Abstract
Extracellular (ex) HSP60 is increasingly recognized as an agent of cell injury. Previously, we reported that low endotoxin exHSP60 causes cardiac myocyte apoptosis. Our findings supported a role for Toll-like receptor (TLR) 4 in HSP60 mediated apoptosis. To further investigate the involvement of TLR4 in cardiac injury, we studied adult cardiac myocytes from C3H/HeJ (HeJ) mice, which have a mutant, nonfunctional TLR4, and compared the results with parallel studies using wild-type (WT) mice. Nuclear factor κB (NFκB) activation is an early step downstream of TLR4. NFκB was activated 1 h after treatment with HSP60 in WT, but not HeJ mouse myocytes. ExHSP60 caused apoptosis in cardiac myocytes from WT mice, but not in myocytes from the HeJ mutants. To further elucidate the importance of exHSP60 in cardiac myocyte injury, both WT and HeJ mutant isolated mouse adult cardiac myocytes were exposed to hypoxia/reoxygenation. Anti-HSP60 antibody treatment reduced apoptosis in the WT group, but had no effect on the HeJ mutant myocytes. Unexpectedly, necrosis was also decreased in the HeJ mutants. Necrosis after hypoxia/reoxygenation in WT cardiac myocytes was mediated in part by TLR2 and TLR4 through rapid activation of PKCα, followed by increased expression of Nox2, and this was ameliorated by blocking antibodies to TLR2/4. These studies provide further evidence that TLR4 mediates exHSP60-associated apoptosis and that exHSP60 has an important role in cardiac myocyte injury, both apoptotic and necrotic.
Keywords: HSP60, TLR4, TLR2, Hypoxia/reoxygenation, Apoptosis, Nox2
Introduction
Heat shock proteins (HSPs) are well known as protective proteins that make cells resistant to stress-induced cell damage (Nollen and Morimoto 2002; Benjamin and McMillan 1998; Knowlton 1995). Among the HSPs, HSP60 is highly conserved intracellular protein that is expressed both constitutively and under stress conditions and that serves as a molecular chaperone to facilitate protein folding (Neupert and Herrmann 2007; Young et al. 2004; Fink 1999). Although the HSPs are protective and the endogenous increase in HSPs in response to injury reduces cell damage, they can lead to inflammation and even to apoptosis, in other words, a paradoxical deleterious response (Kobba et al. 2011; Knowlton and Srivatsa 2008; Nakano et al. 1997). HSPs have been considered to be intracellular proteins; however, HSPs have been found in blood samples at levels of 1 μg to even 100 μg/ml (Srivastava 2002; Lewthwaite et al. 2002; Xu et al. 2000). Previously, we reported that extracellular HSP60 (exHSP60) causes rat cardiac myocyte apoptosis and that this was mediated by Toll-like receptor (TLR) 4 (Kim et al. 2009a). Some work suggests a role for TLR2 in exHSP60-mediated injury (Mathur et al. 2011; de Graaf et al. 2006). Further investigation of the role of exHSP60 and TLR4 in cardiac injury is warranted to understand the contribution of exHSP60 to cardiac injury and to develop methods to mitigate this injury.
The TLRs are part of the innate immune system, providing a rapid response to pathogens. TLRs act as pattern recognition receptors, recognizing pathogen-associated molecular patterns (PAMPs) as well as damage associated-associated molecular patterns (DAMPs). ExHSP60, as well as other extracellular HSPs act as DAMPs, but much remains to be understood about HSPs as DAMPs (Lee et al. 2013). TLRs activate an intracellular signaling response leading to the release of cytotoxic agents, such as pro-inflammatory cytokines like tumor necrosis factor α (TNFα). To further investigate the role of TLR4 in exHSP60-mediated cardiac myocyte injury, we studied the effect of mutation of TLR4 on exHSP60 mediated apoptosis. Furthermore, we found that both TLR2 and TLR4 mediate phosphorylation of protein kinase C alpha (PKCα) leading to increased expression of NADPH oxidase (Nox2) after hypoxia/reoxygenation (H/R). The results of these studies are reported here.
Materials and methods
Isolated adult mouse cardiac myocytes
The murine strains, C3HeB/Fe and C3H/HeJ, were purchased from Jackson Laboratories, Bar Harbor, Maine. The HeJ mice do not express functional TLR4 because of a naturally occurring mutation in the TLR4 gene (Vogel et al. 1999). The C3HeB/Fe mice are the wild type. The animal protocol was approved by the University of California, Davis Animal Research committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Mice cardiac myocytes
In order to prepare adult cardiac myocytes for culture, the heart must be isolated and perfused to digest the extracellular matrix and isolate individual cells for culture. This is a well-established technique for the study of adult heart cells and is widely used (Sun et al. 2000; Ford and Rovetto 1987; Farmer et al. 1983; Burns and Reddy 1977). Mouse cardiac myocytes were isolated and cultured using a method from the Alliance for Cellular Signaling (Sambrano et al. 2002). 2,3-Butanedione monoxime (BDM), which inhibits myosin ATPase and thus blocks contraction, was added to both the isolation solutions and to culture media, as recommended by the Alliance for Cellular Signaling protocol (Sambrano et al. 2002). In our hands, BDM 1 mg/ml for the isolation and 2 mg/ml for cultures worked best. With this protocol an average of 72 % of cells were rods (nonrods are dead/dying myocytes), a percent suitable for study and a good yield for the mouse heart. Following isolation, cells were preincubated at 37 °C for 2 h to allow adherence and then treated with HSP60 (low endotoxin, cat. no. ADI-ESP-540-F, Enzo Life Sciences, Farmingdale, NY), 1 μg/ml. This low endotoxin HSP60 is considered the gold standard for work on extracellular HSP60 (Gao and Tsan 2003). Previously, we have shown that this preparation of low endotoxin HSP60 has less than 1 EU/μg of protein based on an ELISA for endotoxin and that treatment with polymixin B beads, which bind endotoxin, did not inhibit HSP60 mediated apoptosis (Kim et al. 2009a). Furthermore, we found that it requires 13.75 EU of endotoxin to induce apoptosis in adult cardiac myocytes (Kim et al. 2009a). TNFα (cat. no. 210-TA-100, R&D Systems, Minneapolis, MN), 10 μg/ml, was used as an internal reference point for the degree of apoptosis. Untreated cells were used as a control. Isolated adult mouse cardiac myocytes are challenging to maintain in culture. Excellent mouse cardiac myocytes are needed to survive 16 to 19 h in order to study the response of an entire plate of cells, as is done in many of these experiments. To ensure that quality cells were used for studies, the control cells for each experiment were inspected on the second day to verify that healthy rod-shaped cells were present prior to collecting the samples from all the plates for analysis. When this was not the case, the cells were not analyzed and all plates were discarded. This occurred approximately 10 % of the time.
NFκB activity
was measured using a commercial assay similar to an ELISA, where a 96-well plate was coated with the DNA binding domain for nuclear factor κB (NFκB) (cat. no. 898858, Pierce, Rockland, IL), as previously described (Hamilton et al. 2004b). After incubation with nuclear extracts and washing, the plate was incubated with antibody to p50, and then developed for color. Previously, we have shown that these results correlate well with traditional EMSA, but allow quantification of activation (Hamilton et al. 2004a).
Apoptosis endpoints
Three different endpoints of apoptosis were used. The Cell Death ELISA (CDD, cat. no. 11544675001, Roche, Pleasanton, CA) measures oligosome formation and is an index of DNA fragmentation. The TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay (cat. no. 11772457001, Roche) was used to assess the percent of cells undergoing apoptosis. Lastly, caspase-3/7 activity was measured using a kit (cat. no. HTS02, Calbiochem, EMD Millipore, Billerica, MA), following the directions of the manufacturer. Based on our previous findings, caspase-3/7 activity was measured after 16 h of treatment and DNA fragmentation (CDD and TUNEL) was assayed after 19 h of treatment (Kim et al. 2009a).
Hypoxia/reoxygenation
Adult mouse cardiac myocytes were treated with 2 h of hypoxia in a hypoxia workstation (Forma), followed by 1 h of reoxygenation, based on pilot studies, as previously described in detail (Gupta and Knowlton 2002). A subset of cells were treated with either 2 or 5 μg/ml of anti-HSP60 antibodies (ADI-SPA-807E, clone LK2), Enzo Life Sciences, Farmingdale, NY) just prior to reoxygenation. Media LDH was measured as previously described, and data was normalized to total cellular protein to correct for any variation in cell density (Nakano et al. 1997). Media was centrifuged briefly at 500 g to sediment any nonadherent cardiac myocytes, and this pellet was combined with cells scraped from the culture plates to measure total cellular protein. The Live/Dead assay (cat. no. L-3224, Life Technologies, Thermo Fisher, West Sacramento, CA) was used to assess overall cell viability as previously described (Nakano et al. 1997). A minimum of 30 cells were scored per plate.
Westerns
Westerns were performed and analyzed, as previously detailed (Stice et al. 2011). Antibodies to phospho-PKCα (1:90,000, phosphor-T497, cat. no. AB76016, antibody clone EP26084) and PKCα (1:1000, cat. no. AB32376, antibody clone Y124) were obtained from Abcam (Cambridge, MA). Anti-Nox2 antibody (Abcam, cat. no. 129068, clone EPR699) was used at 1:1000. Nitrocellulose (Bio-Rad, Hercules, CA) was used for all blots. Phosphorylation blots were blocked with 3 % BSA following our previously described protocol (Stice et al. 2012). Affinity purified anti-mouse IgG-HRP and anti-rabbit IgG-HRP (both from Amersham, GE Healthcare Biosciences, Piscataway, NJ; anti-mouse cat. no. NA931, anti-rabbit cat. no. NA934) were used at 1:1000 as secondary antibodies.
Statistics
Data is expressed as mean ± standard error of the mean (SEM). Groups were compared by ANOVA and by ANOVA on ranks for normalized data, followed by a Student-Neuman-Keuls or Dunn’s test. For paired data, a Wilcoxin ranks test was performed. A p value <0.05 was considered significant.
Results
Activation of TLR4 leads to NFκB activation, a critical downstream signaling step, leading to increased inflammatory cytokine expression and to activation of apoptotic cascades. The HeJ mutant has a point mutation on the intracellular portion of the receptor, which replaces proline 712 with histidine. This single amino acid substitution renders the TLR4 receptor unresponsive to inflammatory stimuli, like LPS. As NFκB activation is a critical step in TLR4 signaling, we first investigated whether the HeJ mutation affected exHSP activation of NFκB.
HSP60 activates NFκB. NFκB activation was quantified using an assay, which measures binding of p50 from nuclear extracts to a microtiter plate coated with an NFκB consensus binding domain. Previously, we have reported that HSP60 increases NFκB activation as early as 20 min and that activation is still present at 80 min in adult rat cardiac myocytes. In the current study, given the limited number of mouse myocytes, we investigated NFκB activation at 60 min. As shown in Fig. 1a, NFκB activation was doubled in WT cells treated with HSP60 compared to controls. In contrast, there was no change in NFκB activity in cardiac myocytes derived from the HeJ mice.
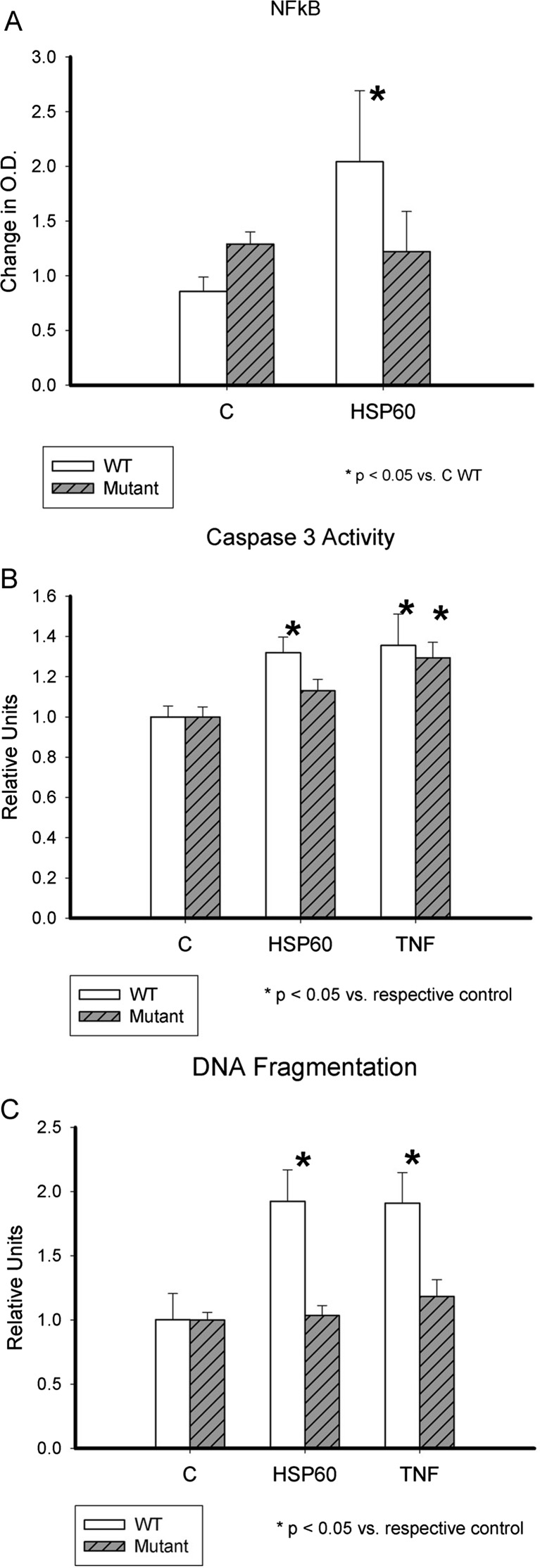
Fig. 1.
ExHSP60, inflammation, and apoptosis. a NFκB activation by HSP60. Graph summarizes effect of HSP60 on NFκB activation in adult cardiac myocytes from TLR4 mutant (HeJ) and WT mice; n = 8–9/group. *p < 0.05 versus WT. b Caspase-3/7 activation by HSP60. Graph summarizes results of experiments measuring caspase-3/7 activity after treatment with HSP60 or TNFα, as a reference control for the amount of apoptosis. Data was normalized to control to correct for variation among mouse myocyte isolation. White bars are WT, and gray-striped bars are TLR4 (HeJ) mutant adult mouse cardiac myocytes; n = 21–32/group. *p < 0.05 versus respective control group. c DNA fragmentation by CDD assay. Graph compares DNA fragmentation in WT and TLR4 mutant (HeJ) adult mouse cardiac myocytes after treatment with HSP60 or TNFα, as a reference control for the amount of apoptosis. White bars are WT, and gray-striped bars are TLR4 (HeJ) mutant adult mouse cardiac myocytes; n = 14–17/group. *p < 0.05 versus respective control group
Apoptosis
Isolated cardiac myocytes from HeJ and WT mice were used to investigate the role of TLR4 in HSP60-mediated apoptosis. The cells were allowed to recover from isolation for 1–2 h and then treated with 1 μg/ml HSP60 or 10 ng/ml TNFα. Activation of caspase-3/7 was measured at 16 h. As shown in Fig. 1b, the HeJ mutant mice myocytes did not have increased caspase-3/7 activity after treatment with HSP60, but did show activation of caspase-3/7 by TNFα. WT cardiac myocytes showed caspase-3/7 activation after either treatment (p < 0.05 vs C), as shown in Fig. 1b.
DNA fragmentation
DNA fragmentation occurs downstream of caspase activation. The Cell Death ELISA (Roche) was used to quantify DNA fragmentation at 19 h. HSP60 did not increase DNA fragmentation in HeJ cardiac myocytes, but TNFα did (Fig. 1c). Both HSP60 and TNFα caused DNA fragmentation in the WT cardiac myocytes (p < 0.05 vs C).
The TUNEL assay
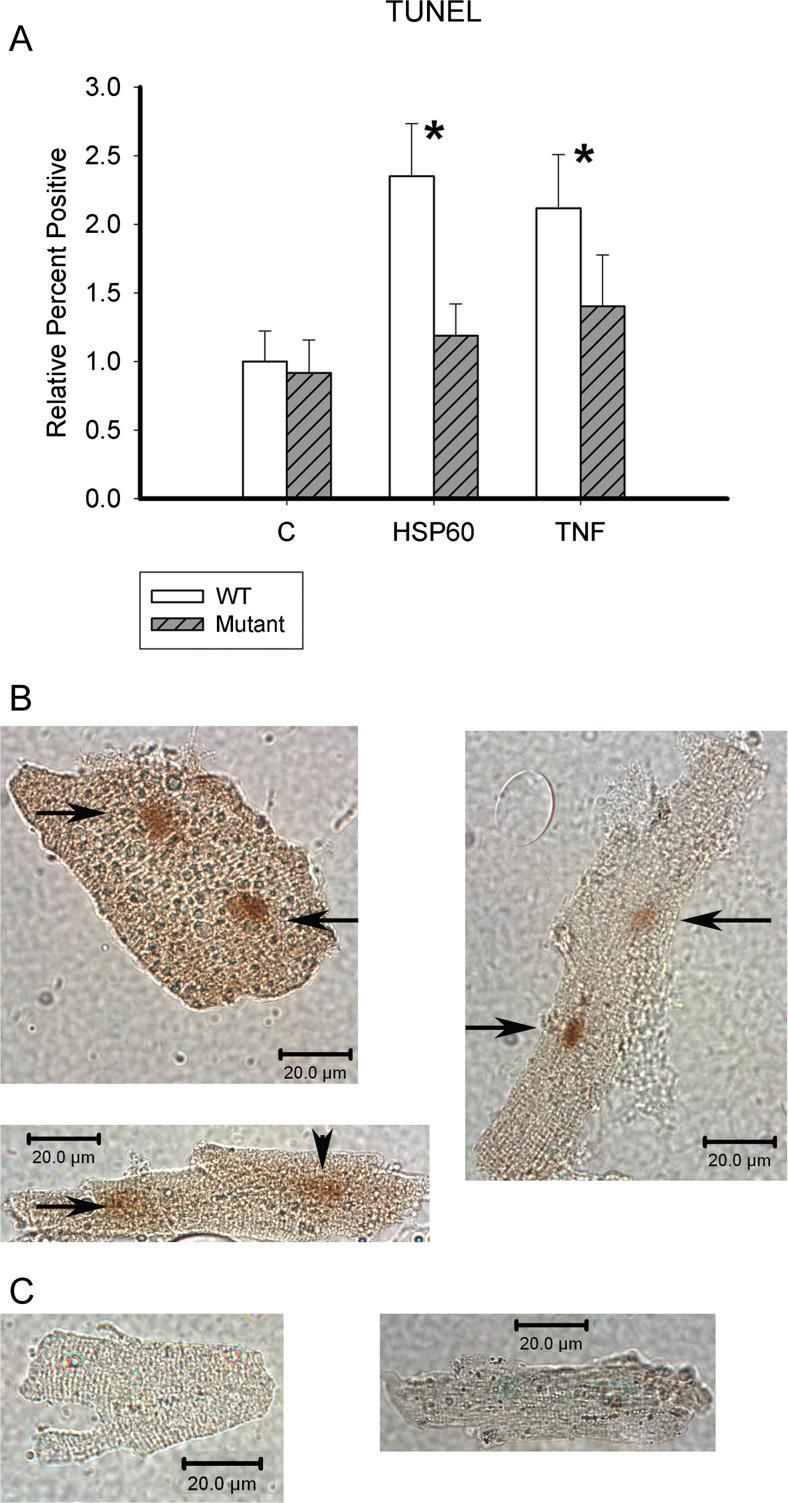
The TUNEL assay was used to assess the percent of cardiac myocytes undergoing apoptosis after 19 h of treatment. ExHSP60 did not increase TUNEL-positive HeJ cardiac myocytes (Fig. 2), and although TNFα increased the percent of TUNEL-positive cells in HeJ myocytes, this was not significant. In contrast, both treatments doubled apoptosis in the WT cardiac myocytes (p < 0.05 vs C). Representative apoptotic and nonapoptotic cells are shown in Fig. 2b and c. There was no difference in the amount of TUNEL-positive cells between the two treatments in WT cardiac myocytes. These results are consistent with the measurements of DNA fragmentation.
Fig. 2.
TUNEL assay. a The TUNEL assay was done to assess relative percent of cardiac myocytes undergoing apoptosis. White bars are WT, and gray-striped bars are TLR4 (HeJ) mutant adult mouse cardiac myocytes. Data was normalized to control to correct for variation among mouse myocyte isolation. White bars are WT, and gray-striped bars are TLR4 (HeJ) mutant adult mouse cardiac myocytes; n = 11–16/group. *p < 0.05 versus respective control group. b Representative TUNEL-positive cells. c Representative TUNEL-negative cells. Arrows point to TUNEL-positive nuclei. Scale bar 20 μM
Hypoxia/reoxygenation
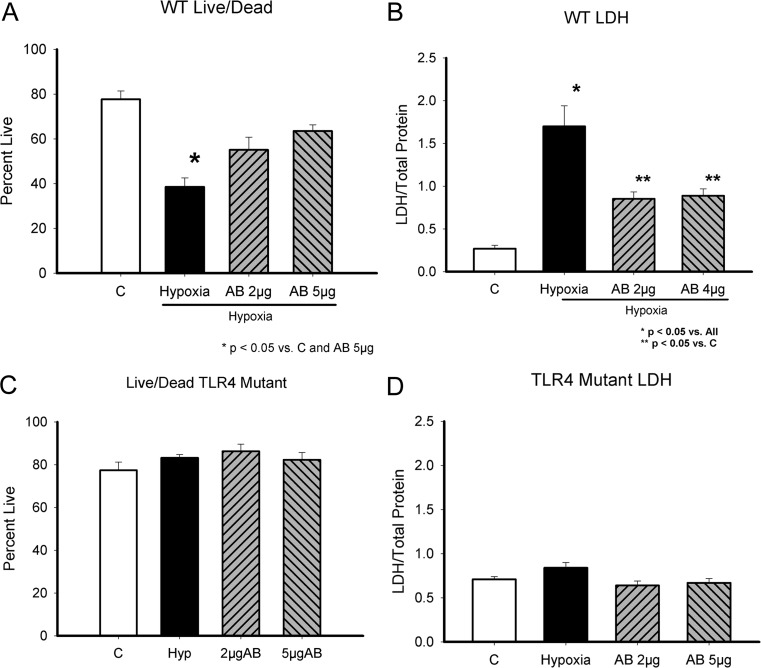
ExHSP60 caused cardiac myocyte cell death. Ischemic injury leads to necrosis and the release of cellular proteins including HSP60. Therefore, we investigated whether hypoxia/reoxygenation injury would be attenuated by treatment with anti-HSP60 antibodies. Cells were subjected to 2 h of hypoxia and 2 h of reoxygenation. Just prior to reoxygenation, 2 or 5 μg/ml of HSP60 antibody was added to the cardiac myocytes. After a 2-h recovery period, cells were evaluated for injury. Cardiac myocyte viability was markedly decreased by hypoxia/reoxygenation (Fig. 3a). HSP60 antibody treatment prevented this significant drop in cell viability. As shown in Fig. 3b, HSP60 antibodies markedly decreased the release of LDH; however, LDH release was still increased in the antibody-treated groups compared to normoxic control cells. For comparison, the same studies were performed using the TLR4 mutant cardiac myocytes. Cell viability was not decreased by hypoxia/reoxygenation (Fig. 3c). There was no LDH release in response to hypoxia/reoxygenation (Fig. 3d). Thus, in contrast to the WT cells, in the mutant cells HSP60 antibody treatment had no benefit, as there was no increase in necrosis with hypoxia/reoxygenation. The experiment was repeated in WT cells, using blocking antibodies to TLR2 and TLR4. As shown in Fig. 4, blocking antibodies to both TLR2 and TLR4 reduced LDH release following hypoxia and reoxygenation. Thus, both TLR2 and TLR4 were involved in the increase in necrosis.
Fig. 3.
HSP60 and hypoxia/reoxygenation-associated injury. a, c Live/Dead assay, which assesses cell viability based on mitochondrial metabolism of calcein and binding of ethidium to cellular DNA. a WT cardiac myocyte viability in control ( C), untreated cells, after 2 h of hypoxia followed by 1 h of reoxygenation (hypoxia), and the effect of treatment with anti-HSP60 antibodies prior to reoxygenation at either 2 or 5 μg/ml concentration. b LDH release after hypoxia/reoxygenation by WT cardiac myocytes. d LDH release after hypoxia/reoxygenation by TLR4 mutant cardiac myocytes. C, control; Hypoxia, treated with 2-h hypoxia/1-h reoxygenation; AB 2 μg and AB 5 μg, 2 or 5 μg/ml of HSP60 antibody added to media just prior to reoxygenation. *p < 0.05 versus C and AB 5 μg in a; *p < 0.05 versus all in b; **p < 0.05 versus C. n = 6–12/group
Fig. 4.
TLR2 and TLR4 blocking antibodies and LDH release after hypoxia/reoxygenation. Cardiac myocytes were pretreated with TLR2 or TLR4 blocking antibodies before hypoxia/reoxygenation. LDH was measured after reoxygenation as an index of necrosis. n = 15/group
Several possible mechanisms were considered for the increased release of LDH following exHSP60 treatment. Based on the location and abundance of Nox2 in the heart and the limited literature in this area involving different cell types, we hypothesized that the increase in necrosis we observed with TLR4/TLR2 activation by exHSP60 was mediated by activation of PKCα leading to a rapid increase in Nox2 expression, followed by increased ROS production (Bae et al. 2009; Simon and Fernandez 2009; Matsuno et al. 2012). PKCα was found to be activated by phosphorylation 10 min after exHSP60 treatment (Fig. 5a and b), and this phosphorylation rapidly disappeared, being no longer present at 60 min (data not shown). Blocking antibodies to either TLR2 or TLR4 reduced PKCα phosphorylation at 10 min. In turn, Nox2 expression increased at 1 h (Fig. 5c and d). This is a rapid response to TLR4 activation and occurs much more quickly than the increase in cytokines in response to TLR4 activation in cardiac myocytes (Kim et al. 2009a).
Fig. 5.
PKCα, Nox2, and exHSP60-mediated necrosis. a TLR2 and TLR4 blocking antibodies and phosphorylation of PKCα after 10 min of reoxygenation. b Representative westerns for phospho-PKCα and total PKCα. Phospho-PKCα densities were normalized to the respective total PKCα. c TLR2 and TLR4 blocking antibodies and Nox2 protein levels at 1-h reoxygenation. Nox2 levels were normalized to GAPDH as a loading control. d Representative westerns for Nox2 and GAPDH. n = 4/group
Discussion
The current study aimed to further investigate the role of TLR4 in cardiac myocyte injury mediated by exHSP60. Previously, we have shown that blocking TLR4 or components of the downstream signaling pathway ameliorated exHSP60-mediated cardiac myocyte apoptosis. TLR4 blocking antibodies decreased but did not completely inhibit HSP60-mediated apoptosis (Kim et al. 2009a). The current work demonstrates directly, through use of the TLR4 mutant mouse, that the TLR4 receptor was essential for exHSP60 mediated apoptosis, and provides new information about the role of TLR2 and TLR4 in necrosis. Our results establish that mutation of the cytoplasmic domain of TLR4 prevents HSP60-induced apoptosis in cardiac myocytes. Furthermore, anti-HSP60 antibodies attenuated myocyte injury after hypoxia and reoxygenation, supporting that HSP60 released during hypoxia/reoxygenation amplifies cell injury. The increase in necrosis was mediated by phosphorylation of PKCα, followed by increased expression of Nox2 at the protein level.
TNFα did not cause significant DNA fragmentation in the TLR4 mutants, which is consistent with our finding in the rat cardiac myocyte that TLR4 blocking antibodies reduced TNFα-mediated DNA fragmentation (Kim et al. 2009a). However, TNFα caused similar amounts of caspase activation, in both WT and mutant cardiac myocytes, again similar to findings in the rat cardiac myocytes. We previously have shown that DNA fragmentation after activation of TLR4 by HSP60 or after TNFα treatment is independent of caspase-3/7 activation (Kim et al. 2009a). The absence of TNFα-mediated DNA fragmentation despite caspase-3/7 activation in the HeJ mutant is congruent with our previous studies demonstrating that TLR4 blocking antibodies unexpectedly reduced TNFα-mediated apoptosis (Kim et al. 2009a).
HSPs as unexpected mediators of injury/inflammation. As more is understood about the HSPs, it has become apparent that they may also be provocateurs of injury, as well as protectors. HSP60 has been thought to be a ligand of the innate immune system, binding TLR4, with evidence slowly accumulating to support this, including the current work. Some have reported that HSP60 binds TLR2, but we have not found this to be the case in cardiac myocytes (Kim et al. 2009a; de Graaf et al. 2006; Zanin-Zhorov et al. 2006; 2003). Innate immunity is a primitive immune system that generates an immediate toxic response to recognized dangerous motifs, such as LPS. Thus, the TLRs (11 identified to date) recognize specific types of molecules as foreign or toxic (Matzinger 2002). Downstream of the TLRs is activation of NFκB and the production of toxic cytokines, such as TNFα, that are released from the cell.
TLR4, HSP60, and myocardial injury. TLR4 mutant and knockout mice have been used to investigate the role of TLR4 in myocardial injury after ischemia or infarction. TLR4 mutant and knockout mice have smaller infarcts, although this was not associated with improved function (Kim et al. 2007; Oyama et al. 2004). In patients, TLR4 levels in monocytes were increased post-infarct and higher TLR4 levels correlated with the development of heart failure. Better recovery of function after global ischemia in the isolated perfused mouse heart was seen with TLR4 deficient mice, and this was associated with lower TNFα and IL1β levels (Cha et al. 2008). In addition, WT mice had fourfold greater activation of NFκB compared to TLR4 deficient. The improved function post-ischemia was reduced by treatment with TNFα and IL1β to match wild-type levels (Cha et al. 2008). TLR4 deficient mice also had reduced post-MI remodeling and long-term better function (Timmers et al. 2008). More recently, it has been shown that treatment with anti-HSP60 antibodies prior to coronary ligation reduced the subsequent production of TNFα and IL-6 (Tian et al. 2013). Thus, in acute ischemia, TLR4 activation contributes significantly to myocardial injury and adverse remodeling.
In the current study, anti-HSP60 antibodies reduced injury after hypoxia/reoxygenation, including LDH release, which is associated with necrosis rather than apoptosis. There was no benefit to anti-HSP60 treatment in the TLR4 mutant cardiac myocytes, as these cells were quite resistant to injury. Others have found that HSP60 released from the ischemic heart enhances cardiac injury and that this is mediated by TLR4 activation (Tian et al. 2013; Li et al. 2011). Thus, HSP60 is potentially an important contributor to ischemic injury.
HSP60, TLR2/4, PKCα, Nox2, and necrosis. Nox2 is one of the seven known Nox family proteins. Nox2 and Nox4 are the most abundant Nox proteins in the heart. Nox2 is associated with the plasma membrane, and Nox4 is associated predominantly with organelle membranes. Overall, Nox2 and Nox4 have been shown to be important and equal contributors of ROS in myocardial ischemia/reperfusion (Matsushima et al. 2014). LPS is commonly used to model sepsis, and several researchers have found that TLR4 has a role in LPS-mediated increased ROS and cell death. TLR4 has been reported to mediate activation of Nox2 and Nox4 in human umbilical vein endothelial cells (HUVECS) (Simon and Fernandez 2009). In HUVECS, cell death from necrosis after LPS treatment occurred prior to any increase in cytokine expression (Simon and Fernandez 2009). Similarly, Nox1, which was expressed at low levels in the heart, and Nox2 expression increased in the heart after treatment with endotoxin (Matsuno et al. 2012). Modeling this in H9c2 cells demonstrated that siRNA to knock down TLR4 prevented the LPS-induced increase in Nox1 mRNA (Matsuno et al. 2012). In macrophages, TLR4-mediated activation of Nox2 by minimally oxidized (mm) LDL (Bae et al. 2009). Based on the location and abundance of Nox2 in the heart, we hypothesized that the increase in necrosis we observed with TLR4 activation by exHSP60 was mediated by an increase in Nox2 expression.
Nox2 is predominantly a membrane bound protein, and it is known to be involved in the stress response in cardiac myocytes (Zhao et al. 2010). Increased Nox2 expression is also found in other cells types in hypoxic conditions (Schroder et al. 2009). Nox2 mediates the increase in ROS seen in HUVECs after treatment with LPS, which activates TLR4 (Simon and Fernandez 2009). Based on a relatively limited literature, Nox2 appeared a likely mediator of TLR mediated necrosis and we investigated its activation after H/R. Overall, there has been limited investigation of Nox2 and hypoxia in cardiac myocytes (Akki et al. 2009).
PKCα is involved in hypoxia-related signaling pathways in other cell types (Hasan et al. 1996), as well as in cardiac myocytes (Mehta et al. 2002). We found that PKCα was phosphorylated after H/R in cardiac myocytes, and this correlated subsequent increased Nox2 activity. PKCα activation was transient and was gone by 1 h.
TLR4 signaling and disease. The TLRs were originally identified as a critical early part of the immune response to infection. However, over time, it has become apparent that in addition to protecting the organism against infection, the TLRs also can promote disease and inflammation. Previously, we have demonstrated in adult rat cardiac myocytes that exHSP60 stimulates apoptosis via TLR4. The current study provides further proof of the important role of TLR4 in HSP60 induced injury post-hypoxia/reoxygenation, as well as in HSP60-induced apoptosis (Kim et al. 2009a). Other work suggests that exHSP60 contributes to neurodegeneration via activation of TLR4 (Lehnardt et al. 2008). However, the role of TLR4 in disease is complex. In cancer, TLRs have been found to have a role promoting cell proliferation, resistance to chemotherapy, and metastasis (Chen et al. 2008). TLR4 signaling in ovarian cancer cell lines activated IRAK4, leading to cJun phosphorylation and activation of NFκB (Szajnik et al. 2009). This promoted VEGF expression and resistance to apoptosis. Similarly, in SW620 cells (colon cancer cell line), TLR4 promoted cell proliferation and migration through phosphorylation of ERK 1/2 (Zhou et al. 2011). Downregulation of TLR4 with siRNA inhibited prostate cancer cell invasion and survival (Hua et al. 2009). Thus, the role of TLR4 in disease is complex.
TLR4, HSP60 experimental caveats. The potential effects of exHSP60 have generated significant interest, particularly with regard to inflammation and as a source of increased injury after myocardial infarction, which results in the release of cellular contents into the blood stream as a result of necrotic cell death. It is important that work in this area be conducted with low endotoxin material and controls to avoid misinterpretation of results.
Conclusions
In the last 5–10 years, observations have been made that suggest a paradoxical, destructive role for HSPs in certain settings. In disease states, the HSPs may be overexpressed and can contribute to disease progression, rather than to protection. In particular, extracellular HSPs can activate inflammatory signaling pathways leading to destructive effects. Others have recently demonstrated a tripling of plasma HSP60 with menopause, and that exHSP60 induced apoptosis in human bone marrow stromal cells via TLR2 (Kim et al. 2009b). In contrast, HSP60 inhibited apoptosis via MyD88 in B cells (Cohen-Sfady et al. 2009). Thus, the role of exHSP60 in cell injury is complex, and at times, exHSP60 is clearly beneficial. Further investigation will be needed to understand the role of exHSP60, TLR2, and TLR4 in the disease.
Acknowledgments
The authors thank S. Gupta, Ph.D. for technical assistance. This study was supported by HL077281 (AAK), HL079071 (AAK), the Department of Veterans Affairs (AAK), and the German Research Foundation DFG KI 1302/1-1 (SCK).
Conflict of interest
The authors have nothing to disclose.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.RES.83.2.117. [DOI] [PubMed] [Google Scholar]
- Burns AH, Reddy WJ. Hexose monophosphate shunt in isolated cardiac myocytes from normal rats. Am J Physiol. 1977;232:E570–E573. doi: 10.1152/ajpendo.1977.232.6.E570. [DOI] [PubMed] [Google Scholar]
- Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, Fullerton DA, Meng X. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg. 2008;85:1678–1685. doi: 10.1016/j.athoracsur.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll—the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27:225–233. doi: 10.1038/sj.onc.1210907. [DOI] [PubMed] [Google Scholar]
- Cohen-Sfady M, Pevsner-Fischer M, Margalit R, Cohen IR. Heat shock protein 60, via MyD88 innate signaling, protects B cells from apoptosis, spontaneous and induced. J Immunol. 2009;183:890–896. doi: 10.4049/jimmunol.0804238. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Kloppenburg G, Kitslaar P, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect. 2006;8:1859–1865. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Farmer BB, Mancina M, Williams ES, Watanabe AM. Isolation of calcium tolerant myocytes from adult rat hearts: review of the literature and description of a method. Life Sci. 1983;33:1–18. doi: 10.1016/0024-3205(83)90706-3. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Ford DA, Rovetto MJ. Rat cardiac myocyte adenosine transport and metabolism. Am J Physiol. 1987;252:H54–H63. doi: 10.1152/ajpheart.1987.252.1.H54. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor {alpha} from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. Cytosolic HSP60, hypoxia and apoptosis. Circulation. 2002;106:2727–2733. doi: 10.1161/01.CIR.0000038112.64503.6E. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFκB signaling. J Mol Cell Cardiol. 2004;36:579–586. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, heat shock proteins, and NF{kappa}B in human vascular endothelium. Arterioscler Thromb Vasc Biol. 2004;24:1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- Hasan NM, Parker PJ, Adams GE. Induction and phosphorylation of protein kinase C-α and mitogen-activated protein kinase by hypoxia and by radiation in Chinese hamster V79 cells. Radiat Res. 1996;145:128–133. doi: 10.2307/3579166. [DOI] [PubMed] [Google Scholar]
- Hua D, et al. Small interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Mol Immunol. 2009;46:2876–2884. doi: 10.1016/j.molimm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Kim SC, et al. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes and apoptosis. Circ Res. 2009;105:1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Koh JM, Lee YS, Kim BJ, Lee SH, Lee KU, Kim GS. Increased circulating heat shock protein 60 induced by menopause, stimulates apoptosis of osteoblast-lineage cells via up-regulation of toll-like receptors. Bone. 2009;45:68–76. doi: 10.1016/j.bone.2009.03.658. [DOI] [PubMed] [Google Scholar]
- Knowlton AA. The role of heat shock proteins in the heart. J Mol Cell Cardiol. 1995;27:121–131. doi: 10.1016/S0022-2828(08)80012-0. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Srivatsa U. Heat-shock protein 60 and cardiovascular disease: a paradoxical role. Futur Cardiol. 2008;4:151–161. doi: 10.2217/14796678.4.2.151. [DOI] [PubMed] [Google Scholar]
- Kobba S, Kim SC, Chen L, Kim E, Tran AL, Knuefermann P, Knowlton AA. The heat shock paradox and cardiac myocytes: role of heat shock factor. Shock. 2011;35:478–484. doi: 10.1097/SHK.0b013e3182094a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AR, Pechenino AS, Dong H, Hammock BD, Knowlton AA. Aging, estrogen loss and epoxyeicosatrienoic acids (EETs) PLoS ONE. 2013;8:e70719. doi: 10.1371/journal.pone.0070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of Toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A. Circulating human heat shock protein 60 in the plasma of British civil servants: relationship to physiological and psychosocial stress. Circulation. 2002;106:196–201. doi: 10.1161/01.CIR.0000021121.26290.2C. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem. 2011;286:31308–31319. doi: 10.1074/jbc.M111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J. 2011;75:2445–2452. doi: 10.1253/circj.CJ-11-0194. [DOI] [PubMed] [Google Scholar]
- Matsuno K, et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic Biol Med. 2012;53:1718–1728. doi: 10.1016/j.freeradbiomed.2012.08.590. [DOI] [PubMed] [Google Scholar]
- Matsushima S, Tsutsui H, Sadoshima J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc Med. 2014;24:202–205. doi: 10.1016/j.tcm.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Chen HJ, Li DY. Protection of myocytes from hypoxia-reoxygenation injury by nitric oxide is mediated by modulation of transforming growth factor-β. Circulation. 2002;105:2206–2211. doi: 10.1161/01.CIR.0000015602.94990.3D. [DOI] [PubMed] [Google Scholar]
- Nakano M, Mann DL, Knowlton AA. Blocking the endogenous increase in HSP72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation. 1997;95:1523–1531. doi: 10.1161/01.CIR.95.6.1523. [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in Toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Fraser I, Han H, Ni Y, O’Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- Simon F, Fernandez R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J Hypertens. 2009;27:1202–1216. doi: 10.1097/HJH.0b013e328329e31c. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells. Chaperoning of the innate and adaptive immune response. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Stice JP, Chen L, Kim SC, Jung JS, Tran AL, Liu TT, Knowlton AA. 17β-Estradiol, aging, inflammation and the stress response in the female heart. Endocrinology. 2011;152:1589–1598. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice JP, Mbai FN, Chen L, Knowlton AA. Rapid activation of nuclear factor-κB by 17β-estradiol and selective estrogen receptor modulators: pathways mediating cellular protection. Shock. 2012;38:128–136. doi: 10.1097/SHK.0b013e31825da754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and selective increase in heat shock proteins by acute dexamethasone treatment. Am J Physiol. 2000;278:H1091–H1096. doi: 10.1152/ajpheart.2000.278.4.H1091. [DOI] [PubMed] [Google Scholar]
- Szajnik M, Szczepanski M, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M, Whiteside TL. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, Knowlton AA, Yuan WJ, Lin L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc Res. 2013;98:391–401. doi: 10.1093/cvr/cvt047. [DOI] [PubMed] [Google Scholar]
- Timmers L, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- Vogel SN, Johnson D, Perera PY, Medvedev A, Lariviere L, Qureshi ST, Malo D. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenser F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.CIR.102.1.14. [DOI] [PubMed] [Google Scholar]
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1579. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ling S, Guo D, Yan Y, Zhou F, Wu Y. Activation of PAR2 or/and TLR4 promotes SW620 cell proliferation and migration via phosphorylation of ERK1/2. Oncol Rep. 2011;25:503–511. doi: 10.3892/or.2010.1077. [DOI] [PubMed] [Google Scholar]