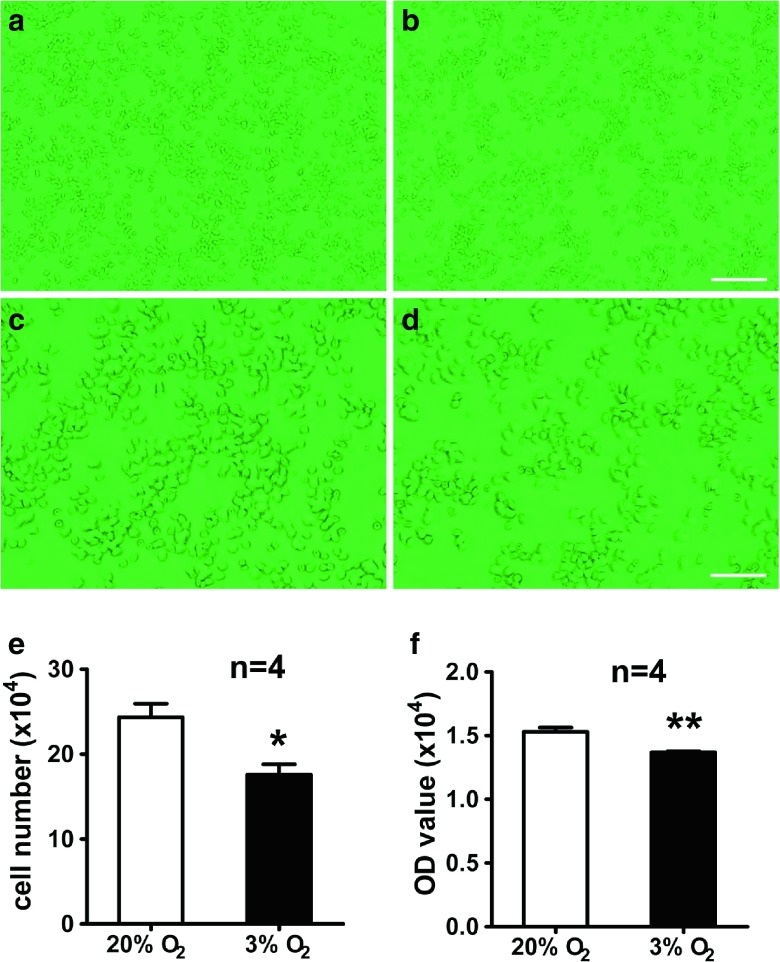
Hypoxia has different effects on various of cell types depending on the oxygen content and exposure time (Santilli et al. 2010b). To confirm the effect of hypoxia on cell survival, PC12 cells were used and cultured under normoxia or hypoxia (3 % O2) for 24 h, and then the number of cells was examined by cell counting and MTS assays. The results of cell counting showed that the number of cells under hypoxia was lower than that under normoxia (17.58 ± 2.13 × 104 vs. 24.33 ± 2.81 × 104) (Fig. 1a–e). To verify the above data, cell viability was quantified by MTS assays. We found that cell viability was reduced under hypoxia compared with that under normoxia (1.40 ± 0.05 vs. 1.60 ± 0.05) (Fig. 1f). These data showed that hypoxia inhibited the proliferation of PC12 cells.
Fig. 1.
Hypoxia (3 % O2) inhibits the proliferation of PC12 cells. PC12 cells were cultured under hypoxia (3 % O2) (b, d) and normoxia (20 % O 2) (a, c) for 24 h. Representative photos under hypoxia (b, d) and normoxia (a, c). Statistic analysis of cell numbers by cell counting (e). Statistic analysis of cell viability by MTS assay (f). Scale bars = 25 μm (a, b) and 100 μm (c, d). *P < 0.05, **P < 0.01; “n” stands for the number of parallel wells
Hypoxia increased [Ca2+]i in PC12 cell
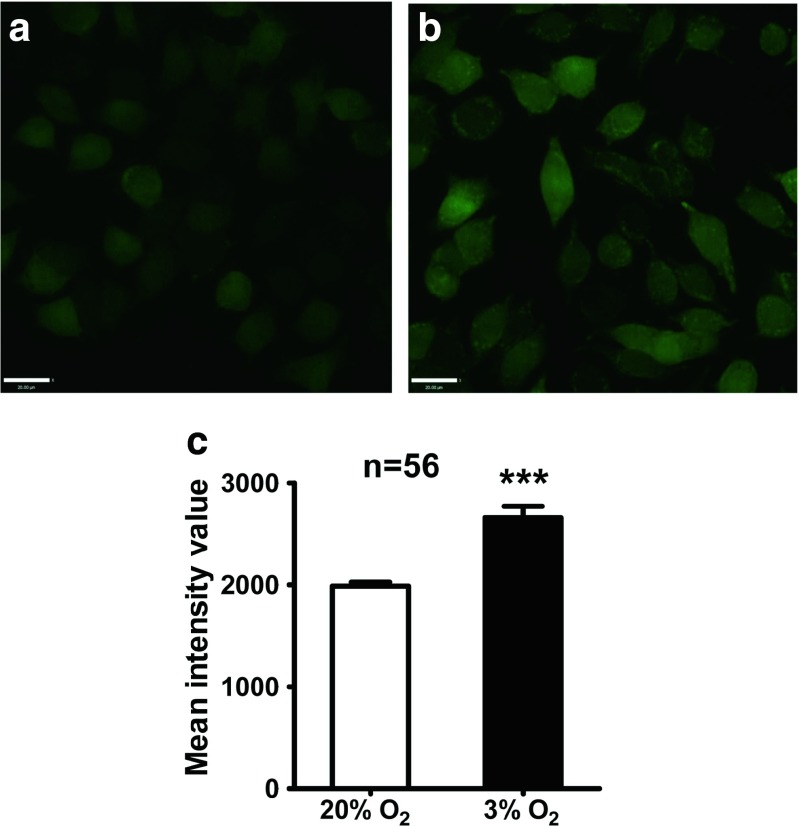
Ca2+ is thought to be important for cell survival. Therefore, we considered that excessive Ca2+ inflow may contribute to the hypoxia-induced inhibition of cell proliferation. [Ca2+]i was examined by loading Fluo4-AM dye into the cells. Ca2+ imaging by CLSM showed that the fluorescence intensities of cells exposed to hypoxia was augmented significantly compared with that of cells under normoxia (Fig. 2), indicating that hypoxia increased [Ca2+]i. Our previous study showed that L-VDCCs are closely associated with hypoxia-induced cell proliferation (Guo et al. 2010a), suggesting that excessive Ca2+ might result from L-VDCCs. Hypoxia induces protein expression of Cav1.2, Cav1.3 and HIF-1α.
Fig. 2.
[Ca2+]i of PC12 cells in 20 and 3 % O2 as detected by calcium imaging. Hypoxic culture in 3 % O2 resulted in elevated [Ca2+]i in most PC12 cells (b), whereas calcium signals were relatively lower in PC12 cells cultured in 20 % O2 (a). The mean intensity value of basal [Ca2+]i in PC12 cells was higher in 3 % O2 compared with that in 20 % O2 (c). Scale bar = 20 μm; ***P < 0.001; “n” indicates the number of analyzed cells
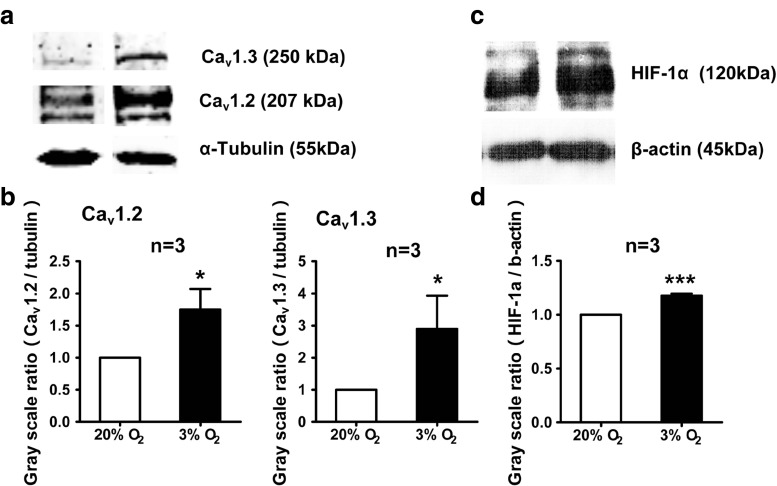
To verify our hypothesis, we detected the protein expression of L-VDCCs. As shown in Fig. 3a and b, both Cav1.2 and Cav1.3 protein expression were upregulated when exposed to hypoxia. This was in accordance with our previous data (Guo et al. 2010b), which may explain the high [Ca2+]i. Furthermore, we tested the protein expression level of HIF-1α, which is one of the key regulators during hypoxia in PC12 cell (Semenza 2000; Baranova et al. 2007). As shown in Fig. 3c and d, HIF-1α protein expression was higher under hypoxia than that under normoxia. Consistent with previous result in neural stem cells (Xiong et al. 2009), the level of HIF-1α protein expression was increased in PC12 cells after hypoxia.
Fig. 3.
Hypoxia induces protein expression of Cav1.2, Cav1.3 and HIF-1α. Expression of Cav1.2, Cav1.3, and HIF-1α protein in PC12 cells under 20 and 3 % O2 were examined by Western blot. Both Cav1.2, Cav1.3 protein (a, b), and HIF-1α protein (c, d) expression in PC12 cells was higher under 3 % O2 than that under 20 % O2. *P < 0.05; “n” stands for the number of repeated experiments
Cytotoxic effects of echinomycin and its inhibitive effects on HIF-1α
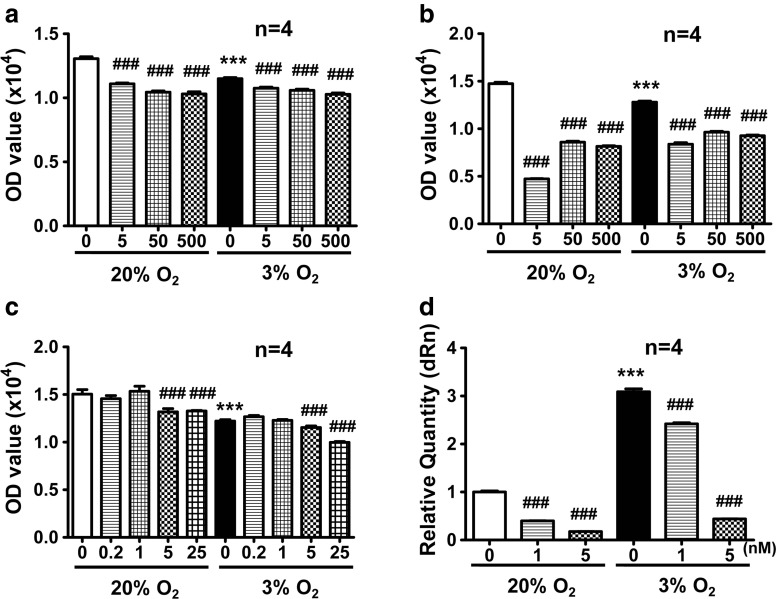
To determine the relationship between HIF-1α and L-VDCCs, echinomycin was used to inhibit the function of HIF-1α under hypoxia. Various concentrations of echinomycin were first tested to determine appropriate doses with minimum cytotoxicity. As shown in Fig. 4a and b, all tested concentrations of echinomycin were toxic to PC12 cells at 24 and 48 h. Therefore, we reduced the amount of echinomycin. As shown in Fig. 4c, 0.2 and 1 nM echinomycin had little effect on cell viability, 5 nM echinomycin had a slight effect, and 25 nM echinomycin was detrimental to cell viability. According to a previous study (Kong et al. 2005), the EC50 of echinomycin is 1.2 nM. Therefore, 1 and 5 nM echinomycin were selected and used in the subsequent experiments.
Fig. 4.
Screening for appropriate concentrations of echinomycin by MTS assays. The viability of PC12 cells was decreased by culturing in 3 % O2 for 24 h. Echinomycin at 5, 50, and 500 nM was toxic to PC12 cells at 24 and 48 h (a, b). Echinomycin at 0.2 and 1 nM had little effect on PC12 cell viability, whereas 5 and 25 nM echinomycin slightly decreased the viability of PC12 cells (c). VEGF mRNA expression was increased in 3 % O2 and echinomycin treatment inhibited VEGF mRNA expression dose dependently (d). ***P < 0.001 vs 20 % O2; ### P < 0.001 vs 20 or 3 % O2; “n” indicates the number of parallel wells
Because VEGF is a recognized target gene of HIF-1α (Carmeliet et al. 1998), in which HIF-1α binds to the HRE of the VEGF gene promoter to promote transcription, we detected the mRNA expression of VEGF in echinomycin-treated cells. As shown in Fig. 4d, hypoxia increased the mRNA expression of VEGF. After treatment with 1 or 5 nM echinomycin for 24 h, VEGF mRNA expression decreased dose dependently. This result suggested that 1 and 5 nM echinomycin mostly inhibited the function of HIF-1α with little effect on the viability of PC12 cells.
Inhibition of HIF-1α regulates the transcription and expression of Cav1.2 and Cav1.3
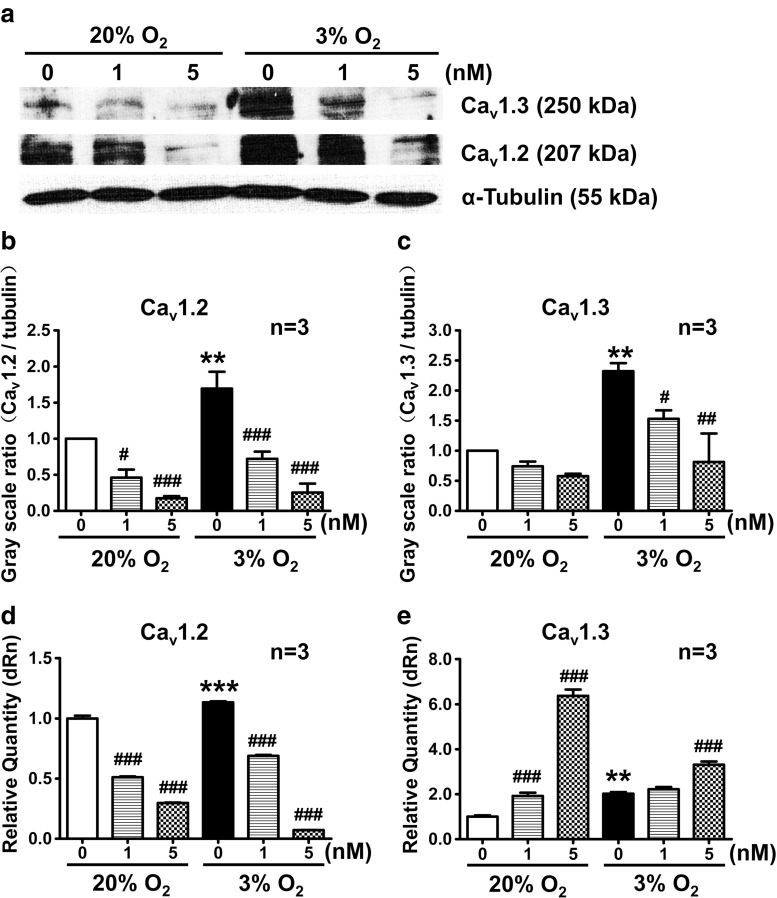
To investigate the relationship between HIF-1α and L-VDCCs, the protein expression of Cav1.2 and Cav1.3 induced by hypoxia was examined with or without echinomycin. The results showed that hypoxia significantly upregulated Cav1.2 and Cav1.3 protein expression (Fig. 5a–c) and mRNA levels (Fig. 5d, e). After HIF-1α inhibition by echinomycin, Cav1.2 and Cav1.3 protein expression was downregulated in a dose-dependent manner (Fig. 5a–c). On the other hand, results showed the echinomycin (HIF-1α inhibitor) downregulated mRNA levels of Cav1.2 but upregulated mRNA levels of Cav1.3.
Fig. 5.
The protein and mRNA expression of Cav1.2 and Cav1.3 during 20 and 3 % O2 was detected by Western blot and real-time PCR. Cav1.2 and Cav1.3 protein expression in PC12 cells was increased in 3 % O2 compared with that in 20 % O2, and decreased in both 3 and 20 % O2 after treatment with echinomycin for 24 h. The mRNA expression of Cav1.2 and Cav1.3 was increased in 3 % O2 compared with 20 % O2. However, when PC12 cells were treated by echinomycin, the mRNA expression of Cav1.2 decreased while the mRNA expression of Cav1.3 increased dose dependently. **P < 0.01 vs 20 % O2; ***P < 0.001 vs 20 % O2; # P < 0.05 vs 20 or 3 % O2; ## P < 0.01 vs 3 % O2; ### P < 0.001 vs 20 or 3 % O2; “n” indicates the number of independent experiments
Inhibition of HIF-1α regulates gene transcription of Cav1.2 and Cav1.3
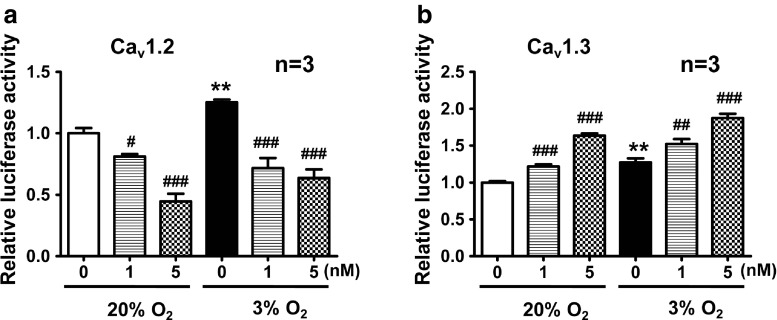
Luciferase reporter assays were designed to verify whether the influence of HIF-1α on protein expression under hypoxia was mediated through binding to the Cav1.2 and Cav1.3 gene promoters, thereby regulating gene transcription. Specific fragments of Cav1.2 and Cav1.3 gene promoters were linked to the luciferase gene in pGL3 plasmids. The results showed that the relative activities of Cav1.2 and Cav1.3 gene promoters had different responses to inhibition of HIF-1α. Under hypoxic conditions, fluorescent signals representing binding of HIF-1α to the Cav1.2 gene promoter were much stronger than that under normoxia. Echinomycin weakened the fluorescent signals dose dependently, regardless of normoxic or hypoxic conditions. In contrast, although hypoxia upregulated Cav1.3 gene expression, echinomycin further increased its expression (Fig. 6). These data indicated that hypoxia might increase the binding of HIF-1α to the gene promoters of Cav1.2 and Cav1.3. However, inhibition HIF-1α by echinomycin only downregulated binding of HIF-1α to the Cav1.2 gene promoter, indicating that Cav1.2 gene transcription might be controlled by HIF-1α, while Cav1.3 might not be directly regulated by HIF-1α.
Fig. 6.
Inhibition of HIF-1α regulates gene transcription of Cav1.2 and Cav1.3 detected by luciferase reporter assays. The binding of HIF-1α to Cav1.2 and Cav1.3 gene promoters was augmented in 3 % O2 compared with that in 20 % O2. After echinomycin treatment, binding of HIF-1α to the Cav1.2 gene promoter (a) was attenuated, while there was an enhancement of the binding of HIF-1α to the Cav1.3gene promoter (b). **P < 0.01 vs 20 % O2; # P < 0.05 vs 20 or 3 % O2; ## P < 0.01 vs 20 or 3 % O2; ### P < 0.001 vs 20 or 3 % O2; “n” stands for the number of independent experiments
Discussion
In the present study, hypoxia at 3 % O2 induced inhibition of PC12 cell proliferation, increased [Ca2+]i, and upregulated the expression of HIF-1α. Moreover, we found upregulated expression levels of L-VDCC subtypes Cav1.2 and Cav1.3 in PC12 cell under hypoxia and proved that Cav1.2 gene was a potential target ofHIF-1α. These data suggest that HIF-1α upregulates L-VDCCs such as Cav1.2 in neurons experiencing hypoxia. Subsequently, upregulation of L-VDCCs resulted in an increase in [Ca2+]i, which finally contributed to hypoxia-induced inhibition of neuronal cell proliferation. This is the first report of a mechanism in which HIF-1α plays a role in hypoxia-induced inhibition of cell proliferation by regulating the expression of L-VDCCs in neurons.
Ca2+ is one of the most important second messengers in regulating various of signaling pathways. Calcium-mediated pathways are essential for cell physiology as well as the pathogenesis of many diseases such as Alzheimer’s disease, brain ischemia, epilepsy, and Parkinson’s disease (Kanazawa et al. 2008; Parekh 2008; Sanz-Blasco et al. 2008; Gandhi et al. 2009; Seo and Seo 2009; Supnet and Bezprozvanny 2010; Tornero et al. 2011). Intracellular calcium overload triggers apoptosis in various cell types including neurons, which is a common event in several pathological situations including neurodegenerative disorders and ischemic stroke (Alberdi et al. 2010; Kanazawa et al. 2008; Gandhi et al. 2009; Seo and Seo 2009; Supnet and Bezprozvanny 2010; Tornero et al. 2011; Zhang 2008; Barsukova et al. 2011). For example, exposure of PC12 cells to staurosporine causes cytosolic and mitochondrial Ca2+ overload that is an essential event for initiation of cell death (Seo and Seo 2009). In endothelial cells, Brown and Davis (2002) found that increased [Ca2+]i inhibits cell proliferation and leads to the breakdown of the blood–brain barrier. In our study, we found that hypoxia (3 % O2) resulted in an increase in [Ca2+]i and inhibition of PC12 cell proliferation. This finding suggests that increased [Ca2+]i may contribute to the inhibition of PC12 cell proliferation.
Hypoxia-induced calcium overload has been reported in neurons and other cell types (Li and Gong 2007; Huang et al. 2009; Lu et al. 2010; Shuangyan et al. 2012). For example, calcium overload is induced by chemical hypoxia in primary cultured rat hippocampal neurons (Huang et al. 2009; Shuangyan et al. 2012). Hypoxia also induces calcium flux through cortical neuron glutamate receptors via protein kinase C (Bickler et al. 2004). Growing evidence suggests that Ca2+ overload is one of the major contributors to myocardial ischemia/reperfusion-induced injury in which hypoxia/reoxygenation occurs (S-s 2012).
In neurons under physiological and pathological conditions, entry of Ca2+ into the cells is mediated through several mechanisms such as overactivation of glutamate receptors (NMDA, AMPA, and KA) or various channels and transporters (TRPM2, TRPM7, NCX, ASICs, CaV1.2, TRPC, L-VGCCs, and hemichannels) (Alberdi et al. 2010; Jia et al. 2007; Szydlowska and Tymianski 2010). Cytoplasmic calcium overload can also occur because of release from internal stores in the mitochondria and endoplasmic reticulum. Such increases in [Ca2+]i can trigger a range of downstream neurotoxic cascades including dysfunction of ATP synthesis, abnormal activation of signaling pathways, and overactivation of enzymes such as calpains and other proteases, protein kinases, nitric oxide synthase, calcineurin, and endonucleases (Jia et al. 2007; Szydlowska and Tymianski 2010). In PC12 cells, T-VDCCs have been reported to contribute to cytotoxic calcium overload during ischemia, which is mediated at least in part by mitochondrial Ca2+ uptake (Gouriou et al. 2013). In our study, we found that hypoxia induced upregulation of L-VDCCs Cav1.2 and Cav1.3. This observation suggests that upregulated Cav1.2 and Cav1.3 expression may contribute to calcium overload that subsequently results in the inhibition of PC12 cell proliferation.
HIFs are the principal transcription factors regulating gene transcription in response to hypoxia (Zhao et al. 2008) and play important roles in the mechanism of cellular adaptation to oxygen deprivation. During hypoxia, HIF-1α levels accumulate and directly upregulate gene expression in neurons and other cell types (Loor and Schumacker 2008; Zhu et al. 2006; Rankin and Giaccia 2008). More than 100 direct HIF target genes have been identified, which regulate a number of cellular processes including cell survival, apoptosis, resistance to oxidative stress, mitochondrial function, glucose metabolism, angiogenesis, erythropoiesis, proliferation, and invasion (Wenger 2002). HIF also indirectly regulates cellular processes such as proliferation and differentiation through interactions with other signaling proteins such as C-Myc and Notch (Loor and Schumacker 2008; Rankin and Giaccia 2008). For example, HIF can directly activate the expression of a number of genes including VEGF, VEGF receptors FLT-1 and FLK-1, plasminogen activator inhibitor-1, angiopoietin-1 and angiopoietin-2, platelet-derived growth factor B, the TIE-2 receptor, matrix metalloproteinase-2 and matrix metalloproteinase-9, heme oxygenase-1, nitric oxide synthase, and cyclooxygenase-2 (Loor and Schumacker 2008; Rankin and Giaccia 2008). In neurons, HIF-1α-induced activation of the Notch pathway is essential for hypoxia-mediated maintenance of glioblastoma stem cells (Qiang et al. 2011).
Our study showed that the expression levels of Cav1.2 and Cav1.3 were upregulated in response to hypoxia (3 % O2). This finding is consistent with the reported upregulation of L-VDCC expression in PC12 cells (Peers et al. 2005; Webster et al. 2006). In addition, expression of Cav1.2 and Cav1.3 was dose dependently decreased in PC12 cells treated with echinomycin. Interestingly, we found that the luciferase activity indicating Cav1.2 transcription was dose dependently decreased by echinomycin, whereas Cav1.3 gene transciption was increased dose dependently. Similarly, echinomycin (HIF-1α inhibitor) also downregulated mRNA levels of Cav1.2 but upregulated mRNA levels of Cav1.3. These data suggest that, in response to hypoxia, HIF-1α may directly regulate Cav1.2 transcription but not Cav1.3. The Cav1.3 gene might be an indirect downstream target gene of activated HIF-1α, and data of decreased expression of Cav1.3 suggest that Cav1.3 protein might be strongly degraded in hypoxia.
In conclusion, we found that HIF-1α might play a role in hypoxia-induced inhibition of cell proliferation by regulating the expression of L-VDCCs in neurons. On the other hand, the gene expression pattern in response to HIF activation is cell type specific. HIF activation in one cell lineage may not be evident in other cell types (Loor and Schumacker 2008). Mechanisms of HIF-mediated calcium overload and inhibition of neuronal cell proliferation in response to hypoxia need to be investigated further in other cell lineages in vitro and in vivo.
Acknowledgments
This work was supported by the National Basic Research Program of the China-973 Project (2011CB910800 and 2010CB944801), the Natural Science Foundation of Beijing (5102010), and the National Natural Science Foundation of China (30831160514 and 31271205).
Footnotes
Ran Li and Yong Wang contributed equally to this work.
Contributor Information
Lingling Zhu, Phone: +86-10-66931315, Email: linglingzhu@hotmail.com.
Xiaomin Wang, Phone: +86-10-83911707, Email: xmwang@ccmu.edu.cn.
References
- Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1α increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27(23):6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsukova AG, Bourdette D, Forte M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur J Neurosci. 2011;34(3):437–447. doi: 10.1111/j.1460-9568.2011.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Ferriero DM. Hypoxia increases calcium flux through cortical neuron glutamate receptors via protein kinase C. J Neurochem. 2004;88(4):878–884. doi: 10.1046/j.1471-4159.2003.02203.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke. 2002;33(6):1706–1711. doi: 10.1161/01.STR.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim H-W, Kim RH, Seta K, Millhorn DE (2001) The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol Part B Biochem Mol Biol 128(2):187–204 [DOI] [PubMed]
- Del Toro R, Levitsky KL, López-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. Int J Biol Chem. 2003;278(25):22316–22324. doi: 10.1074/jbc.M212576200. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19(3):237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouriou Y, Bijlenga P, Demaurex N. Mitochondrial Ca2+ uptake from plasma membrane Cav3.2 protein channels contributes to ischemic toxicity in PC12 cells. J Biol Chem. 2013;288(18):12459–12468. doi: 10.1074/jbc.M112.428128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M, Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8(4):367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- Guo Z, Shi F, Zhang L, Zhang H, Yang J, Li B, Jia J, Wang X, Wang X. Critical role of L-type voltage-dependent Ca2+ channels in neural progenitor cell proliferation induced by hypoxia. Neurosci Lett. 2010;478(3):156–160. doi: 10.1016/j.neulet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Guo Z, Shi F, Zhang L, Zhang H, Yang J, Li B, Jia J, Wang X, Wang X (2010b) Critical role of L-type voltage-dependent Ca2+channels in neural progenitor cell proliferation induced by hypoxia. Neurosci Lett 478(3):156–160 [DOI] [PubMed]
- Huang L, Li Q, Li H, He Z, Cheng Z, Chen J, Guo L. Inhibition of intracellular Ca2+ release by a Rho-kinase inhibitor for the treatment of ischemic damage in primary cultured rat hippocampal neurons. Eur J Pharmacol. 2009;602(2–3):238–244. doi: 10.1016/j.ejphar.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10(5):559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, Makino M, Morishima Y, Yamada K, Nabeshima T, Shirasaki Y. Degradation of PEP-19, a calmodulin-binding protein, by calpain is implicated in neuronal cell death induced by intracellular Ca2+ overload. Neuroscience. 2008;154(2):473–481. doi: 10.1016/j.neuroscience.2008.03.044. [DOI] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65(19):9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Li Y-H, Gong P-L. Neuroprotective effect of dauricine in cortical neuron culture exposed to hypoxia and hypoglycemia: involvement of correcting perturbed calcium homeostasis. Can J Physiol Pharmacol. 2007;85(6):621–627. doi: 10.1139/Y07-056. [DOI] [PubMed] [Google Scholar]
- Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia–reperfusion. Cell Death Differ. 2008;15(4):686–690. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- Lu F, Tian Z, Zhang W, Zhao Y, Bai S, Ren H, Chen H, Yu X, Wang J, Wang L, et al. Calcium-sensing receptors induce apoptosis in Rat cardiomyocytes via the endo (sarco)plasmic reticulum pathway during hypoxia/reoxygenation. Basic Clin Pharmacol Toxicol. 2010;106(5):396–405. doi: 10.1111/j.1742-7843.2009.00502.x. [DOI] [PubMed] [Google Scholar]
- Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586(13):3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15(4):628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Scragg JL, Boyle JP, Fearon IM, Taylor SC, Green KN, Webster NJ, Ramsden M, Pearson HA. A central role for ROS in the functional remodelling of L-type Ca2+ channels by hypoxia. Phil Trans R Soc B Biol Sci. 2005;360(1464):2247–2254. doi: 10.1098/rstb.2005.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wu T, Zhang H-W, Lu N, Hu R, Wang Y-J, Zhao L, Chen F-H, Wang X-T, You Q-D, et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2011;19(2):284–294. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15(4):678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE. 2010;5(1):e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli G, Lamorte G, Carlessi L, Ferrari D, Nodari LR, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5(1):e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Blasco S, Valero RA, Rodríguez-Crespo I, Villalobos C, Núñez L. Mitochondrial Ca2+ overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE. 2008;3(7):e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Gene Dev. 2000;14(16):1983–1991. [PubMed] [Google Scholar]
- Seo SR, Seo JT (2009) Calcium overload is essential for the acceleration of staurosporine-induced cell death following neuronal differentiation in PC12 cells. Exp Mol Med 41:269–276 [DOI] [PMC free article] [PubMed]
- Shi F, Liang Z, Guo Z, Li R, Yu F, Zhang Z, Wang X, Wang X. Senegenin promotes in vitro prol iferation of human neural progenitor cells. Neural Regen Res. 2011;6(3):171–176. [Google Scholar]
- Shuangyan W, Ruowu S, Hongli N, Bei Z, Yong S. Protective effects of Rg2 on hypoxia-induced neuronal damage in hippocampal neurons. Artif Cells Blood Substit Immobil Biotechnol. 2012;40(1–2):142–145. doi: 10.3109/10731199.2011.611474. [DOI] [PubMed] [Google Scholar]
- Spicer Z, DE M. Oxygen sensing in neuroendocrine cells and other cell types: pheochromocytoma (PC12) cells as an experimental model. Endocr Pathol. 2003;14:277–291. doi: 10.1385/EP:14:4:277. [DOI] [PubMed] [Google Scholar]
- S-s Z, He F, Chen A-h, Hao P-y, Song X-d (2012) Suppression of rat frizzled-2 attenuates hypoxia/reoxygenation-induced Ca2+ accumulation in rat H9c2 cells. Exp Cell Res 318(13):1480–1491 [DOI] [PubMed]
- Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010;47(2):183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Tornero D, Posadas I, Ceña V (2011) Bcl-x(L) blocks a mitochondrial inner membrane channel and prevents Ca2+ overload-mediated cell death. PLoS ONE 6(6):e20423 [DOI] [PMC free article] [PubMed]
- Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein α1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Mol Brain Res. 2003;110(2):193–202. doi: 10.1016/S0169-328X(02)00643-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98(12):1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Li R, Yang ZF, Wang YZ, Gong XL, Wang XM. A DNA methyltransferase inhibitor, 5‐Aza‐2′‐deoxycytidine, exacerbates neurotoxicity and upregulates Parkinson’s disease‐related genes in dopaminergic neurons. CNS Neurosci Ther. 2013;19(3):183–190. doi: 10.1111/cns.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NJ, Ramsden M, Boyle JP, Pearson HA, Peers C. Amyloid peptides mediate hypoxic increase of L-type Ca2+ channels in central neurones. Neurobiol Aging. 2006;27(3):439–445. doi: 10.1016/j.neurobiolaging.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J Off Publ Fed Am Soc Exp Biol. 2002;16(10):1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zhao T, Huang X, Liu Z-h, Zhao H, Li M-m, Wu L-y, Shu H-b, Zhu L-l, Fan M. Heat shock protein 90 is involved in regulation of hypoxia-driven proliferation of embryonic neural stem/progenitor cells. Cell Stress Chaperones. 2009;14(2):183–192. doi: 10.1007/s12192-008-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365(6442):153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ma J-x. SERPINA3K prevents oxidative stress induced necrotic cell death by inhibiting calcium overload. PLoS ONE. 2008;3(12):e4077. doi: 10.1371/journal.pone.0004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang C-p, Liu Z-h, Wu L-y, Huang X, Wu H-t, Xiong L, Wang X, Wang X-m, Zhu L-l, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells—role of hypoxia-inducible transcription factor-1α. FEBS J. 2008;275(8):1824–1834. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson P, Hagberg H, Culmsee C. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia–ischemia. Cell Death Differ. 2006;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]