Abstract
Thermal stress has a significant adverse effect on commercial swine production but it is not easy to measure. Animals may adapt to stress conditions by an alteration in the expression of stress-related genes such as heat shock proteins (HSPs) and monocarboxylate transporters (MCTs). The present study presents a comparative analysis of seasonally varied effects on the expression profiles of HSPs (27, 70, and 90) and MCTs (1, 2, and 4) transcripts in thigh muscle and colon tissue of Ghungroo and Large White Yorkshire (LWY) breeds of pig. By real-time polymerase chain reaction, the mRNA expression of HSP27 and HSP90 genes was found to be higher in both thigh muscle and colon tissue in Ghungroo compared to Large White Yorkshire pigs during the summer. However, the relative expression of HSP70 was significantly higher (P < 0.01) in Ghungroo compared to Large White Yorkshire pigs during both seasons in both thigh muscle and colon tissue. The expression of HSP90 was higher in Ghungroo when compared to LWY though the variation was non-significant (P > 0.05) in the colon during different seasons. However, in Ghungroo, the mRNA expression of MCT1 was found to be significantly (P < 0.05) higher in thigh muscle and colon regions during the summer compared to LWY, whereas MCT2 was expressed more in the colon in LWY compared to Ghungroo during the summer. The relative expression of mRNA of MCT4 was found to be significantly (P < 0.05) higher in thigh region in both summer and winter in Ghungroo compared with LWY. Thus, the study demonstrated that both HSPs and MCTs gene expression during thermal stress suggests the possible involvement of these genes in reducing the deleterious effect of thermal stress, thus maintaining cellular integrity and homeostasis in pigs. These genes could be used as suitable markers for the assessment of stress in pigs.
Keywords: Thermal stress, HSP, MCT, Pig, Summer, Winter
Introduction
Thermal stresses trigger a complex program of gene expression and biochemical adaptive responses (Fujita 1999 and Lindquist 1986) in humans as well as in livestock. The reduction of productivity and the devastating economic consequences to the global livestock industry due to both hot and cold environments have been documented previously (St-Pierre et al. 2003; Bernabucci et al. 2010). Among all livestock, pigs are more susceptible to temperature changes, with subsequent effects on commercial swine production. Hot weather adversely affects pig performance, reproduction, feed conversion, and the health and welfare of the animals (Lucas et al. 2000). Cellular responses to stress are of great interest with respect to animal health and production. Biologically, the ability to survive and adapt to thermal stress appears to be a fundamental requirement of cellular life, as cell stress responses are ubiquitous (Sonna et al. 2002). Interestingly, many of the physiological effects of cold exposure that are observed on the cellular level are similar to those seen in heat-stressed cells, including decelerated protein synthesis and cell cycle progression, reduced membrane permeability and cytoskeletal structure changes, as well as protein denaturation (Sonna et al. 2002). However, there are few studies, either in vitro or in vivo, that have focused on low temperatures. In principle, cold should reduce rates of enzymatic reactions, diffusion, and membrane transport, whereas heat would tend to accelerate these processes. Particularly in mammals, exposure to hypothermia or hyperthermia has been related to morphological and physiological modifications.
The highly anaerobic muscles of pigs are easily acidified due to accumulation of lactate and protons. At the cellular level, this acidification of the muscle during stress triggers a stress reaction, which leads to the synthesis of special stress proteins, heat shock proteins (HSPs) (Weitzel et al. 1985). HSPs help other proteins to maintain their conformation and even assist in their repair (Hendrick and Hartl 1993; Kiang and Tsokos 1998). HSPs are essential molecular chaperones in protein assembly and disassembly, protein folding and unfolding, and refolding of damaged proteins (Schmitt et al. 2007). HSPs represent several families of cellular stress response proteins, some of which are expressed constitutively and others expressed largely under conditions of stress. Expression of inducible HSPs has been associated with protection of cells from the adverse effects of various types of stressors. HSPs form a protein family classified according to their molecular weights (Locke 1997; Liu and Steinacker 2001). One of the most abundant and best characterized is the 70-kDa family (HSP70). The Hsp70 family of proteins is highly conserved in all organisms. HSP70 helps cells respond to stress, and its expression is induced by various cellular stresses (Lindquist and Craig 1988). Heat shock protein 90, found in both the cytoplasm and the nucleus, is one of the most abundant proteins in mammalian cells. Mammalian cells also contain several small HSPs, including Hsp25/27. Hsp25 is found in murine cells, whereas its homolog, Hsp27, is found in human cells. The cytoprotective heat shock protein 27 (Hsp27), one of the major inducible chaperones present in intestinal epithelial cells, is known to be regulated by various factors, including diet and gut microbiota and its metabolites (Ren et al. 2001; Arvans et al. 2005).
The oxidative capacity of the domesticated pig is low (Essen-Gustavsson 1986; Karlsson 1993; Ruusunen 1994), and the muscles contain mainly thick fast glycolytic fibers (IIB), which in stressful situations easily switch their energy metabolism to anaerobic glycolysis. Generally, lactate formed in glycolytic (white) muscle fibers is transported to the liver or to oxidative (red) fibers to be used as a fuel. In domestic pigs, however, the transport of lactate out of muscles is limited by poor capillarization (Ruusunen and Puolanne 2004) and its utilization as a fuel is limited by the small number of oxidative fibers (Essen-Gustavsson 1986). Since the mitochondrial density in the white muscles is lower than in red muscles (Beecher et al. 1969), lactate and protons may accumulate in muscles, causing pain in living muscle and, perimortally, quality defects in meat. The muscle fibers protect themselves from acidification by transporting both lactate and protons out of the cell (Poole and Halestrap 1993). In all species studied, efflux of lactate and protons is facilitated mainly by monocarboxylate transporters (MCTs), which co-transport a proton and a lactate anion through the cell membrane (Juel 1998; Halestrap and Price 1999). If lactate and protons are not transported efficiently out of the cells, animals experience stress caused by acidification. The low oxidative capacity, sparse capillarization, and fiber-type distribution of the muscles of the domestic pig render it an interesting animal for studies of MCT isoform composition. In the gastrointestinal tract, MCTs are important in the transport of short-chain fatty acids (SCFAs) and lactate (Koho et al. 2005). SCFAs are weak acids, but because the pH of the gastrointestinal tract, with the exception of the stomach, is nearly neutral, 90–99 % of SCFAs are present as anions rather than as free acids. In pigs, as in other monogastric species, the main site for SCFA production and absorption is the colon (Sepponen 2008). In addition, lactate is mostly absorbed in the small intestine (Argenzio and Southworth 1974). In the gut, the abundance of the MCT protein (colonic luminal membrane) declines during transition from normality to malignancy, which in turn reduces the SCFA required to maintain regulation of normal differentiation and proliferation in the colonic mucosa (Ritzhaupt et al. 1998b). In pigs, this is a normal situation when stressful conditions change the vascular resistance, which results in decreased blood supply in the splanchnic area. This leads easily to ischemia in the gastrointestinal system (Niewold et al. 2000), which has been shown to be associated with intestinal acidosis in pigs (Ljungdahl et al. 1997). Thus, during stress, it is important that MCT proteins function properly to maintain a sufficiently high intracellular pH for HSP production (Sepponen 2008). The MCT family has 14 members (Halestrap 2012), among which MCT1, MCT2, and MCT4 were reported to be present in muscles as well as the intestine of both domestic and wild pigs (Sepponen 2008; Ritzhaupt et al. 1998).
To the best of our knowledge, no studies have been designed so far to gain an insight into the comparative analysis of seasonal variation effects on HSPs and MCT gene expression profile in thigh muscle as well as colon tissue in the Indian native breed Ghungroo and the exotic breed, Large White Yorkshire pigs. Thus, this study was designed to find out the relative expression profile of HSPs (HSP27, HSP70, and HSP90) and MCTs (MCT1, MCT2, and MCT4) in thigh muscles and colon tissue of both breeds during the winter and summer seasons and to discover possible variations between the breeds.
Materials and methods
Animals and sampling
The present study was conducted in 30 animals each from two different breeds of pig, i.e., indigenous native breed Ghungroo and exotic Large White Yorkshire (LWY). The animals were divided equally into three groups and the experiment was carried out during three distinct phases according to three different season of the year, viz, thermo-neutral (mid-January to mid-February), winter (December to January), and summer (mid-April to mid-June). The pigs were raised at a Regional Pig breeding station, Haringhata Farm (West Bengal, India, latitude 22°56′ N and longitude 88°32′ E), until they attained an average weight of 80 ± 10 kg. On the day of slaughter, pigs were transported from the farm to the meat plant located nearby and were allowed to rest 3 h before exsanguination.
Environmental conditions
The data on meteorological variables, i.e., average daily temperature, relative humidity, were recorded by observatory installed at Department of Agricultural Meteorology & Physics, Bidhan Chandra Krishi Viswavidhyalaya (BCKV), Mohanpur (latitude 22°56′ N and longitude 88°32′ E), West Bengal. The samples were collected once a week and the environmental conditions during the experimental days of collection were recorded and considered during data analysis. Temperature Humidity Index (THI) was calculated as per Jonson et al. 1963, i.e.,
where Tdb is dry bulb temperature (°C) and Twb is wet bulb temperature (°C)
Tissue collection and total RNA extraction
Samples from the proximal colon tissue and thigh muscles were taken approximately 1 and 2 h, respectively, after stunning. The tissues were washed with phosphate buffer saline (PBS), snap frozen using liquid nitrogen, and preserved at −80 °C until RNA extraction. Total RNA from tissue was extracted using TRIZOL reagent (Sigma-Aldrich, USA) as per the manufacturer’s instructions. The extracted RNA was dissolved in 30 μl nuclease-free water and the concentration was measured using Nanodrop (Thermo Scientific, USA). The quality and integrity of the extracted RNA was analyzed by 1.5 % agarose gel electrophoresis. Absorbance of the isolated RNA samples were measured at OD 260/OD 280 to check for protein contamination.
Gene expression (HSP and MCT) analysis by quantitative real-time PCR
Reverse transcription
Approximately 1 μg of total RNA was reverse transcribed to cDNA using iScript™ select cDNA synthesis kit (Bio-Rad Laboratory, CA) as per the manufacturer’s instructions. RPS15a was used as the housekeeping gene. Primers were designed for HSP27, HSP70, HSP90, MCT1, MCT2, MCT4, and RPS15a on the basis of prior sequence information available at the National Centre for Biotechnology Information (NCBI) and synthesized by GCC Biotech (India) Pvt. Ltd. The primer sequences and expected amplicon size of the targeted genes are given in Table 1.
Table 1.
Gene transcripts, primer sequence, and resulting fragment size
| Gene | Sequence of nucleotide (5′–3′) | Fragment size (bp) | Annealing temp (°C) | NCBI/reference |
|---|---|---|---|---|
| MCT1 | F: ATGGGCATCAACTACCGACTTC R: CTCTTTGGGGCTTCCTTCTATG |
168 | 55 | EU404088.1 |
| MCT2 | F: CAAGCCTGGTGGTATATGC R: CAAGAAGAACTGGGCAACAC |
153 | 60 | EU650275.1 |
| MCT4 | F: CCCGTGTTCGTGGTGAGCTA R: TGAAGAGGTAGACGGAGTAA |
160 | 58 | EU650276.1 |
| HSP27 | F: AGGAGCGGCAGGATGAG R: GGACAGGGAGGAGGAGAC |
101 | 60 | Kamanga-Sollo et al. (2011) |
| HSP70 | F: GTGGCTCTACCCGCATCCC R: GCACAGCAGCACCATAGGC |
114 | 62 | Kamanga-Sollo et al. (2011) |
| HSP90 | F: CGCTGAGAAAGTGACCGTTATC R: ACCTTTGTTCCACGACCCATAG |
126 | 60 | Kamanga-Sollo et al. (2011) |
| RPS15a | F: AATGGTGCGCATGAATGTC R: GACTTTGGAGCACGGCCTAA |
100 | 60 | XM_005679050.1 |
EMBL accession number or reference of published sequence
Quantitative real-time PCR
Relative expression levels of mRNA transcripts of HSPs (HSP27, HSP70, and HSP90) and MCTs (MCT1, MCT2, and MCT4) genes were measured by quantitative real-time PCR (qPCR) in Strategene real-time qPCR (Mx3005P, Agilent Technology, USA) using SsoFast™ Eva green® qPCR kit (Bio-Rad, USA). A master mix as per the following components was prepared: SsoFast™ Eva Green® super mix—10 μl, forward primer (0.2 mM)—0.5 μl, reverse primer (0.2 mM)—0.5 μl, cDNA sample for each gene—1 μl, and nuclease-free water (NFW) up to 20 μl. RPS15a was used as a housekeeping gene for this experiment. The thermal profile was an initial 30-s denaturation step at 95 °C followed by 40 cycles including denaturation at 95 °C for 5 s, gene-specific annealing temperature (Table 1) for 10–12 s and elongation at 72 °C for 10 s followed by a final elongation step at 72 °C for 10 min and last cycle at 95 °C for 1 min with a gradual increase from 60 to 65 °C for 10 s to 95 °C for 15 and 30 s at 0.58 °C per second and continuous fluorescence measurement, and a final cooling down to 40 °C. After the end of the run, a cycle threshold (Ct) values and amplification plot for all determined factors were acquired. Real-time PCR efficiencies were determined by amplification of a standardized dilution series and slopes were obtained. The specificity of the desired products was documented using an analysis of the melting temperature, which was product-specific. The amplified PCR products were resolved in 1.5 % electrophoresis through agarose gel containing Lab safe Nucleic acid stain (G Biosciences, USA) and visualized in gel documentation system (GELDOC, USA). The relative expression of PCR products was determined as per the method of Pfaffl (2001).
Statistical analysis
The statistical significance of differences in mRNA expression of the genes during different season in respective breeds in different tissues was assessed by two-way ANOVA using GraphPad Prism 4.03 software whereas the relative expression values of mRNA for different genes between breeds during a particular season in a particular tissue were carried out by unpaired t test. A difference with value P < 0.05 was considered statistically significant.
Results
Climatic conditions
The average environmental parameters recorded during the experimental periods are presented in Table 2. THI during thermo-neutral seasons was 70, whereas it was decreased to 63 during the winter and increased to 80 during the summer.
Table 2.
Climatic conditions during experimental days at Haringhata Farm, Mohanpur, West Bengal, India
| Season | Average temperature (°C) | Relative humidity (%) | THI |
|---|---|---|---|
| Thermo-neutral | 22 | 78 | 70 |
| Winter | 16 | 81 | 63 |
| Summer | 34 | 75 | 82 |
Thermo-neutral (mid-January to mid-March), winter (mid-November to mid-January), and summer (mid-April to mid-June)
RNA integrity and purity
Two intact bands of 28 s and 18 s indicated good quality and intactness of isolated RNA. The concentrations of RNA samples were in the range of 200–2000 ng/μl with an absorbance ratio (OD 260/OD 280) of more than 1.8, which indicated that isolated RNA samples were free from protein contamination.
Gene expression analysis
Efficiency-corrected relative quantification of mRNA was obtained by Pfaffl method (2001). The relative expression of mRNA for HSPs (HSP27, HSP70, and HSP90) as well as MCTs (MCT1, MCT2, MCT4) of Ghungroo and LWY during peak winter and summer months is presented in Table 3 and Fig. 1. The thermo-neutral season values were used as calibrator.
Table 3.
Relative expression values of mRNA for different genes
| Genes | Location | Summer | Winter | ||
|---|---|---|---|---|---|
| Ghungroo | Large White Yorkshire | Ghungroo | Large White Yorkshire | ||
| MCT1 | Thigh | (5.97 ± 0.54)x | (5.20 ± 0.67)x | (3.80 ± 0.37)y | (1.66 ± 0.30)x |
| Colon | (4.65 ± 0.48)y | (3.04 ± 0.59)x | (0.43 ± 0.10)x | (0.47 ± 0.27)x | |
| MCT2 | Thigh | (4.46 ± 0.49)x | (4.15 ± 0.75)x | (3.99 ± 0.57)x | (3.58 ± 0.64)x |
| Colon | (0.62 ± 0.07)x | (1.93 ± 0.19)y | (0.15 ± 0.09)x | (0.28 ± 0.12)x | |
| MCT4 | Thigh | (4.62 ± 0.81)y | (2.03 ± 0.26)x | (3.58 ± 0.66)y | (1.29 ± 0.24)x |
| Colon | (0.46 ± 0.19)x | (0.43 ± 0.23)x | (0.36 ± 0.17)x | (0.68 ± 0.24)x | |
| HSP27 | Thigh | (7.87 ± 1.16)y | (2.28 ± 0.32)x | (1.3 ± 0.13)y | (0.5 ± 0.17)x |
| Colon | (2.19 ± 0.83)x | (1.17 ± 0.54)x | (3.16 ± 1.67)x | (1.18 ± 0.08)x | |
| HSP70 | Thigh | (4.22 ± 0.73)y | (2.50 ± 0.11)x | (2.23 ± 0.79)x | (0.80 ± 0.18)x |
| Colon | (5.72 ± 0.35)y | (2.31 ± 0.11)x | (1.08 ± 0.12)y | (0.39 ± 0.15)x | |
| HSP90 | Thigh | (2.74 ± 0.62)x | (1.91 ± 0.40)x | (0.22 ± 0.05)x | (0.42 ± 0.15)x |
| Colon | (1.42 ± 0.40)x | (0.99 ± 0.34)x | (0.44 ± 0.06)x | (0.34 ± 0.12)x | |
Values bearing different letters (x, y) vary significantly (P < 0.05) between breeds during a particular season
MCTs monocarboxylate transporters, HSPs heat shock proteins
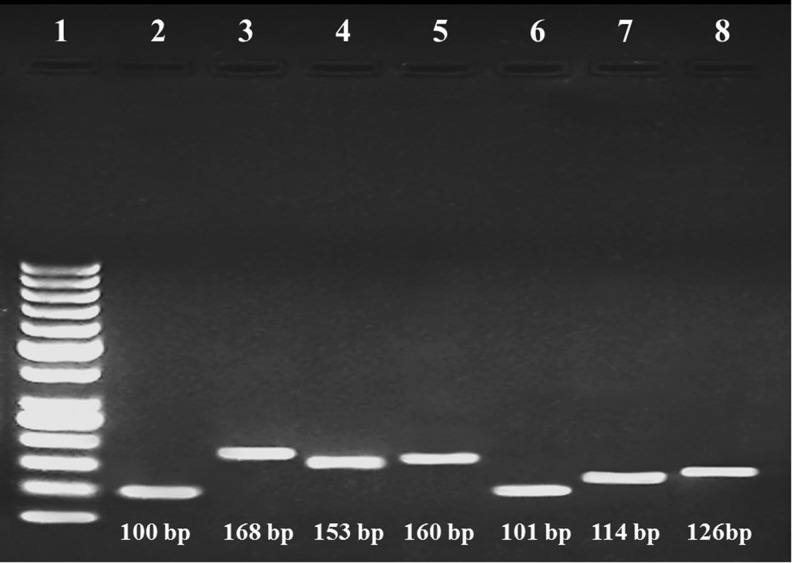
Fig. 1.
Two percent agarose gel electrophoresis showing the expression of HSPs and MCTs in pig muscle and colon tissue by real-time qPCR. Lane 1, 50 bp ladder; lane 2, RPS15a as internal control; lane 3, MCT1; lane 4, MCT2; lane 5, MCT4; lane 6, HSP27; lane 7, HSP70; lane 8, HSP90
Expression of mRNA of HSP27
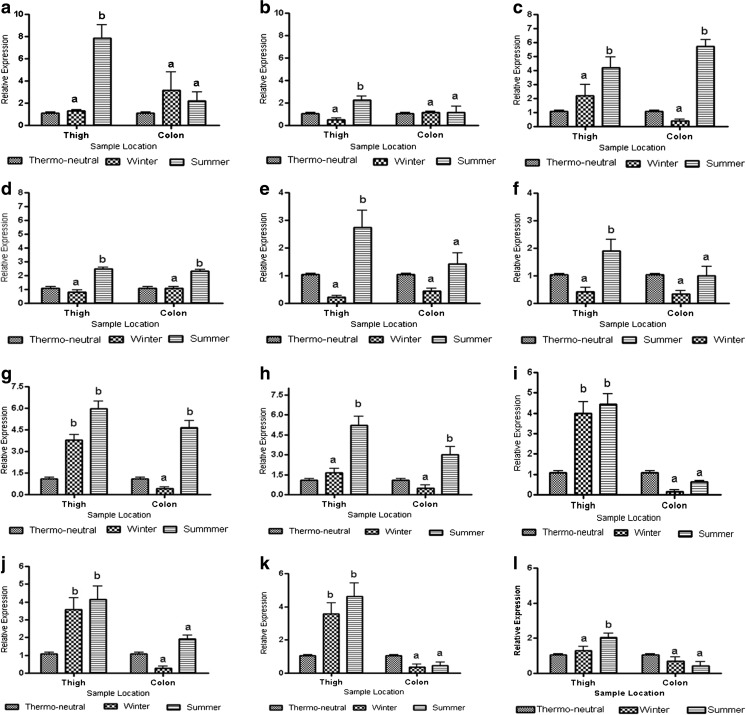
During the summer season, the relative expression of mRNA of HSP27 in thigh region was found to be increased significantly (P < 0.05) compared to that in the winter season in both Ghungroo (Fig. 2a) and LWY (Fig. 2b). However, a comparatively higher expression of this gene was found in both thigh muscle and colon tissue in Ghungroo compared to LWY during the summer season (Table 3), although the variation in colon-derived tissue was non-significant (P > 0.05).
Fig. 2.
a–l Relative expression profile of MCTs and HSPs mRNA during different seasons (a HSP27; c HSP70; e HSP90; g MCT1; i MCT2; k MCT4) in Ghungroo and (b HSP27; d HSP70; f HSP90; h MCT1; j MCT2; l MCT4) in Large White Yorkshire breed of pig by quantitative polymerase chain reaction (qPCR). Different superscripts denote statistically different values (P < 0.05) in comparison to thermo-neutral season
Expression of mRNA of HSP70
In both Ghungroo (Fig. 2c) and LWY (Fig. 2d), the HSP70 mRNA expression was found to be increased significantly (P < 0.05) during the summer season in thigh as well as in colon region. However, the expression value was significantly higher (P < 0.01) in Ghungroo compared to LWY during both seasons (Table 3) in both thigh muscle and colon tissue.
Expression of mRNA of HSP90
In Ghungroo (Fig. 2e) and LWY (Fig. 2f) breeds, the mRNA expression for HSP90 during summer seasons was found to be higher in thigh region compared to that in the winter season, but the mRNA expression in the colon region during different seasons was found to be non-significant (P > 0.05). Although the variation in expression was non-significant (P > 0.05) between the breeds during different seasons, the expression was higher in Ghungroo when compared to LWY (Table 3).
Expression of mRNA of MCT1
In both the breeds, the relative expression of MCT1 mRNA was found to have increased significantly (P < 0.05) during the summer seasons in both thigh muscle and colon region (Fig. 2g, h). However, in Ghungroo, the mRNA expression value was found to be significantly (P < 0.05) higher in thigh muscle during the winter and in the colon region during the summer compared to LWY (Table 3). The mRNA expression of MCT1 remained almost stable in colon tissue during the winter and in thigh muscle during the summer season when compared between the breeds.
Expression of mRNA of MCT2
In both Ghungroo (Fig. 2i) and LWY (Fig. 2j), the MCT2 mRNA expression was significantly increased (P < 0.05) in colon tissue whereas it remained almost stable in thigh muscles during the summer. When the relative expression was compared between breeds, this gene was found to be expressed more in the colon in LWY compared to Ghungroo during the summer season (Table 3).
Expression of mRNA of MCT4
The relative expression of mRNA for MCT4 of Ghungroo and LWY during winter and summer are presented in Table 3 and Fig. 2k, l, respectively. In Ghungroo, the relative expression of mRNA of MCT4 was found to be significantly (P < 0.05) higher in thigh region in both summer and winter compared to LWY. Moreover, the expression value of MCT4 in colon tissue remained stable during the summer in both the breeds, whereas it was two times more expressed in colon tissue of LWY compared to Ghungroo.
Discussion
To the best of our knowledge, no study has so far given an insight into the comparative analysis of seasonal variation in the relative expression of HSPs and MCTs gene between Ghungroo and Large White Yorkshire breed of pigs. Thermal stress triggers a complex cellular response including altering gene expression (Sonna et al. 2002; Kultz 2005). To gain insight into the molecular mechanisms by which the animal body reacts to thermal stress and exerts its complex biological effects, we performed relative mRNA expression analysis of HSPs (HSP27, HSP70, and HSP90) and MCTs (MCT1, MCT2, and MCT4) in thigh muscle and colon tissue of both breeds during thermo-neutral, summer, and winter seasons.
In the present investigation, the environmental temperature during the thermo-neutral season, winter, and summer was 22, 16, and 34 °C, respectively. An environmental temperature range of 18–21 °C has generally been found to support optimal productive performance of pigs bred for production (Huynh et al. 2005). During the summer and winter, animals were exposed to ambient temperature beyond their comfort zone, putting them under heat and cold stress. Our THI showed that a THI above 80 is stressful to pigs as reported previously (Pourouchottamane et al. 2013).
HSPs
HSPs are the major components of molecular chaperones that facilitate the folding of newly synthesized proteins, prevent the aggregation of misfolded proteins, and promote their refolding in different cellular compartments such as the cytosol or endoplasmic reticulum (Mohamed 2012). Stressors are broadly classified into environmental, pathological, and physiological, which induce the up-regulation of HSP gene expression in a stressed cell which is called as stress response (Lindquist and Craig 1988).
HSP27
HSP27 is the smallest cytosolic protein that is induced by heat shock (Rylander et al. 2005; Rembold and Zhang 2001). We observed that during the summer, the relative expression of mRNA of HSP27 in the thigh region was found to be increased significantly (P < 0.05) compared to the thermo-neutral and winter seasons in Ghungroo and LWY, whereas its mRNA expression value was higher during the summer in both thigh and colon tissue in Ghungroo compared to LWY. HSP27 has been reported to be triggered by a variety of physiologically stressful stimuli such as elevated temperature, ischemia, and oxidative stress (Rylander et al. 2005). In our study, increased temperatures during the summer triggers HSP27 expression in active thigh muscles. In Ghungroo, the increased HSP27 expression in the colon during winter may be due to the fact that HSP27 was reported to play a major role in protecting the intestinal epithelial cell from damage by up-regulating its expression (Liu et al. 2012). The variation in HSP expression due to breed difference was also reported in other species (Dangi et al. 2012; Banerjee et al. 2014) and HSPs expression also acts as a potential indicator of animal adaptation to harsh environmental stress (Valenzuela 2000; Hansen 2004).
HSP70
HSP70 is found in cellular compartments such as the cytoplasm, nucleus, endoplasmic reticulum, and mitochondria (Rylander et al. 2005). Its major functions are protein folding, cytoprotection, and as molecular chaperones (Dangi et al. 2012). In the present study, we observed that HSP70 mRNA expression was found to be significantly higher (P < 0.05) during the summer in both breeds in comparison with the winter season. Our findings are in accordance with previous studies in which heat stress-induced HSP70 expression was observed in the goat PBMCs (Banerjee et al. 2014; Dangi et al. 2012) and bovine lymphocytes (Lacetera et al. 2006; Patir and Upadhyay 2007, 2010). During the winter, HSP70 expression was approximately three times higher in Ghungroo compared to LWY. It is not surprising to see the HSP70 up-regulation in response to cold in Ghungroo as the denaturation of proteins is a general phenomenon when cells are exposed to cold (Kostal and Tollarova-Borovanska 2009; Tsai et al. 2002), and in turn, partially denatured or misfolded proteins are potent triggers of rapid HSP70 accumulation (Feder and Hofmann 1999; Welch 1993).
HSP90
HSP90 is mostly found in the cytoplasm, endoplasmic reticulum, and nucleus (Kregel 2002). The major functions are protein translocation and regulation of steroid hormone receptors (Dangi et al. 2012). Our present study found that the mRNA expression of HSP90 in both the breeds during the summer season was significantly higher (P < 0.05) than the corresponding values in thigh muscle during the winter season. Our findings are in accordance with previous studies in which heat stress-induced HSP90 expression was observed in the goat PBMCs (Dangi et al. 2012), in human blood lymphocytes (Schimidt and Abdulla 1988), and in lung, heart, spleen, liver, and brain of human (Sareh et al. 2011). The increased expression during the summer season could provoke its transcription to protect cells from the damaging effects of heat stress such as the denaturation of proteins, help in protein refolding, and prevent aggregation of denatured proteins (Dangi et al. 2012).
MCTs
The important anatomical and physiological constraints of pigs are small heart size, lower plasma and blood volume to their body weight ratio (Yang and Lin 1997), poor blood circulation with sparse capillarization (Ruusunen and Puolanne 2004), and higher percentage of white (type IIB fibers or fast glycolytic) fibers (Ruusunen and Puolanne 2004). Due to the above properties, even moderate physical activity derives its energy by anaerobic metabolism (Essen-Gustavsson 1986) whose end product is lactic acid (Nelson and Cox 2005). Lactic acid and other monocarboxylate compounds such as pyruvate, oxo acids, SCFA, etc. almost dissociates to its respective anion at physiological pH (Poole and Halestrap 1993). These anions cannot cross the plasma membrane by free diffusion and require a specific transport mechanism called MCTs (Halestrap and Price 1999).
MCT1
In both breeds, the relative expression of MCT1 mRNA was found to be increased significantly (P < 0.05) during the summer season in both thigh muscles and colon regions. MCT1 has been thought to transport lactate into the muscle fibers for oxidation (McCullagh et al. 1996; Halestrap and Price 1999; Juel and Halestrap 1999). Our finding was in contrast to findings of Sepponen (2008) where MCT1 was almost undetectable or insignificant in all the five muscles he studied. The mRNA expression of MCT1 remains almost stable in colon tissue during the winter season. This might be due to relatively lower concentrations of lactate production in the intestine during the winter.
MCT2
In our study, a significant (P < 0.05) increase in MCT2 mRNA expression in thigh region in both Ghungroo and LWY was found during the summer season. MCT2 has a very low Km for lactate (Lin et al. 1998; Broer et al. 1999), and therefore, it is tempting to speculate that this isoform is needed for pH regulation in tissues that rely on glycolysis in their energy production, thus producing small amounts of lactate continuously (Sepponen 2008). Therefore, one may expect that a pig, which has a high percentage of type IIB muscle fibers that easily produce lactic acid, regulates the acidification of its muscles through the action of MCT2 (Sepponen 2008). But when relative expression was compared between breeds, this gene was found to be expressed more in colon in LWY than in Ghungroo during the summer. The reason might be that there is more production of lactate in colon of LWY compared to Ghungroo.
MCT4
In Ghungroo, the relative expression of mRNA of MCT4 was found to be significantly (P < 0.05) higher in the thigh region in both summer and winter seasons but significantly (P < 0.05) higher only during the summer season in LWY. It has been speculated that at high lactate concentrations MCT4 is the principal MCT isoform to transport lactate. The higher expression of MCT4 in thigh muscles in this study was the same as that reported previously (Sepponen 2008) as MCT4 may function in the efflux of lactate during stressful situations, when the production of lactate in the muscle is high. In the colon region, however, the expression of MCT4 was increased during the summer but the variation was non-significant between the breeds. The relatively low level of expression of MCT4 in colon needs further study to understand the exact isoforms collectively expressed during thermal stress.
This study concluded that the mRNA expression pattern for HSP and MCT genes is breed-specific, most likely due to variations in thermal tolerance and adaptation to different climatic conditions. Ghungroo pigs, a breed native to India, expressed higher levels of HSPs and MCTs transcripts indicating their more adaptive capacity towards thermal stress in comparison to the exotic breed Large White Yorkshire. The exact co-relation between the expression of MCTs and HSPs gene in a specific breed needs further study. Additionally, it would be of interest to undertake future studies with regard to protein profiling of these two genes.
Acknowledgments
Financial help from the Department of Biotechnology, Government of India, is duly acknowledged. The authors are thankful to the Director, Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India, and to the Vice-Chancellor, WBUAFS, Kolkata, India, for providing the necessary infrastructure facility for conducting this research work
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- Argenzio RA, Southworth M. Sites of organic acid production and absorption in the gastrointestinal tract of the pig. Am J Physiol. 1974;228:454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- Arvans DL, Vavricka SR, Ren HY, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 2005;288:G696–G704. doi: 10.1152/ajpgi.00206.2004. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh, JaganMohanarao G, et al. Seasonal variation in expression pattern of genes under HSP70. Cell Stress Chaperones. 2014;19:401–408. doi: 10.1007/s12192-013-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher GR, Kastenschmidt LL, Hoeksta WG, Cassens RG, Briskey EJ. Energy metabolites in red and white striated muscles of the pig. J Agric Food Chem. 1969;17:29–33. doi: 10.1021/jf60161a009. [DOI] [Google Scholar]
- Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A. Metabolic and hormonal adaptations to heat stress in domesticated ruminants. Animal. 2010;4:1167–1183. doi: 10.1017/S175173111000090X. [DOI] [PubMed] [Google Scholar]
- Broer S, Broer A, Schneider HP, Stegan C, Halestrap AP, Deitmer JW (1999) Characterisation of the high affinity monocarboxylate transporter MCT2 in Xenopus leavis oocytes. Biochem J 341:529–535 [DOI] [PMC free article] [PubMed]
- Dangi SS, Gupta M, Maurya D, Yadv VP, Panda RP, Singh G, Mohan NH, et al. Expression profile of HSP genes during different seasons in goats (Capra hircus) Trop Anim Health Prod. 2012;44:1905–1912. doi: 10.1007/s11250-012-0155-8. [DOI] [PubMed] [Google Scholar]
- Essen-Gustavsson B. Activity and inactivity-related muscle adaption in the animal kingdom. In: Saltin B, editor. Biochemistry of exercise VI. Campaigne: Human Kinetic Publishers; 1986. pp. 435–444. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;6:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fujita J. Cold shock response in mammalian cells. J Mol Microb Biotechnol. 1999;1:243–255. [PubMed] [Google Scholar]
- Halestrap AP. The monocarboxylate transporter family—structure and functional characterization. IUBMB Life. 2012;64(1):1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. doi: 10.1042/0264-6021:3430281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PJ. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci. 2004;82:349–360. doi: 10.1016/j.anireprosci.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Huynh TTT, Aarnink AJA, Verstegen MWA, et al. Effects of increasing temperature on physiological changes in pigs at different relative humidities. J Anim Sci. 2005;83:385–1396. doi: 10.2527/2005.8361385x. [DOI] [PubMed] [Google Scholar]
- Juel C. Muscle pH regulation: role of training. Acta Physiol Scand. 1998;162:359–366. doi: 10.1046/j.1365-201X.1998.0305f.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle-role and regulation of the monocarboxylate transporter. J Physiol. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanga-Sollo E, Pampusch MS, White ME, Hathaway MR, Dayton WR. Effects of heat stress on proliferation, protein turnover, and abundance of heat shock protein messenger ribonucleic acid in cultured porcine muscle satellite cells. J Anim Sci. 2011;89:3473–3480. doi: 10.2527/jas.2011-4123. [DOI] [PubMed] [Google Scholar]
- Karlsson A (1993) Porcine muscle fibres—biochemical and histochemical properties in relation to meat quality. Dissertation. Swedish University of Agricultural Sciences
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/S0163-7258(98)00028-X. [DOI] [PubMed] [Google Scholar]
- Koho N, Maijala V, Norberg H, NieminenM PAR. Expression of MCT1, MCT2 and MCT4 in the rumen, small intestine and liver of reindeer (Rangifer tarandus tarandus L.) Comp Biochem Physiol A. 2005;141:29–34. doi: 10.1016/j.cbpb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kostal V, Tollarova-Borovanska M. The 70 kDa heat shock protein assists during the repair of chilling injury in the insect, Pyrrhocoris apterus. PLoS ONE. 2009 doi: 10.1371/journal.pone.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Molecular biology of thermoregulation. Invited review: heat shock proteins modifying factors in physiological stress responses and acquired thermo tolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Lacetera N, Bernabucci U, Scalia D, Basirico L, Morera P, Nardone A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown-Swiss and Holstein cows. J Dairy Sci. 2006;89:4606–4612. doi: 10.3168/jds.S0022-0302(06)72510-3. [DOI] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RSK, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci. 2001;6:D12–D25. doi: 10.2741/Liu. [DOI] [PubMed] [Google Scholar]
- Liu HY, Lundh T, Dicksved J, Lindberg JE. Expression of heat shock protein 27 in gut tissue of growing pigs fed diets without and with inclusion of chicory fiber. J Anim Sci. 2012;90:25–27. doi: 10.2527/jas.53724. [DOI] [PubMed] [Google Scholar]
- Ljungdahl M, Rasmussen I, Raab Y, Hillered L, Haglund U. Small intestinal mucosal pH and lactate production during experimental ischemia-reperfusion and fecal peritonitis in pigs. Shock. 1997;2:131–138. doi: 10.1097/00024382-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–136. doi: 10.1249/00003677-199700250-00007. [DOI] [PubMed] [Google Scholar]
- Lucas EM, Randall JM, Meneses JF. Potential for evaporative cooling during heat stress periods in pig production in Portugal (Alentejo) J Agric Eng Res. 2000;76:363–371. doi: 10.1006/jaer.2000.0550. [DOI] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O’Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol Endocrinol Metab. 1996;271:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- Mohamed BA. Targeted disruption of Hspa4 gene leads to cardiac hypertrophy and fibrosis. J Mol Cell. 2012;53:459–568. doi: 10.1016/j.yjmcc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM (2005) Lehninger principles of biochemistry. In: Glycolysis, gluconeogenesis, and the pentose phosphate pathway, 5th edn, W. H. Freeman and Company, New York, pp 738
- Niewold TA, van Essen GJ, Nabuurs MJA, Stockhofezurwieden N, van der Meulen J. A review of porcine pathophysiology: a different approach to disease. Vet Q. 2000;22:209–212. doi: 10.1080/01652176.2000.9695060. [DOI] [PubMed] [Google Scholar]
- Patir H, Upadhyay RC. Interrelationship between heat shock protein 70 (HSP70) and lymphocyte proliferation in thermal exposed buffalo heifers. Ital J Anim Sci. 2007;6:1344–1346. [Google Scholar]
- Patir H, Upadhyay RC. Purification, characterization and expression kinetics of heat shock protein 70 from Bubalus bubalis. Res Vet Sci. 2010;88:258–262. doi: 10.1016/j.rvsc.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acid Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across the mammalian plasma membranes. Am J Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Pourouchottamane R, Pankaj PK, Banik S, Naskar S, Venkatsubramanian V, Ramana DBV. Effect of micro-environmental variations on biomolecular profile and performance of pig. J Agrometerol. 2013;15:1–6. [Google Scholar]
- Rembold CM, Zhang E. Localisation of heat shock protein 20 in swine carotid artery. BMC Physiol. 2001 doi: 10.1186/1472-6793-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HY, Musch MW, Kojima K, Boone D, Ma A, Chang EB. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–639. doi: 10.1053/gast.2001.27028. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol. 1998;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusunen M (1994) Muscle histochemical properties of different pig breeds in relation to meat quality. Dissertation. University of Helsinki
- Ruusunen M, Puolanne E. Histochemical properties of fiber types in muscles of wild and domestic pigs and the effects of growth rate on muscle fiber properties. Meat Sci. 2004;67:533–539. doi: 10.1016/j.meatsci.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Rylander MN, Feng Y, Bass J, Diller K. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann N Y Acad Sci. 2005;1066:222–242. doi: 10.1196/annals.1363.009. [DOI] [PubMed] [Google Scholar]
- Sareh H, Tulaurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones. 2011;16:297–307. doi: 10.1007/s12192-010-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimidt JA, Abdulla E. Down regulation of IL-1 biosynthesis by inducers of heat shock response. J Immunol. 1988;141:2027–2034. [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Sepponen K (2008) Monocarboxylate transporters and heat shock proteins in domestic pigs in relation to stress and meat quality. Academic dissertation. University of Helsinki, Finland
- Sonna LA, Fujita J, Gaffin SL, Lilly CM. Invited review: effects of heat and cold stress on mammalian gene expressions. J Appl Physiol. 2002;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci. 2003;86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5. [DOI] [Google Scholar]
- Tsai CJ, Maizel JV, Nussinov R. The hydrophobic effect a new insight from cold denaturation and two-state water structure. Crit Rev Biochem Mol Biol. 2002;37:55–69. doi: 10.1080/10409230290771456. [DOI] [PubMed] [Google Scholar]
- Valenzuela RB (2000). Genotype differences in heat shock protein (Hsp70) expression in bovine lymphocytes exposed to temperature treatments. J Animal Sci. 78:151
- Weitzel G, Pilatus U, Rensing L. Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp Cell Res. 1985;159:252–256. doi: 10.1016/S0014-4827(85)80054-9. [DOI] [PubMed] [Google Scholar]
- Welch WJ. How cells respond to stress. Sci Am. 1993;1993:34–41. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- Yang TS, Lin JH. Variation of heart size and its correlation with growth performance and vascular space in domestic pigs. J Anim Sci. 1997;64:523–528. doi: 10.1017/S1357729800016155. [DOI] [Google Scholar]