Abstract
This case-control study aimed to investigate whether the levels of Hsp70 (HSPA1A) and Hsp27 (HSPB1) in plasma and lymphocytes were associated with the risk of chronic obstructive pulmonary disease (COPD) among coal workers. A total of 76 COPD cases and 48 age-matched healthy controls from a group of coal workers were included. The case group consisted of 35 COPD patients whose condition was complicated with coal workers’ pneumoconiosis (CWP) and 41 COPD patients without CWP. Heat shock proteins (Hsps) in plasma and lymphocytes were detected by ELISA and flow cytometry, respectively. Multiple logistic regression models were applied to estimate the association between Hsp levels and COPD risk. Our results showed that plasma Hsp70 and lymphocyte Hsp27 levels were significantly higher and plasma Hsp27 levels were significantly lower in COPD cases than in controls (p < 0.01). No significant differences in lymphocyte Hsp70 levels were found between COPD cases and the matched subjects. Higher plasma Hsp70 levels (odds ratio (OR) = 13.8, 95 % confidence interval (CI) = 5.7–33.5) and lower plasma Hsp27 levels (OR = 4.6, 95 % CI = 2.0–10.5) were significantly associated with an increased risk of COPD after adjusting for confounders. Higher lymphocyte Hsp27 levels were only associated with an increased risk of COPD with CWP (OR = 6.6, 95 % CI = 2.0–22.1) but not with an increased risk of COPD without CWP (OR = 3.0, 95 % CI = 0.9–8.9). Additionally, there were strong joint effects of different Hsps on COPD risk. These results showed that higher levels of plasma Hsp70 and lower levels of plasma Hsp27 might be associated with an increased risk of COPD among coal workers. They may have the potential to serve as monitoring markers for COPD in coal workers.
Keywords: COPD, Heat shock proteins, Coal workers, Biomarker, Coal workers’ pneumoconiosis
Introduction
Chronic obstructive pulmonary disease (COPD) continues to be a major cause of morbidity and mortality worldwide (Mannino and Buist 2007). It is a syndrome characterized by progressive and irreversible airflow limitation, with clinical symptoms such as chronic and progressive dyspnea, cough, and sputum production (Vestbo et al. 2013). In addition to cigarette smoking, epidemiological evidence has shown that exposure to coal mine dust is a major cause of COPD among coal workers. It has been suggested that intense and prolonged exposure to workplace dust in coal mining contributes to the development of COPD (Kuempel et al. 2009; Meijers et al. 1997). A 22-year follow-up cohort study of 25,000 British coal miners reported a consistent increase in mortality from COPD (Miller and Jacobsen 1985). The exposure-response relationship between coal mine dust and COPD mortality was confirmed by Kuempel et al (Kuempel et al. 1995). Although the exact pathogenesis of COPD is not completely understood, it is acknowledged that persistent oxidative damage and inflammation contribute considerably to COPD occurrence and development (Oudijk et al. 2003; Chung and Adcock 2008; MacNee and Rahman 2001). Previous studies found that patients with COPD had both local and systemic inflammatory reactions, such as elevated specific inflammatory cell numbers and altered inflammatory cytokine levels (Saetta et al. 1993; Gan et al. 2004). Scholars also observed up-regulated neutrophil peroxide anion levels and impaired antioxidant balance in the blood of COPD patients (Rahman et al. 1996; Nicks et al. 2011). Until now, the diagnosis and monitoring of COPD mainly relied on the use of a spirometry test. But this test is not recommended for COPD patients during an acute phase, because it is too difficult for them to perform. In addition, COPD is a complex syndrome with both pulmonary and extra-pulmonary effects, while the spirometry parameters only reflect the severity of airflow limitation. Research on blood sensitive protein levels will help to explain the mechanism or to monitor the development of COPD.
Heat shock proteins (Hsps) are a large group of proteins, which are highly conserved and ubiquitously expressed (Lindquist and Craig 1988). They are generally considered to be intracellular proteins with molecular chaperone functions (Hendrick and Hartl 1993). A proportion of them have been found to exist in both lesion sites and the blood stream (Zhang et al. 2010; Gruden et al. 2013; Morteza et al. 2013). Many studies have explored the potential roles of circulating Hsps as biomarkers for diabetes and acute coronary syndrome (Morteza et al. 2013; Zhang et al. 2010). Hsp70 (HSPA1A) and Hsp27 (HSPB1) are two important members of the Hsps. Hsp70 has both important intracellular and extracellular functions. Apart from its intercellular functions as a molecular chaperone and anti-apoptotic protein, Hsp70 located in extracellular medium has powerful effects on the regulation of the immune system (Asea et al. 2000, 2002; Srivastava 2002; Panjwani et al. 2002). Exogenous Hsp70 can stimulate cytokine release by acting as endogenous ligand for CD14 and toll-like receptor 4, which suggests its dual role as a chaperone and cytokine (Asea et al. 2000, 2002). Hsp27 is an important small heat shock protein. Under stress conditions, such as hyperthermia and oxidative stress, intracellular Hsp27 can act as an ATP-independent chaperone to protect against protein aggregation and can play key roles in keeping the cytoskeleton stable and resisting cell apoptosis (Rogalla et al. 1999; Hino et al. 2000; Arrigo 2007). In addition, Salari and De et al. have identified the anti-inflammatory function of extracellular Hsp27 via NF-κB and p38 signaling pathways (Salari et al. 2013; De et al. 2000).
Previous studies indicated that altered levels of Hsps in serum and peripheral blood cells were associated with increased COPD risk in the general population (Hacker et al. 2009; Jan Ankersmit et al. 2012; Dong et al. 2013). However, few similar studies have been conducted among coal workers. It would be useful to identify the associations between Hsp levels and the risk of COPD among coal workers. Hence, we developed this case-control study. Because long exposure to coal dust may result in coal workers’ pneumoconiosis (CWP), which is an occupational lung disease characterized by pulmonary inflammation and fibrosis, we have distinguished COPD patients with CWP from COPD patients without CWP in this study (Castranova and Vallyathan 2000). The objectives of this study were (1) to assess the possible associations between levels of peripheral Hsp70 and Hsp27 and the risk of COPD and (2) to explore the potential of peripheral Hsp70 and Hsp27 as biomarkers for COPD monitoring in coal workers.
Subjects and methods
Study subjects
All subjects were male coal workers recruited from one coal mining company in Shanxi province, China. The products of this company are coke coal and meager lean coal, and the primary operation is underground coal excavation. Coal workers were usually exposed to coal mine dust for more than 30 h a week. There was no other obvious toxicant exposure in the workplace. In this study, we recruited 76 newly diagnosed COPD coal workers and 48 age-matched healthy coal workers. The COPD cases included 35 COPD patients whose condition was complicated with CWP and 41 COPD patients without CWP. All the cases and controls, who were identified by the hospital of the company, were free from other known diseases or infections in the previous 6 months. COPD was diagnosed based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Gómez and Rodriguez-Roisin 2002). CWP was diagnosed based on the Chinese Pneumoconiosis Roentgen Diagnostic Criteria, which is similar to the International Labour Organization classification (Chen et al. 2001). After the obtaining signatures indicating informed consent from the participants, face to face interviews were conducted by trained investigators using unified questionnaires, which included personal information, medical history, life habits as well as work history. Each item of the questionnaire was examined and integrated uniformly after the interviews. The amount of cigarette smoking was calculated in terms of packs-year (smoking 20 cigarettes per day for 1 year). This study was approved by the Ethics and Human Subject Committees of the coal mine’s General Hospital and of Tongji Medical College, Huazhong University of Science and Technology (China).
Cumulative dust exposure data collection and spirometry tests
Total dust concentrations (mg/m3) for each post in the workplace were available dating from the establishment of the coal mining company. All the concentrations were assessed monthly based on a gravimetric method and were reported in the form of average level per year. Cumulative exposure to dust (mg/m3-years) was calculated for each participant by multiplying the intensity of dust exposure by the duration of work (Chen et al. 2012). Spirometry tests were performed by trained technicians using a portable spirometer (Chestgraph, HI-101, CHEST MI Inc., Tokyo, Japan) according to the criteria of the American Thoracic Society (Miller et al. 2005).
Blood sampling and isolation of plasma and lymphocytes
For each participant, approximately 6-ml fasting venous blood was collected in heparinized tubes. Plasma and lymphocytes were separated from each sample immediately after blood collection. Plasma was isolated by centrifugating 3-ml blood at 1000×g for 10 min at room temperature and then stored at −80 °C until analysis. Lymphocytes were isolated and purified according to Bøyum and Serneri et al. with some modifications (Bøyum 1976; Serneri et al. 1992). First, peripheral blood mononuclear cells (PBMCs) were isolated from the fresh whole blood by Ficoll density gradient centrifugation. Five milliliters of a 1:1 mixture of whole blood and ice-cold physiological saline were gently applied on an equal volume of Ficoll-Hypaque (Biochemical Reagent Co, Shanghai, China) in centrifuge tubes. The tubes were then centrifuged at 400×g for 30 min at room temperature. After centrifugation, the PBMCs band, the buffy coat between the plasma and the Ficoll-Hypaque layers, was removed and washed twice with phosphate-buffered saline (PBS). The PBMCs isolated by this method contained about 20 % monocytes; hence, another incubation step was conducted to obtain lymphocytes (Serneri et al. 1992). The collected PBMCs were resuspended and adjusted to 1 × 106/ml with RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 1 % penicillin/streptomycin. The cell suspensions were transferred to 2 % gelatin-precoated flasks and cultured in a 37 °C 5 % CO2 incubator for 1 h to make the monocytes adhere. The non-adherent cells were collected as lymphocytes by centrifuging the suspensions at 900×g for 3 min. The collected lymphocytes were then fixed in 500 ul PBS containing 4 % paraformaldehyde and stored at 4 °C for detection of Hsps.
Detection of Hsp70 and Hsp27 levels in lymphocytes
Flow cytometry was used for the detection of Hsp70 and Hsp27 in lymphocytes (Xiao et al. 2003; Bachelet et al. 1998). Each fixed lymphocyte sample was divided into two to detect Hsp70 and Hsp27 separately as follows. Briefly, the fixed lymphocytes were centrifuged at 600×g for 5 min and then were resuspended in 100-ul diluted antibody solutions (1:500 dilution of rabbit-anti-human Hsp70 (SPA-812E) or 1:300 dilution of rabbit-anti-human Hsp27 (SPA-803E), Stressgen Bioreagents, Victoria, BC, Canada) in a permeabilization solution (PBS containing 1 % bovine serum albumin (BSA) and 0.04 % Triton X-100) (Wang et al. 2009). After a 30-min incubation at 4 °C, cells were washed by 0.5 ml PBS containing 1 % BSA and were incubated with 100 μl of fluorescein-isothiocyanate-labeled (FITC) anti-rabbit immunoglobulin G (IgG, diluted 1:100, Kirkegaard & Perry Lboratories, Gaithersburg, MD, USA) for another 30 min. Lymphocytes were washed by 0.5 ml PBS containing 1 % BSA and resuspended in 0.3 ml cold PBS. Meanwhile, two negative controls without anti-human Hsp antibodies or without the secondary FITC-labeled anti-rabbit IgG were performed. The stained lymphocytes were analyzed by flow cytometry (FACS CALIBUR, Beckton Dickinson Company, San José, CA, USA). A total of 10,000 cells were counted and the mean fluorescence intensity (MFI) detected at 525 nm was used for quantitation of Hsp70 and Hsp27.
Detection of Hsp70 and Hsp27 levels in plasma
Plasma Hsp70 and Hsp27 levels were measured by ELISA using commercially available kits (EKS-715 and EKS-500, Stressgen Bioreagents, Victoria, BC Canada, now Assay Designs, Ann Arbor, USA) according to the manufacturer’s recommendations. For the detection of plasma Hsp27, 1:10 diluted plasma was used. For the detection of Hsp70, plasma samples were diluted to 1:2~1:10 before detection, because pre-experiments found that Hsp70 levels in some 1:2 diluted plasma samples were still beyond the detection range of Hsp70 ELISA kits (0.20–12.5 ng/ml). The Hsp70 ELISA kit detected recombinant and native Hsp70 (HSPA1A) and did not detect other Hsp70 family members. The sensitivity of Hsp70 and Hsp27 kits were 0.09 and 0.39 ng/ml respectively according to the manufacturer. Both the inter- and intra-assay coefficients of variations of the two kits were under 10 %.
Statistical analysis
All statistical analyses were performed using SAS version 9.1(SAS Institute). Shapiro-Wilk’s W test was employed to assess the normality of continuous variables. The Hsp70 and Hsp27 were log-normally distributed, and other continuous variables were normally distributed, which were presented as mean ± standard deviation (SD). Qualitative variables were presented as number and percent. Analysis of variance (ANOVA) and chi-square test were used for statistical comparisons between groups. Spearman’s correlation test was performed to investigate the relationship between different levels of HSPs. Multiple logistic regression analysis was used to calculate odds ratios (ORs) and 95 % confidence intervals (CIs) to estimate single and joint effects of Hsp70 and Hsp27 on COPD. The variables of age, amount of cigarette smoking, and cumulative coal mine dust exposure were simultaneously incorporated into the regression models to adjust for the effects of each factor. Statistical inferences were based on two statistical levels (p < 0.05 or p < 0.01 on two sides).
Results
Basic characteristics of COPD cases and controls
The characteristics of COPD cases and controls are presented in Table 1. Cases and controls were well matched for age, dust exposure duration, proportion of cigarette smoker, and amount of smoking per person. Average dust concentrations and cumulative dust exposure levels were significantly higher in the COPD group than in the matched group (p < 0.01). As expected, COPD cases had significantly lower levels of spirometry parameters (VC and FEV1% pred) (p < 0.01). No statistically significant differences of any observed characters were observed between the COPD without CWP subgroup, and the COPD with CWP subgroup were observed (p > 0.05).
Table 1.
Characteristics of COPD cases and controls
| Variables | Controls (n = 48) | Cases | ||
|---|---|---|---|---|
| COPD without CWP patients (n = 41) | COPD with CWP patients (n = 35) | All COPD patients (n = 76) | ||
| Age (year) | 68.7 ± 6.7 | 69.5 ± 6.5 | 68.9 ± 6.8 | 69.2 ± 6.6 |
| Dust exposure duration (year) | 27.5 ± 9.1 | 28.2 ± 10.3 | 28.4 ± 5.3 | 28.3 ± 8.4 |
| Average dust concentration (mg/m3) | 20.7 ± 8.5 | 26.7 ± 4.2* | 27.9 ± 6.5* | 27.3 ± 5.4* |
| Cumulative dust exposure (mg/m3-years) | 556.2 ± 257.4 | 757.2 ± 306.7* | 796.9 ± 267.7* | 775.5 ± 288.2* |
| Smokers (%yes) | 39 (81.8) | 35 (85.4) | 28 (80.0) | 63 (82.9) |
| Amount of cigarette smoking (packs-year) | 26.8 ± 26.3 | 32.0 ± 25.1 | 26.7 ± 24.9 | 29.53 ± 24.9 |
| VC(L) | 26.3 ± 8.4 | 19.7 ± 7.4* | 17.2 ± 7.5* | 18.55 ± 7.5 |
| FEV1% pred(%) | 73.0 ± 20.1 | 45.8 ± 27.3* | 46.2 ± 14.8* | 46.0 ± 22.3* |
Data are presented as “mean ± SD” for continuous variables and as number (percent) for categorical variables
*p < 0.01, compared with controls
Hsp Levels in cases and controls
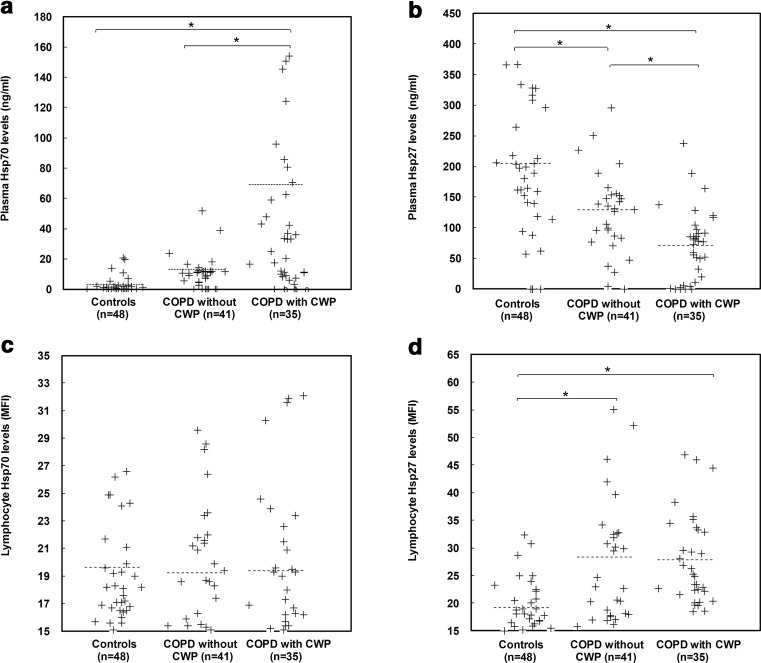
Hsp levels between controls and all the COPD patients were firstly compared. Plasma Hsp70 levels and lymphocyte Hsp27 levels were significantly higher in all the COPD cases than in the controls (44.0 vs 3.3 ng/ml for plasma Hsp70; 28.1 vs 19.2 MFI for lymphocyte Hsp27; p < 0.01). In contrast, plasma Hsp27 levels were significantly lower in all the COPD cases than in the controls (97.1 vs 204.7 ng/ml, p < 0.01). There were no differences of lymphocyte Hsp70 levels between all the COPD cases and controls (19.3 vs 19.6 MFI, p > 0.05).
Hsp levels between the matched group and the two COPD subgroups were also compared (Fig. 1). Plasma Hsp27 levels significantly differed among the three groups (p < 0.01), while lymphocyte Hsp70 levels showed no difference between any two of the three groups (p > 0.05). Plasma Hsp70 levels were found to be significantly higher in the COPD with CWP patients when compared with the healthy ones or with the COPD without CWP patients. But its levels did not significantly differ between the controls and the COPD without CWP patients. Additionally, lymphocyte Hsp27 levels were significantly higher in both the two COPD subgroups than in the matched healthy group, while they showed no difference between the two COPD subgroups.
Fig. 1.
Scatter plots of Hsp70 and Hsp27 located in plasma and lymphocytes by different groups including controls, COPD without CWP patients and COPD with CWP patients. Horizontal dotted lines indicate the mean. Asterisks mark significant differences (p < 0.01) between different groups
Correlations between levels of Hsp27 and Hsp70 in plasma and lymphocytes
The correlation coefficients between the levels of four Hsps are summarized in Table 2. Results showed that lymphocyte Hsp27 levels were significantly correlated with the other three Hsp levels (positively correlated with plasma Hsp70 levels and lymphocyte Hsp70 levels, and negatively correlated with plasma Hsp27 levels). Moreover, plasma Hsp27 levels were observed to be negatively correlated with plasma Hsp70 levels (r = −0.48, p < 0.01).
Table 2.
Correlations between Hsp70 and Hsp27 levels in plasma and lymphocytes
| Variables | Plasma Hsp70 | Plasma Hsp27 | Lymphocyte Hsp70 | Lymphocyte Hsp27 |
|---|---|---|---|---|
| Plasma Hsp70 | – | −0.48a | −0.09 | 0.38a |
| Plasma Hsp27 | – | -0.15 | −0.39a | |
| Lymphocyte Hsp70 | – | 0.61a | ||
| Lymphocyte Hsp27 | – |
The correlation coefficients between Hsp27 and Hsp70 levels were calculated by Spearman’s rank correlation test
aCorrelation is significant at the 0.05 level
Association between Hsp levels and other factors and COPD risk
As shown in Table 3, we found that high levels of plasma Hsp70 were associated with a >12-fold (OR = 13.8, 95 % CI = 5.7–33.5) higher risk of COPD than subjects with low plasma Hsp70 levels after adjusting for age, cumulative dust exposure, and amount of smoking. A similar effect was observed for the association between high levels of lymphocyte Hsp27 and COPD risk (OR = 4.5, 95 % CI = 2.1–9.7). Compared with subjects with high levels of plasma Hsp27, the risk of COPD for subjects with low plasma Hsp27 levels was increased more than three fold (OR = 4.6, 95 % CI = 2.0–10.5) after adjusting for confounders.
Table 3.
Multiple Logistic regression analysis for the association between Hsp levels and the risk of COPD without CWP, COPD with CWP, and all COPD among coal workers
| Variables | COPD without CWP | COPD with CWP | All COPD | |||
|---|---|---|---|---|---|---|
| n (%) | OR (95 % CI) | n (%) | OR (95 % CI) | n (%) | OR (95 % CI) | |
| Agea | ||||||
| ᅟ~60 | 8 (9.0) | 1.0 | 7 (8.4) | 1.0 | 11 (8.9) | 1.0 |
| ᅟ61~70 | 34 (38.2) | 0.6 (0.1–3.3) | 37 (44.6) | 0.4 (0.0–3.9) | 52 (41.9) | 0.9 (0.2-3.5) |
| ᅟ71~ | 47 (52.8) | 0.5 (0.1–2.8) | 39 (47.0) | 0.2 (0.0–1.8) | 61 (49.2) | 0.5 (0.1-1.8) |
| Amount of cigarette smokingb | ||||||
| ᅟNever | 15 (16.9) | 1.0 | 16 (19.3) | 1.0 | 22 (17.7) | 1.0 |
| ᅟLow (>0 and ≤25 pack-year) | 28 (34.5) | 1.4 (0.3–6.0) | 28 (33.7) | 1.9 (0.4–8.9) | 40 (32.3) | 1.2 (0.4-3.4) |
| ᅟHigh (>25 pack-year) | 46 (51.7) | 2.2 (0.6–8.5) | 39 (47.0) | 1.5 (0.4–6.0) | 62 (50.0) | 1.3 (0.5-3.4) |
| Cumulative coal mine dust exposurec | ||||||
| ᅟLow (>0 and ≤500 mg/m3-year) | 30 (33.7) | 1.00 | 24 (29.0) | 1.0 | 33 (26.6) | 1.0 |
| ᅟMiddle (>501 and ≤800 mg/m3-year) | 30 (33.7) | 2.3 (0.7–7.5) | 32 (38.6) | 12.3 (2.2–69.3) | 45 (36.3) | 4.6 (1.7-12.1) |
| ᅟHigh (>801 mg/m3-year) | 29 (32.6) | 5.9 (1.7–20.6) | 27 (32.5) | 20.4 (3.5–118.8) | 46 (37.1) | 6.7 (2.5-18.0) |
| Plasma Hsp70d | ||||||
| ᅟLow (≤11.2 ng/ml) | 69 (77.5) | 1.0 | 51 (61.5) | 1.0 | 76 (61.3) | 1.0 |
| ᅟHigh (>11.2 ng/ml) | 20 (22.5) | 9.5 (2.2–40.5) | 32 (38.6) | 134.1 (12.6–709.7) | 48 (38.7) | 13.8 (5.7-33.5) |
| Plasma Hsp27d | ||||||
| ᅟHigh (>120.3 ng/ml) | 49 (55.1) | 1.0 | 38 (45.8) | 1.0 | 55 (44.4) | 1.0 |
| ᅟLow (≤120.3 ng/ml) | 40 (45.0) | 3.1 (1.0–9.5) | 45 (54.2) | 10.7 (2.6–44.4) | 69 (55.7) | 4.6 (2.0-10.5) |
| Lymphocyte Hsp70d | ||||||
| ᅟLow (≤18.2 MFI) | 52 (58.4) | 1.0 | 49 (59.0) | 1.0 | 72 (58.1) | 1.0 |
| ᅟHigh (>18.2 MFI) | 37 (41.6) | 1.1 (0.4–3.0) | 34 (41.0) | 0.7 (0.2–2.1) | 52 (41.9) | 1.1 (0.6-2.3) |
| Lymphocyte Hsp27d | ||||||
| ᅟLow (≤22.3 MFI) | 61 (68.5) | 1.0 | 49 (59.0) | 1.0 | 72 (58.1) | 1.0 |
| ᅟHigh (>22.3 MFI) | 28 (31.5) | 3.0 (0.9–8.9) | 34 (41.0) | 6.6 (2.0–22.1) | 52 (41.9) | 4.5 (2.1-9.7) |
Multiple logistic regressions were employed to analyze the associations of risk factors and COPD risk in coal workers. Continuous variables were categorized according to their distribution, and the dividing values were listed following the corresponding valuables
aAdjusted for amount of cigarette smoking, cumulative coal mine dust exposure among subjects including healthy controls and patients with corresponding disease
bAdjusted for age and cumulative coal mine dust exposure among subjects including healthy controls and patients with corresponding disease
cAdjusted for age and amount of cigarette smoking among subjects including healthy controls and patients with corresponding disease
dAdjusted for age, cumulative coal mine dust exposure and amount of cigarette smoking among subjects including healthy controls and patients with corresponding disease
For further analysis, associations between Hsp levels and the risk of COPD with or without CWP were estimated (Table 3). Results showed that higher plasma Hsp70 levels were associated with both an increased risk of COPD without CWP and an increased risk of COPD with CWP, with ORs of 9.5 (95 % CI = 2.2–40.5) and 134.1 (95 % CI = 12.6–709.7), respectively. Similarly, low plasma Hsp27 levels were found to be associated with not only an increased risk of COPD without CWP, but also with an increased risk of COPD with CWP, with ORs of 3.1 (95 % CI = 1.0–9.5) and 10.7 (95 % CI = 2.6–44.4), respectively. However, high lymphocyte Hsp27 levels were observed to be associated only with the risk of COPD with CWP (OR = 6.6, 95 % CI = 2.0–22.1), but not with the risk of COPD without CWP (OR = 3.0, 95 % CI = 0.9–8.9). Logistic regression results showed no significant associations between lymphocyte Hsp70 levels and COPD.
To understand the impact of cigarette smoking on the associations between Hsps and COPD, we conducted a stratified analysis. All participants were divided into two subgroups of cigarette smokers (current and previous smokers) or non-smokers. The associations between HSP and COPD obtained by multiple logistic regression models among smokers were similar to those of all participants. As the number of non-smokers (22 subjects) in this study was not big enough to establish a regression model, we did not show these results.
Joint effects of different Hsps on COPD risk
Results of the joint effects of Hsp70 and Hsp27 on COPD by regression analysis are shown in Table 4. The ORs of three joint forms were estimated by multiple logistic regressions after adjusting for age, cumulative dust exposure, and amount of cigarette smoking. Lymphocyte Hsp70 was excluded because no significant associations between its levels and risk of COPD were observed in the above analysis (Table 3). All three joint forms were associated with a higher risk of COPD compared with the form of certain single Hsps. The adjusted OR of the joint form “high plasma Hsp70 and low plasma Hsp27” (OR = 24.9, 95 % CI = 9.5–91.0) was higher than the other two joint ORs.
Table 4.
Multiple logistic regression analysis for the association between joint Hsp levels and COPD risk among coal workers
| Variables | All COPD | |
|---|---|---|
| n (%) | OR (95 % CI) | |
| Plasma Hsp70 and plasma Hsp27 | ||
| ᅟLow plasma Hsp70 or high plasma Hsp27 | 90 (72.6) | 1 |
| ᅟHigh plasma Hsp70 and low plasma Hsp27 | 34 (27.4) | 29.4 (9.5–91.0) |
| Plasma Hsp70 and lymphocyte Hsp27 | ||
| ᅟLow plasma Hsp70 or low lymphocyte Hsp27 | 95 (76.6) | 1 |
| ᅟHigh plasma Hsp70 and high lymphocyte Hsp27 | 29 (23.4) | 22.9 (7.2–72.6) |
| Plasma Hsp27 and lymphocyte Hsp27 | ||
| ᅟHigh plasma Hsp27 or low lymphocyte Hsp27 | 100 (80.7) | 1 |
| ᅟLow plasma Hsp27 and high lymphocyte Hsp27 | 24 (19.4) | 27.0 (6.8–106.9) |
Multiple logistic regressions were employed to analyze the associations between joint Hsp levels and risk of COPD among all the participants after adjusting for age, cumulative coal mine dust exposure, and amount of cigarette smoking; Hsp levels were categorized according to medians (plasma Hsp70, 11.2 ng/ml; lymphocyte Hsp27, 22.3 MFI; plasma Hsp27, 120.3 ng/ml)
Discussion
In the present study, we found that plasma Hsp70 and lymphocyte Hsp27 levels were significantly higher and that plasma Hsp27 levels were significantly lower in COPD patients than in controls. After adjusting for confounders including amount of smoking and coal dust exposure, there was an increased risk of COPD when plasma Hsp70 levels increased or when plasma Hsp27 levels decreased. Higher lymphocyte Hsp27 levels were only associated with an increased risk of COPD with CWP, but not with an increased risk of COPD without CWP.
COPD is a systemic, oxidative, stressful and inflammatory disease with both pulmonary and extra-pulmonary effects (Agustı et al. 2003). Previous studies have suggested that inhaled coal mine dust particles could induce a stress response and start the chronic inflammation, which are associated with the development of COPD (Gosset et al. 1991; Morfeld et al. 2001; Júnior et al. 2009). This concurs with our finding that elevated cumulative coal mine dust exposure was associated with an increased COPD risk. Stressful cellular environments were found to induce extracellular Hsp70 release (Vega et al. 2008; Lancaster et al. 2004). Previous in vitro studies found that extracellular Hsp70 could modulate immune responses, such as the production of inflammatory cytokines and inducible nitric oxides (Panjwani et al. 2002; Asea et al. 2000). Campisi et al. thought extracellular Hsp70 might have an improved immune response to pathogenic challenges, especially those involved in proinflammatory responses (Campisi et al. 2003). In this study, although the increase of plasma Hsp70 levels in patients with COPD without CWP was not significant as compared with the controls, plasma Hsp70 levels were significantly higher in patients with COPD with CWP. Moreover, plasma Hsp70 levels were associated both with an increased risk of COPD without CWP and with an increased risk of COPD with CWP. We speculated that the higher plasma Hsp70 levels in COPD patients might result from the stressful cellular environments caused by inhalation of coal mine dust, and the upregulated plasma Hsp70 might play an important role as an inflammatory regulator in the development of COPD. In the relationship between intracellular and extracellular Hsps, the general view was that intracellular Hsps could be released into an extracellular environment and induce a range of proinflammatory responses (Pockley 2003). The sources of elevated plasma Hsp70 in COPD patients might be necrotic lung cells or peripheral blood mononuclear cells such as lymphocytes (Njemini et al. 2006). In the present study, our results showed no significant correlation between lymphocyte Hsp70 and plasma Hsp70, and no differences of lymphocyte Hsp70 levels were found between COPD cases and controls. It might be possible that the source of elevated plasma Hsp70 was from necrotic lung cells, but further studies of this mechanism are needed to prove this view. From the results above, we considered that higher plasma Hsp70 levels might represent a danger signal for COPD among coal workers, but this inference needs further epidemiology and mechanism evidences.
Extracellular Hsp27 can act as a signaling molecule in stress conditions, but was found to be decreased in some diseases (Salari et al. 2013). In our previous research among coal workers, plasma Hsp27 levels were found to be lower in lung cancer patients when compared with healthy ones (Wang et al. 2010). Analogous changes of Hsp27 levels in coronary artery disease were found by Seibert and his colleagues, and lower serum Hsp27 levels were associated with the presence of coronary heart disease in humans (Seibert et al. 2013). In the present study, plasma Hsp27 levels were lower in coal workers with COPD compared to those in the matched group. Its downregulation may be associated with the progress of COPD. Previous studies have found the important pathogenic role of protease-antiprotease imbalance in the development of COPD, while extracellular Hsp27 was found to be degraded by enzymes and might reflect a proteolytic imbalance (Abboud and Vimalanathan 2008; Martin-Ventura et al. 2006). In this view, plasma Hsp27 might be a potential biomarker for COPD monitoring among coal workers. Our results showed an increase of lymphocyte HSP27 levels in all COPD cases when compared with controls, and lymphocyte Hsp27 levels were found to be negatively associated with plasma Hsp27 levels. The increase of lymphocyte Hsp27 levels seemed to have a regenerative feedback effect in peripheral lymphocytes when plasma Hsp27 levels in COPD patients were low. An in vitro study also found that heat shock stress could cause an increase of Hsp27 levels in lymphocytes (Njemini et al. 2006). However, it was noted that the OR of high lymphocyte Hsp27 levels on COPD without CWP was high but without statistical significance (OR = 3.0, 95 % CI = 0.9–8.9). Therefore, we thought that lymphocyte 27 levels might have the potential to be a monitoring marker for COPD. To further evaluate the potential of Hsps as monitoring markers, we estimated the joint effects of Hsp70 and Hsp27 on COPD. The higher OR value of the joint form “high plasma Hsp70 and low plasma Hsp27” reminded us that the combination of plasma Hsp70 levels and plasma Hsp27 levels might be a more effective and sensitive way to monitor COPD.
The advantage of the current study was that plasma and lymphocyte Hsp levels were considered together. Moreover, more than one Hsp was included. The combination might be more powerful in clarifying the relationship between Hsps and COPD among coal workers. The main limitation was that this study was a cross-sectional epidemiology study. Our findings needed larger prospective cohort studies and mechanism studies to be further proved.
Conclusion
In summary, this case-control study showed that higher levels of plasma Hsp70 and lower levels of plasma Hsp27 might be associated with an increased risk of COPD among coal workers. They may have the potential to be monitoring markers for COPD in coal workers.
Acknowledgments
We are particularly grateful to all individuals who voluntarily participated in the study and to the doctors in the Central Hospital of Xishan Coal & Power Company for their generous help in the health examination and sampling of subjects. This study was supported by research funds from the National Basic Research Program of China (2011CB503804) and the National Natural Science Foundation of China (81372967).
References
- Abboud R, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema [State of the Art Series. Chronic obstructive pulmonary disease in high-and low-income countries. Edited by G. Marks and M. Chan-Yeung. Number 3 in the series] Int J Tuberc Lung Dis. 2008;12(4):361–367. [PubMed] [Google Scholar]
- Agustı A, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. The cellular "networking" of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70 role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bachelet M, Mariéthoz E, Banzet N, Souil E, Pinot F, Pollal CZ, Durand P, Bouchaert I, Polla BS. Flow cytometry is a rapid and reliable method for evaluating heat shock protein 70 expression in human monocytes. Cell Stress Chaperones. 1998;3(3):168. doi: 10.1379/1466-1268(1998)003<0168:FCIARA>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;5(s5):9–15. doi: 10.1111/j.1365-3083.1976.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8(3):272. doi: 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castranova V, Vallyathan V. Silicosis and coal workers' pneumoconiosis. Environ Health Perspect. 2000;108(Suppl 4):675. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhuang Z, Attfield M, Chen B, Gao P, Harrison J, Fu C, Chen J, Wallace W. Exposure to silica and silicosis among tin miners in China: exposure-response analyses and risk assessment. Occup Environ Med. 2001;58(1):31–37. doi: 10.1136/oem.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu Y, Wang H, Hnizdo E, Sun Y, Su L, Zhang X, Weng S, Bochmann F, Hearl FJ. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Med. 2012;9(4):e1001206. doi: 10.1371/journal.pmed.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Adcock I. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-α induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165(7):3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- Dong J, Guo L, Liao Z, Zhang M, Zhang M, Wang T, Chen L, Xu D, Feng Y, Wen F. Increased expression of heat shock protein 70 in chronic obstructive pulmonary disease. Int Immunopharmacol. 2013;17(3):885–893. doi: 10.1016/j.intimp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Gan W, Man S, Senthilselvan A, Sin D. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez FP, Rodriguez-Roisin R. Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2002;8(2):81–86. doi: 10.1097/00063198-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Gosset P, Lassalle P, Vanhee D, Wallaert B, Aerts C, Voisin C, Tonnel AB. Production of tumor necrosis factor-alpha and interleukin-6 by human alveolar macrophages exposed in vitro to coal mine dust. Am J Respir Cell Mol Biol. 1991;5(5):431–436. doi: 10.1165/ajrcmb/5.5.431. [DOI] [PubMed] [Google Scholar]
- Gruden G, Barutta F, Catto I, Bosco G, Caprioli MG, Pinach S, Fornengo P, Cavallo-Perin P, Davini O, Cerrato P. Serum levels of heat shock protein 27 in patients with acute ischemic stroke. Cell Stress Chaperones. 2013;18(4):531–533. doi: 10.1007/s12192-013-0403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Taghavi S. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55(1–2):31–40. [PubMed] [Google Scholar]
- Hendrick JP, Hartl F. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62(1):349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000;271(1):164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Jan Ankersmit H, Nickl S, Hoeltl E, Toepker M, Lambers C, Mitterbauer A, Kortuem B, Zimmermann M, Moser B, Bekos C, Steinlechner B, Hofbauer H, Klepetko W, Schenk P, Dome B. Increased serum levels of HSP27 as a marker for incipient chronic obstructive pulmonary disease in young smokers. Respiration. 2012;83(5):391–399. doi: 10.1159/000336557. [DOI] [PubMed] [Google Scholar]
- Júnior SÁ, Possamai F, Budni P, Backes P, Parisotto E, Rizelio V, Torres M, Colepicolo P, Wilhelm Filho D. Occupational airborne contamination in south Brazil: 1. Oxidative stress detected in the blood of coal miners. Ecotoxicology. 2009;18(8):1150–1157. doi: 10.1007/s10646-009-0364-8. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Stayner LT, Attfield MD, Buncher CR. Exposure‐response analysis of mortality among coal miners in the United States. Am J Ind Med. 1995;28(2):167–184. doi: 10.1002/ajim.4700280203. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Wheeler MW, Smith RJ, Vallyathan V, Green FH. Contributions of dust exposure and cigarette smoking to emphysema severity in coal miners in the United States. Am J Respir Crit Care Med. 2009;180(3):257–264. doi: 10.1164/rccm.200806-840OC. [DOI] [PubMed] [Google Scholar]
- Lancaster G, Møller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9(3):276. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig E. The heat-shock proteins. Annu Rev Genet. 1988;22(1):631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- MacNee W, Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol Med. 2001;7(2):55–62. doi: 10.1016/S1471-4914(01)01912-8. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Nicolas V, Houard X, Blanco-Colio LM, Leclercq A, Egido J, Vranckx R, Michel J-B, Meilhac O. Biological significance of decreased HSP27 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(6):1337–1343. doi: 10.1161/01.ATV.0000220108.97208.67. [DOI] [PubMed] [Google Scholar]
- Meijers J, Swaen G, Slangen J. Mortality of Dutch coal miners in relation to pneumoconiosis, chronic obstructive pulmonary disease, and lung function. Occup Environ Med. 1997;54(10):708–713. doi: 10.1136/oem.54.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Jacobsen M. Dust exposure, pneumoconiosis, and mortality of coalminers. Br J Ind Med. 1985;42(11):723–733. doi: 10.1136/oem.42.11.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Morfeld PB, Schins RPF, Lenaerts H, Witte B, Derwall R, Piekarski C. Cross sectional study on cytokine production (TNF-α, IL-8) in German coalminers with progressive massive fibrosis and in control miners using a rapid wholeblood assay. Biomarkers. 2001;6(6):428–439. doi: 10.1080/13547500110066623. [DOI] [PubMed] [Google Scholar]
- Morteza A, Nakhjavani M, Larry M, Nargesi AA, Esteghamati A. Heat shock protein 70 and albuminuria in patients with type 2 diabetes: a matched case control study. Cell Stress Chaperones. 2013;18(6):815–819. doi: 10.1007/s12192-013-0435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicks ME, O'Brien MM, Bowler RP. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD. 2011;8(4):264–269. doi: 10.3109/15412555.2011.579202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. The effect of aging and inflammation on heat shock protein 27 in human monocytes and lymphocytes. Exp Gerontol. 2006;41(3):312–319. doi: 10.1016/j.exger.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Oudijk ED, Lammers JJ, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(46 suppl):5s–13s. doi: 10.1183/09031936.03.00004603a. [DOI] [PubMed] [Google Scholar]
- Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168(6):2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362(9382):469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154(4):1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo A-P, Buchner J. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Maestrelli P, Ferraresso A, Drigo R, Potena A, Ciaccia A, Fabbri LM. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis. 1993;147(2):301–306. doi: 10.1164/ajrccm/147.2.301. [DOI] [PubMed] [Google Scholar]
- Salari S, Seibert T, Chen Y-X, Hu T, Shi C, Zhao X, Cuerrier CM, Raizman JE, O’Brien ER. Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages. Cell Stress Chaperones. 2013;18(1):53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert TA, Hibbert B, Chen YX, Rayner K, Simard T, Hu T, Cuerrier CM, Zhao X, de Belleroche J, Chow BJ, Hawken S, Wilson KR, O'Brien ER. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: observations from a human cohort and treatment of female mice. J Am Coll Cardiol. 2013;62(16):1446–1454. doi: 10.1016/j.jacc.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Serneri G, Abbate R, Gori A, Attanasio M, Martini F, Giusti B, Dabizzi P, Poggesi L, Modesti P, Trotta F. Transient intermittent lymphocyte activation is responsible for the instability of angina. Circulation. 1992;86(3):790–797. doi: 10.1161/01.CIR.86.3.790. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20(1):395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180(6):4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Wang F, Feng M, Xu P, Xiao H, Niu P, Yang X, Bai Y, Peng Y, Yao P, Tan H. The level of Hsp27 in lymphocytes is negatively associated with a higher risk of lung cancer. Cell Stress Chaperones. 2009;14(3):245–251. doi: 10.1007/s12192-008-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xing J, Wang F, Han W, Ren H, Wu T, Chen W. Expression of Hsp27 and Hsp70 in lymphocytes and plasma in healthy workers and coal miners with lung cancer. Huazhong Univ Science and Technolog Med Sci. 2010;30:415–420. doi: 10.1007/s11596-010-0441-5. [DOI] [PubMed] [Google Scholar]
- Xiao C, Wu T, Ren A, Pan Q, Chen S, Wu F, Li X, Wang R, Hightower LE, Tanguay RM. Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. Cell Stress Chaperones. 2003;8(1):86–92. doi: 10.1379/1466-1268(2003)8<86:BAILOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, Hu FB, Tanguay RM, Wu T. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15(5):675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]