Abstract
A Primer on the reptile brain, in particular the light it sheds on the structural and functional evolution of vertebrate neural circuits.
Main Text
“Deep inside the skull of every one of us there is something like a brain of a crocodile. Surrounding the R-complex is the limbic system or mammalian brain, which evolved tens of millions of years ago in ancestors who were mammal but not yet primates. It is a major source of our moods and emotions, of our concern and care for the young. And finally, on the outside, living in uneasy truce with the more primitive brains beneath, is the cerebral cortex; civilization is a product of the cerebral cortex.”
— Carl Sagan, Cosmos p.276–277
Carl Sagan’s amusing words of wisdom notwithstanding — is the H-bomb not also a product of the cerebral cortex? — is the reptilian brain really just a mammalian brain missing most of the parts? Some 320 million years ago, the evolution of a protective membrane surrounding the embryo, the amnion, enabled vertebrates to develop outside of water and thus to invade new terrestrial niches. These amniotes were the ancestors of today’s mammals and sauropsids (reptiles and birds). Present-day reptiles are a diverse group of more than 10,000 species that comprise sphenodons (‘Tuatara’), lizards, snakes, turtles and crocodilians. Although turtles were once thought to be the most ‘primitive’ among reptiles, current genomic data point toward two major groupings: the Squamata (lizards and snakes); and a group comprising both the turtles and the Archosauria (dinosaurs, modern birds and crocodilians) (Figure 1). Dinosaurs inhabited the Earth from the Triassic (230 million years ago), at a time when the entire landmass formed a single Pangaea. They flourished from the beginning of the Jurassic to the mass extinction at the end of the Cretaceous (65 million years ago), and birds are their only survivors.
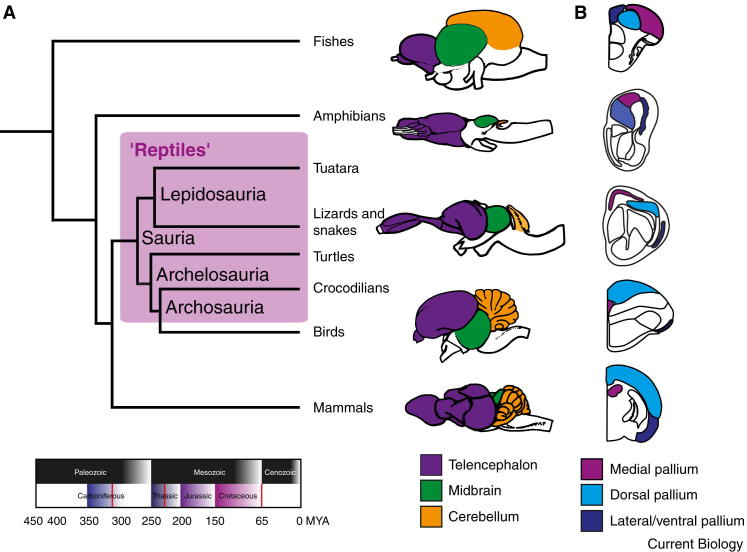
Figure 1.
Vertebrate phylogeny, gross brain morphology, and homologous regions in the forebrain.
(A) Phylogenetic tree of vertebrates and timeline of major events in amniote evolution. Shaded area in the phylogenetic tree indicates ‘reptiles’ as defined in the text. Red lines (lower panel) indicate the origin of amniotes (320 million years ago), the origin of dinosaurs (230 million years ago) and their extinction (65 million years ago). Right panel: schematic drawings showing brains (lateral view, anterior left) of vertebrate representatives: from top to bottom, a fish (knife-fish), an amphibian (tiger salamander), a reptile (monitor lizard), a bird (pigeon) and a mammal (hedgehog tenrec). Major subdivisions (examples in color) are present in all species but appear in different proportions. Adapted from Nieuwenhuys et al. (1998), with kind permission from Springer Science and Business Media. (B) Transverse section of the right hemisphere of vertebrate representatives: from top to bottom, a fish (zebrafish), an amphibian (frog), a reptile (lizard), a bird (pigeon) and a mammal (rat). Colors represent conserved pallial subdivisions. Adapted from Bruce and Neary (1995), Bruce (2007) and Mueller et al. (2011).
What people generally call reptiles is thus a group defined in part by exclusion: it gathers amniote species that are neither mammals nor birds (Figure 1), making the reptiles technically a paraphyletic grouping. Despite this technical point, the so-defined reptiles share many evolutionary, anatomical, developmental, physiological (for example, ectothermia), and functional features. It is thus reasonable to talk about a ‘reptilian brain’ — the subject of this Primer.
Structure and evolution of the reptilian brain
The diversity of reptiles and their evolutionary relationship to mammals make reptilian brains great models to explore questions related to the structural and functional evolution of vertebrate neural circuits. To this end, comparative studies seek to identify homologies — structural or molecular similarities that are due to common ancestry — at a variety of levels, for example, brain regions, circuits or cell types. Homologies can be inferred from extant species by using a comparative approach within a phylogenetic framework. Vertebrate brains have been classically compared in terms of morphology, connectivity, and neurochemistry; however, adult neuroanatomy may not be sufficient to determine homologies without ambiguity.
Vertebrates share among themselves the highest morphological resemblance not as adults, but at their so-called ‘phylotypic stage’ of embryonic development, a resemblance mirrored by similarity across transcriptomes. Identification of conserved brain subdivisions, established by conserved signaling centers and uniquely defined by the combinatorial expression of transcription factors during development, demonstrates that all of the general brain regions found in mammals, including the cerebral cortex, have homologies in reptiles. The cerebral cortex is part of the pallium, a developmental subdivision of the telencephalon delineated by the expression of transcription factors such as Pax6, Emx1 and Tbr1, which is conserved in all vertebrates (Figure 2). Gene expression data show that the same fundamental subdivisions of the pallium — lateral, ventral, medial and dorsal, the latter giving rise to the neocortex in mammals — occur in developing vertebrates, despite the divergent morphologies of pallial structures in adults (Figure 1B). The cerebral cortex is thus not a mammalian invention, but rather an ancient pallial structure that predates the split between sauropsids and theropsids, the mammals’ precursors.
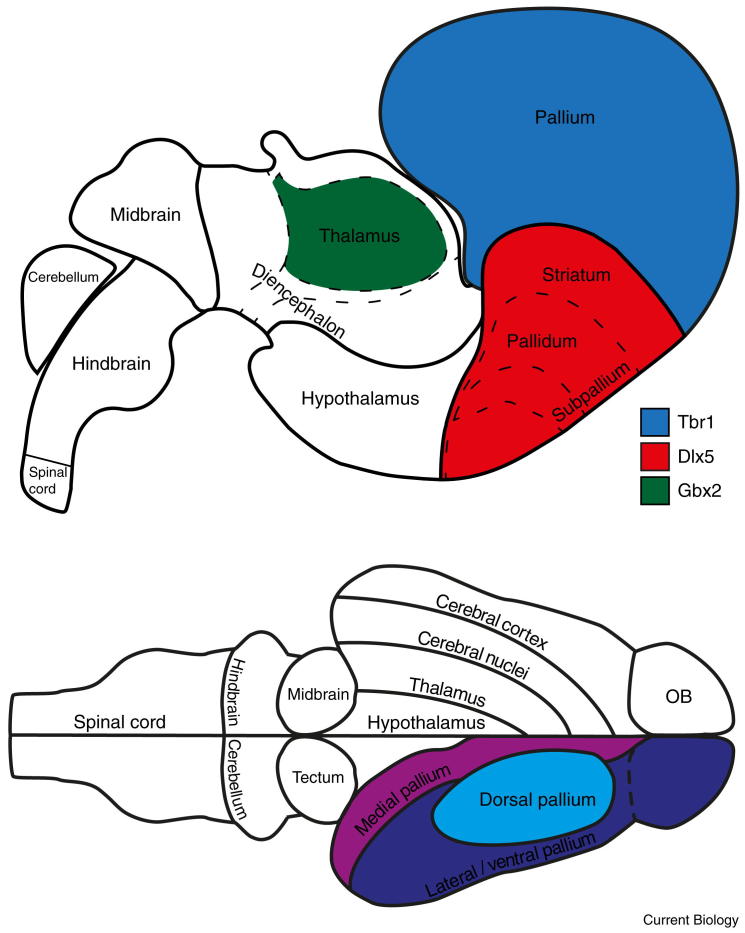
Figure 2.
Developmental and adult bauplan of the vertebrate brain.
Upper panel: simplified developmental scheme of vertebrate brain regions. Combinatorial expression of transcription factors such as Tbr1, Dlx5, and Gbx2 defines brain regions during development and sets the stage for further differentiation in adults. Note that Tbr1 and Dlx5 delineate further regions not shown here. Lower panel: schematic illustration of the adult turtle brain showing major subdivisions present in all vertebrates. Lateral and medial pallium express different sets of molecular markers during development. Which regions of the adult brain correspond to lateral and medial pallium is an active topic of research and thus we combine both pallial subdivisions into a single region. Adapted from Puelles et al. (2013) and Swanson (2011) by permission of Oxford University Press, USA.
Comparative studies of brain structure and development have revealed a general Bauplan (Figure 2), an outline of the fundamental large-scale architecture of the vertebrate brain, reflecting its basic functional organization. The telencephalon integrates and stores multimodal information, and is also the higher center of action selection and motor control (basal ganglia). The hypothalamus is a conserved area controlling homeostasis and behaviors essential for survival, such as feeding and reproduction. In all vertebrates, behavioral states are controlled by common brainstem neuromodulatory circuits, such as the serotoninergic system. Finally, vertebrates harbor a diverse set of sense organs, and their brains share pathways for processing incoming sensory inputs. For example, in all vertebrates, visual information from the retina is relayed and processed to the pallium through the tectum and the thalamus, while olfactory input from the nose first reaches the olfactory bulb and then the pallium.
Although pallial structures exist in amphibians and fish, reptiles and mammals are the only vertebrates to have a cerebral cortex with a clear, though simple, three-layered structure, similar to that of mammalian allocortex. The reptilian ventral pallium also gives rise to the dorsal ventricular ridge, a structure that dominates the bird pallium and contributes to the complex cognitive abilities of birds, but whose mammalian equivalent is still the subject of debate among comparative anatomists. The reptilian cortex contains far fewer subdivisions than that of rodents, carnivores, or primates: it is subdivided into a medial cortex, often called hippocampus by anatomists; a lateral cortex, equivalent to the mammalian piriform cortex; and a dorsal cortex in between, which receives multimodal inputs (for example, visual inputs in turtles). There is little evidence for motor and somatosensory areas in the reptilian cortex, but pallial motor control may have evolved early in vertebrate evolution. Owing to this simplicity (Figure 3), the reptilian brain facilitates the study of primordial cortical function as a whole, and points to the origins of cortex as fulfilling general associative functions.
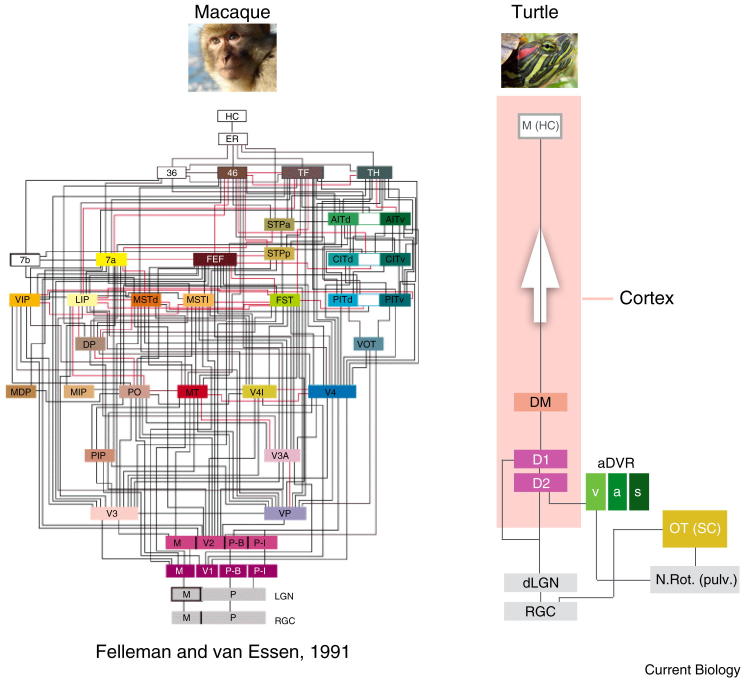
Figure 3.
Visual circuits in monkey and turtle.
Comparison of known visual cortical circuits between macaque (left) and freshwater turtle (right), emphasizing the great simplicity of reptilian cortex. Arrow indicates direction of information flow from sensory periphery. aDVR, anterior dorsal ventricular ridge (a: auditory, s: somatosensory, v: visual); dLGN, dorsal lateral geniculate nucleus; D1 and D2, dorsal cortex areas D1 and D2; DM, dorsomedial cortex; M (HC), medial cortex (‘hippocampus’); N.Rot., nucleus rotundus (‘pulvinar’); OT, optic tectum (‘superior colliculus’); RGC, retinal ganglion cells. Adapted from Felleman and van Essen (1991), by permission of Oxford University Press. Monkey photo: RedCoat, Wikimedia Commons; turtle photo: Eurogroup for Animals.
Besides sharing pallial modules, mammals and reptiles also share a complement of cortical cell types, suggesting that some structural elements of cortical circuits arose early in amniote evolution. Like the mammalian cortex, the reptilian cortex contains excitatory, glutamatergic neurons and inhibitory (GABAergic) interneurons. In both mammals and reptiles, these neurons have a common developmental origin: excitatory neurons are generated by multipotent cortical progenitors, whereas inhibitory neurons are born in the subpallium before migrating to the cortex.
Classical studies suggest that the reptilian main cortical cell layer (layer 2, L2) corresponds to the deep, output layers of mammalian neocortex, whereas its layer 1 (L1) is equivalent to mammalian layer I. According to this view, mammalian cortical evolution would have included the incorporation of new, intermediate cell layers acting as input stations and internal circuitry. Morphologically, L2 pyramidal neurons of the reptilian dorsal cortex are most similar to mammalian hippocampal excitatory neurons. Indeed, reptilian pyramidal neurons have, depending on the area, little to no basal dendritic field, and several densely spine-studded apical dendrites, quite different from the single, long, apical dendrite of neocortical pyramidal neurons. Consistent with this correspondence between layers, reptilian subpallial cells transplanted into mammalian embryos generate GABAergic neurons that can populate only the deeper cortical layers.
Challenging this view, however, recent molecular studies have found that turtle and lizard cortical neuroblasts generate neurons that express upper layer molecular markers, in a developmental sequence similar to that observed in mammals. Although the molecular characterization of neuronal types in the reptilian cortex is still in its infancy, it is possible that the reptilian cortex represents an ancestral blueprint for the more elaborate mammalian cortical circuits. For example, reptilian cortical neurons, or subsets of them, might share molecular (and functional) features with both upper and lower layer mammalian cells.
Cortical function
Cortical circuitry is where most of the comparative work on the reptilian brain has been done. In the three-layered reptilian cortex, afferent inputs travel medially through superficial L1, where they fan out in a non-topographic manner. Their targets are GABAergic interneurons (with cell bodies in L1 and L3) and glutamatergic pyramidal neurons, with cell bodies packed in L2, and dendrites in L1 and L3. Interneurons project locally within cortex, providing feedforward and feedback inhibition to specific regions of the pyramidal cells’ dendrites. Several interneuron subtypes can be defined based on the expression of a subset of common genetic markers used in mammals. For example, in turtle cortex, some interneuron types express calbindin, others express neuropeptide-Y, while parvalbumin (PV)-positive interneurons appear to be absent. Pyramidal cells make reciprocal connections with each other and with interneurons locally, with subcortical afferent structures, and with other cortical areas.
The lateral cortex receives olfactory input from the olfactory bulb, and projects to the medial cortex (hippocampus). The dorsal cortex receives input from the thalamus; in many species this input is visual, originating in the thalamic lateral geniculate nucleus (LGN) and also eventually reaches the hippocampus. Pyramidal cells of the hippocampus project back to dorsal and lateral cortices, forming an internal cortical loop. Different mammalian species can exhibit vast elaborations in the number and connectivity of cortical subregions (Figure 3). However, by examining homologous structures, shared circuit motifs can be recognized (Figure 4).
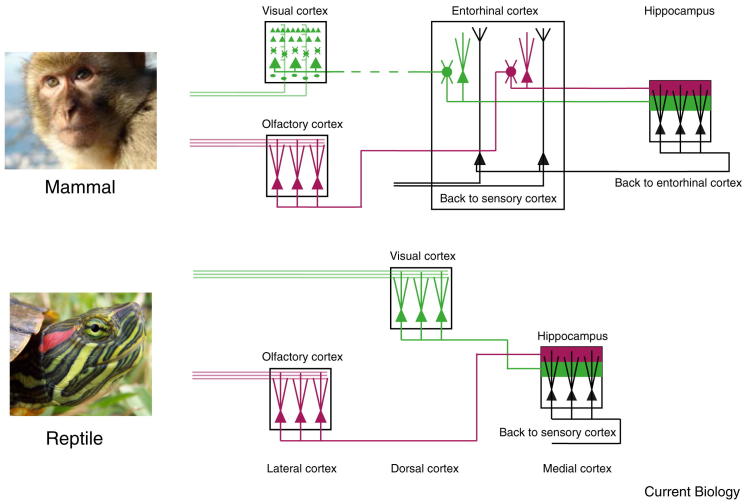
Figure 4.
Shared circuit motifs between reptilian and mammalian cortex.
Whereas processing steps are fewer in reptiles, they reach the same target as in mammalian cortex. Cortical architecture is more similar across regions in reptiles, suggesting similar and possibly general computations are performed on different sensory inputs. Adapted from: Igarashi et al. (2014). Monkey photo: RedCoat, Wikimedia Commons; turtle photo: Eurogroup for Animals.
In turtles, visual stimulation triggers propagating waves of neural activity that travel across the cortex. These waves are slower and simpler than those observed in mammalian neocortex. They are accompanied by relatively slow oscillations, which are most prominent in the 20 Hz frequency band. Whereas the so-called gamma oscillations in mammalian cortex are typically around and above 40 Hz, recent results in mice indicate that the 20 Hz band dominates when PV-interneuron development is artificially arrested, consistent with the above observation that turtle cortex lacks PV interneurons. The computational role, if any, of such dynamics is unknown at present. Progress will require new experimental approaches that allow the simultaneous sampling of large neuronal populations. Specific and data-driven theories of computation in reptilian cortex thus await further study. To the extent that modern reptilian cortex resembles that in the common ancestor of reptiles and mammals, understanding reptilian cortex function may reveal some of the fundamental associative computations that early cortical circuits evolved to carry out.
Physiological adaptations of the reptilian brain
Reptiles are ectotherms and must behaviorally or physiologically adapt to varying temperatures, making reptile brains notable for their adaptation to extreme conditions. The turtle brain, for example, has evolved remarkable adaptations to hypoxic conditions, which have long been known to experimentalists interested in hearing, olfaction, motor control or cerebellar physiology. Semi-aquatic freshwater turtles, such as Chrysemys picta, are remarkable for their ability to survive hibernation in hypoxic waters during cold northern winters, and have been reported to survive anoxic periods of over four months at 3°C. This resilience points to several physiological adaptations to survive cold, anoxic conditions during hibernation and subsequent re-oxygenation in the spring. The mechanisms underlying such tolerance are interesting — and also possibly applicable to the treatment of brain injury due to ischemia.
Anoxia tolerance critically depends on the ability to reduce energy expenditure while elevating anaerobic ATP production. ATP consumption in neural tissues, particularly those involved in sensory functions, requires high ATP turnover. Free glucose and glycogen can serve as metabolic substrates in anoxic conditions, and stores of glycogen are released from the liver and skeletal muscles to facilitate ATP production during hibernation. But, by shifting to anaerobic glycolysis, hibernating turtles must buffer the resulting metabolic product lactic acid to prevent fatal acidosis. In turtles, extracellular buffering of lactic acid is achieved by having large volumes of pericardial and peritoneal fluids, and through sequestration in the mineralized shell and skeleton.
During hibernation, turtles reduce ATP consumption in neural tissue by reducing membrane permeability and thus the need for pump-mediated restoration of ionic balance. This phenomenon has been observed in turtle neurons for voltage-gated Na+ channels, the oxygen-sensitive calcium-activated K+ channels, as well as AMPA and NMDA receptors. Neuronal depolarization and NMDA receptor over-activation are a hallmark of anoxia in mammalian neural tissue and lead to excitotoxic cell death via increased intracellular Ca2+ concentrations. In turtle neurons, NMDA receptor opening times are reduced by 65% after 60 minutes of anoxia, and NMDA receptor currents are actually silenced by anoxia-triggered cytosolic Ca2+ release from mitochondrial stores. Despite reduced channel activity and consequent reduced electrical activity during anoxia, turtles appear to remain vigilant and responsive to visual stimuli during hibernation (but less so to tactile stimuli). This maintenance of visual responses in the absence of tactile responses during hibernation suggests a differential down-regulation of sensory modalities. Because changes in daily illumination contribute to signaling the end of hibernation, it may be a selective advantage for energy-conserving mechanisms to be applied less to the visual system than to other brain areas.
When oxygen again becomes available, the re-oxygenation of tissue, such as that observed after ischemia-reperfusion events caused by myocardial infarction or stroke, is problematic in mammals because it is associated with the excessive production of reactive oxygen species (ROS) that damage cells through protein oxidation and lipid peroxidation. Turtles have evolved effective physiological mechanisms that allow for repeated cycles of anoxia and re-oxygenation without the free-radical damage induced by ischemia-reperfusion of tissues observed in mammals. These mechanisms involve an evolutionarily old up-regulation of nitric oxide and its metabolites, which limit ROS generation and tissue damage in cardiac and brain tissue. Thus, the turtle brain, with these physiological adaptations, is a very useful model for the study of neural circuit function. Neural tissue remains alive and viable for days ex vivo, and tissue can be routinely stored overnight in a refrigerator at 3–5°C.
Brain and behavior
Reptiles express a number of complex behaviors normally attributed to mammals. They can, for example, learn to navigate mazes as well as birds or mammals do and likely use a hippocampal structure to do so. Remarkably, sea turtles navigate across the ocean to their home beach, possibly using magnetic, olfactory, and visual cues. Reptiles also express interesting social behaviors: pythons and crocodiles, for example, incubate their eggs after hatching, and mothers guard the nest and provide infant care. Some lizard species exhibit pair bonding, parental care, and form families. Others construct networks of tunnels and may live in social communities like those of naked-mole rats.
Reptiles inhabit a variety of ecological niches, such as terrestrial deserts, temperate forests, tropical jungles, the sea, and fresh waters. They are found in all oceans and on all continents except Antarctica. Semi-aquatic turtles, which live in primarily aquatic habitats but make terrestrial excursions to lay eggs, offer an interesting model animal in which to study navigation. Many turtles are known for their impressive navigational skills and high nest-site fidelity, or the tendency for individuals to return to the same geographic location or microhabitat to nest. The mechanisms underlying these behaviors are not entirely understood, but navigational ability in the fresh water turtle Chrysemys picta seems to depend on experience gained during a critical period (when younger than four years of age). Translocated resident adults, for example, can quickly and consistently find new aquatic habitats within 33 hours, often using precise and predictable routes, whereas translocated non-resident adults appear unable to find new aquatic habitats even after 21 days.
The hippocampus is critical for map-like or relational memory representations of allocentric space, where objects are represented in relation to other objects. In birds and mammals, lesions to the hippocampal formation produce selective impairments in spatial tasks that require the encoding of relationships among multiple environmental features (place learning) but not in tasks that require approaching a single cue or simple non-spatial discriminations. Whereas extensive comparative research supports the idea that the reptilian medial cortex is homologous to the hippocampal formation of mammals and birds, only a few studies have examined the neural function of this brain structure or its role in place learning. In one such study, Rodriguez et al. (2002) evaluated the effects of lesions to the hippocampus of turtles in place and cue-maze tasks. Hippocampal lesioned (and sham-lesioned) animals performed cue-discrimination tasks correctly, but failed at the place learning that relied on allocentric space learning. These results indicate that lesions to the hippocampus of turtles selectively impair map-like memory representations of the environmental space, mirroring the effect of hippocampal lesions in mammals and birds. Thus, reptilian hippocampus may also share a central role in navigation.
In conclusion, the observation that mammalian and reptilian brains share both ancestry and a large number of functional attributes suggests that the identification of primordial (and possibly general) algorithmic principles of brain function could be helped by comparative approaches. To this end the reptilian brain, with its simpler structure, may prove invaluable to decipher fundamental questions of modern neuroscience.
Further Reading
- Belgard T.G., Montiel J.F., Wang W.Z., García-Moreno F., Margulies E.H., Ponting C.P., Molnár Z. Adult pallium transcriptomes surprise in not reflecting predicted homologies across diverse chicken and mouse pallial sectors. Proc. Natl. Acad. Sci. USA. 2013;110:13150–13155. doi: 10.1073/pnas.1307444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce L.L. Evolution of the nervous system in reptiles. In: Kaas J.H., editor. Volume 2. Academic Press; Oxford: 2007. pp. 125–156. (Evolution of Nervous Systems). [Google Scholar]
- Bruce L.L., Neary T.J. The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain Behav. Evol. 1995;46:224–234. doi: 10.1159/000113276. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J., Rowell J., Ragsdale C. Cell-type homologies and the origins of the neocortex. Proc. Natl. Acad. Sci. USA. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman D.J., van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fournier J., Müller C.M., Laurent G. Looking for the roots of cortical sensory computation in three-layered cortices. Curr. Opin. Neurobiol. 2015;31:119–126. doi: 10.1016/j.conb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K.M., Ito H.T., Moser E.I., Moser M.B. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 2014;588:2470–2476. doi: 10.1016/j.febslet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Jackson D.C., Ultsch G.R. Physiology of hibernation under the ice by turtles and frogs. J. Exp. Zool. A Ecol. Genet. Physiol. 2010;313:311–327. doi: 10.1002/jez.603. [DOI] [PubMed] [Google Scholar]
- López J.C., Vargas J., Gómez Y., Salas C. Spatial and non-spatial learning in turtles: the role of medial cortex. Behav. Brain Res. 2003;143:109–120. doi: 10.1016/s0166-4328(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Luts P., Milton S.L. Negotiating brain anoxia survival in the turtle. J. Exp. Biol. 2004;207:3141–3147. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- Madsen J.G., Wang T., Beedholm K., Madsen P.T. Detecting spring after a long winter: coma or slow vigilance in cold, hypoxic turtles? Biol. Lett. 2013;9:20130602. doi: 10.1098/rsbl.2013.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T., Dong Z., Berberoglu M.A., Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei) Brain Res. 2011;1381:95–105. doi: 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R., ten Donkelaar H.J., Nicholson C. Berlin Heidelberg, Springer-Verlag; 1998. The Central Nervous System of Vertebrates. [Google Scholar]
- Nomura T., Gotoh H., Ono K. Changes in the regulation of cortical neurogenesis contribute to encephalization during amniote brain evolution. Nat. Commun. 2013;4:2206. doi: 10.1038/ncomms3206. [DOI] [PubMed] [Google Scholar]
- Northcutt R.G. Variation in reptilian brains and cognition. Brain Behav. Evol. 2012;82:45–54. doi: 10.1159/000351996. [DOI] [PubMed] [Google Scholar]
- Prechtl J.C., Choen L.B., Pesaran B., Mitra P.P., Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc. Natl. Acad. Sci. USA. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L., Harrison M., Paxinos G., Watson C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci. 2013;36:570–578. doi: 10.1016/j.tins.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rodríguez F., López J.C., Vargas J.P., Gómez Y., Broglio C., Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 2002;22:2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.C., Krochmal A.R. The role of age-specific learning and experience for turtles navigating a changing landscape. Curr. Biol. 2015;25:333–337. doi: 10.1016/j.cub.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Striedter G.F. Sinauer Associates; 2005. Principles of Brain Evolution. [Google Scholar]
- Swanson L.W. Oxford University Press; 2011. Brain Architecture: Understanding the Basic Plan. [Google Scholar]
- Ulinski P.S. Visual cortex of turtles. In: Kaas J.H., editor. Volume 2. Oxford Academic Press; 2007. pp. 195–203. (Evolution of Nervous Systems). [Google Scholar]