Abstract
Recent studies have established the interaction between APOA2 −256T>C polymorphism and dietary saturated fatty acids intake in relation to obesity on healthy individuals. In the current study, we investigate the effects of this interaction on anthropometric variables and serum levels of leptin and ghrelin in patients with type 2 diabetes. In this cross-sectional study, 737 patients with type 2 diabetes mellitus (290 males and 447 females) were recruited from diabetes clinics in Tehran. The usual dietary intake of all participants during the last year was obtained by validated semiquantitative food frequency questionnaire. APOA2 genotyping was performed by real-time PCR on genomic DNA. No significant relation was obtained by univariate analysis between anthropometric variables and APOA2 genotypes. However, after adjusting for age, gender, physical activity and total energy intake, we identified a significant interaction between APOA2-saturated fatty acids intake and body mass index (BMI). After adjusting for potential confounders, serum levels of ghrelin in CC genotype patients were significantly higher than T allele carriers (p = 0.03), whereas the case with leptin did not reveal a significant difference. The result of this study confirmed the interaction between APOA2 −256T>C polymorphism and SFAs intake with BMI in type 2 diabetic patients. In fact, homozygous patients for the C allele with high saturated fatty acids intake had higher BMI. The APOA2 −256T>C polymorphism was associated with elevated levels of serum ghrelin.
Keywords: Apolipoprotein A2; Obesity; Fatty acids; Ghrelin; Diabetes mellitus, type 2
Introduction
Obesity is a major health problem, with an increasing prevalence in Southeast Asia, Mediterranean and Iran (Klein et al. 2002; Mohamadnejad et al. 2003). Overweight and obesity are among the most common health concerns in patients with type 2 diabetes, affecting more than 85 % of all cases. Obesity, through increasing insulin resistance and serum glucose concentration, adds more complexity to the progression of type 2 diabetes (Maggio and Pi-Sunyer 1997). Dyslipidemia and the increase in abdominal adipose tissue are the most common metabolic disorders in type 2 diabetic patients (Sam and Haffner 2008).
Racial differences of obesity in comparable environments (Wang and Beydoun 2007; Ogden et al. 2006) suggest that obesity is the result of complex interactions among genetic, behavioral, social and environmental factors (Hill 2006; Prentice 2005; Speakman 2004; Ravussin and Bouchard 2000). Nutrigenetics evaluates the effect of genome variations in the individual’s response to nutrients (Ordovas and Corella 2004). Single-nucleotide polymorphism (SNP) studies provide a powerful molecular tool for investigating the role of nutrition on health and diseases, which leads to the determination of optimal diets for individuals (Ames 1999). The obesity gene map shows the specific loci on all chromosomes except chromosome Y which are related to obesity (Ellsworth et al. 2005). There are many genes that regulate the energy balance, appetite, lipid metabolism and adipogenesis (Martinez et al. 2008).
One notable gene is Apolipoprotein A2, with many identified genetic variants (Fullerton et al. 2002). APOA2 is the second most important protein in HDL, comprising approximately 20 % of total HDL protein (Blanco-Vaca et al. 2001). Previous studies indicate a new association between −256T>C APOA2 polymorphism and saturated fatty acids intake in relation to BMI and obesity (Corella et al. 2007, 2009, 2011).
A recent study examined the association between this SNP and serum level of leptin and ghrelin, as an effective mechanism underlying the obesity. In this study, significant APOA2-SFAs interaction with regard to ghrelin levels was observed. Serum ghrelin for CC patients with low SFA intake was lower than CC patients with high SFA intake and T allele carriers (TT + TC) (Smith et al. 2011).
In fact, diet is one of the most important environmental factors, which interacts with the genome to modulate the risk of diseases. Understanding these interactions could lead us to prevent from chronic diseases, through dietary recommendation (Ordovas and Corella 2004). Due to the few studies in this field, and no record in Iran, we conduct the current research with the aim of investigating the distribution of −256T>C APOA2 polymorphism and then the interaction between APOA2 genotypes and saturated fatty acids intake with regard to anthropometric variables, ghrelin and leptin serum levels in type 2 diabetic patients.
Materials and methods
Study design and subjects
In this cross-sectional study, which was conducted from October 2011 to June 2012, 737 type 2 diabetic patients (290 men and 447 women) aged 35–65 years who were randomly sampled through the Iranian Diabetes Society, Gabric Diabetes Association, Tehran, East Medical Center and Health Centers all around the city.
After written and verbal explanation of the aim and methodology of this study, informed consent was obtained from all subjects. Individuals under 35 or over 65 years of age, insulin administrating patients, pregnant or lactating women, and those who consumed alcohol 24 h before blood sample collection were excluded. This study was approved by the Ethics Committee of Tehran University of Medical Sciences.
Assessment of anthropometric measures and physical activity
Weight was measured in the fasting state, with the minimal clothing, by using Seca falcon scales, with an accuracy of 100 gr. Height was measured using a Seca height gauge with an accuracy of 0.5 cm. Waist circumference was measured with an accuracy of 0.5 cm from the midpoint between the upper edge of the iliac crest and the lower edge of the chest and the last rib (Lohman et al. 1988). Measurements were taken by a dietitian. BMI was calculated by dividing weight (in kg) to the square of height (in m).
The amount of daily physical activity was assessed by the classified physical activity questionnaire according to metabolic equivalent task (MET) which includes nine activity levels from sleep/rest (METs = 0.9) to high-intensity physical activities (METs > 6). This questionnaire was developed and validated in previous studies in Europe (Aadalh and Jorgensen 2003). In Iran, reliability and validity of this questionnaire have been confirmed by Kelishadi et al. (2004). Hours spent on each physical activity were multiplied by their MET quantities, and the numbers obtained were summed together to calculate MET hour/day (MET h/day) value.
Assessment of dietary intake
The participant’s usual dietary intake during the last year was evaluated through face-to-face interviews conducted by a trained dietitian using a semiquantitative food frequency questionnaire for 147 food items. The validity and reliability of the questionnaire were previously confirmed in Iran (Esmaillzadeh et al. 2004). The subjects were asked to report the frequency of food item consumption in a day, a week, a month or a year. The amounts listed for each food were converted to grams per day using household Handbook Scale (Ghaffarpour et al. 1999). Finally, the equivalent grams of each food item were determined. All foods and beverages were coded. In order to assess calorie and nutrient intake, Nutritionist-III (N3) Software program designed for Iranian foods was used.
Biochemical analysis
Venous blood samples were collected after 12-h overnight fasting. Serums were obtained, and hormonal evaluations were conducted on 258 patients with simple random sampling from each of the genotype groups.
Serum ghrelin and leptin concentrations were measured by ELISA method (Bioassay Technology Co, China and Mediagnost, Germany, respectively).
Genetic analysis
Genomic DNA was extracted from whole blood using salting-out method (Miller et al. 1988). APOA2 −265T>C genotyping (rs5082) was performed by StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA; Alvandi and Koohdani 2014).
Statistical analysis
The Kolmogorov–Smirnov test was used to examine the normal distribution of data. Weight, BMI and waist circumference were log-transformed. Chi-square test was used to compare percentages. ANOVA and independent t test were applied to compare crude means. The relation between APOA2 polymorphism and anthropometric variables was tested in an analysis of covariance (ANCOVA) multivariate model, controlling for confounding factors (age, gender, physical activity and total energy intake). The interaction between APOA2 −256T>C polymorphism and SFA intake for anthropometric variables was tested in an ANCOVA multivariate interaction model, controlling for confounding factors (age, gender, physical activity and total energy intake). In addition, adjustment for BMI was carried out when indicated. Mann–Whitney test was used in order to examine the relationship between genotypes and ghrelin levels (nonparametric variable). p value <0.05 was considered statistically significant. For all analyses, the SPSS software (version 21; SPSS Inc., Chicago, IL) was used.
Results
The APOA2 polymorphism was genotyped in a total of 737 patients (age 54.1 ± 6.5 years). In this population, the distribution of −256T>C APOA2 polymorphism was 38.9, 48.6 and 12.5 percent for TT, TC and CC genotypes, respectively. The allelic distribution conforms to the Hardy–Weinberg equilibrium. Based on these genotypes, the general characteristics of individuals are shown in Table 1. These data indicate that CC subjects were significantly older than T allele carriers (TT + TC; p = 0.03) and smoking is significantly more common in CC group (p = 0.03). No statistically significant difference was identified in dietary intake between the two genotype groups (Table 2).
Table 1.
General characteristics of the study population
| APOA2 genotype | p value | ||
|---|---|---|---|
| TT + TC (n = 645) | CC (n = 92) | ||
| Age, year | 53.9 ± 6.6 | 55.5 ± 5.6 | 0.03 |
| Physical activity, MET h/day | 37.9 ± 5.5 | 37.2 ± 4.5 | 0.3 |
| Smoking, n (%) | 125 (19.4) | 10 (10.9) | 0.03 |
| Family history of diabetes, n (%) | 269 (41.7) | 35 (38) | 0.3 |
| History of CVD, n (%) | 241 (37.4) | 37 (40.2) | 0.3 |
| Lipid lowering drugs intake, n (%) | 367 (56.9) | 56 (60.9) | 0.3 |
| Glucose lowering drugs intake, n (%) | 599 (92.9) | 86 (93.5) | 0.5 |
Data are mean ± SD except where noted
Table 2.
Association between the APOA2 polymorphism and dietary intake
| APOA2 genotype | p valuea | p valueb | ||
|---|---|---|---|---|
| TT + TC (n = 645) | CC (n = 92) | |||
| Grains (g/day) | 420 ± 216.7 | 394.3 ± 207.1 | 0.29 | 0.13 |
| Fruits (g/day) | 464.1 ± 268 | 474 ± 239.5 | 0.74 | 0.69 |
| Vegetables (g/day) | 454 ± 264 | 444.8 ± 250 | 0.75 | 0.91 |
| Low-fat dairy (g/day) | 183.7 ± 142 | 166.1 ± 127.9 | 0.26 | 0.27 |
| High-fat dairy (g/day) | 105.6 ± 156 | 99.6 ± 162 | 0.73 | 0.85 |
| Red meat (g/day) | 20.4 ± 22 | 23 ± 37.6 | 0.34 | 0.25 |
| Organ meat (g/day) | 2.4 ± 8.6 | 1.1 ± 1.6 | 0.17 | 0.2 |
| Poultry (g/day) | 39.7 ± 37.6 | 36.1 ± 38.6 | 0.39 | 0.35 |
| Fish (g/day) | 13.8 ± 14.8 | 16.2 ± 16.2 | 0.16 | 0.17 |
| Legumes (g/day) | 43.5 ± 45 | 41.7 ± 30.1 | 0.7 | 0.69 |
| Vegetable oils (g/day) | 21.1 ± 23.2 | 19.4 ± 21.6 | 0.5 | 0.41 |
| Hydrogenated fats (g/day) | 11.5 ± 24.6 | 9 ± 22.3 | 0.37 | 0.37 |
| Olive (g/day) | 7.1 ± 13.1 | 6.7 ± 7.1 | 0.79 | 0.8 |
| Butter (g/day) | 3 ± 6.6 | 2.7 ± 5 | 0.63 | 0.65 |
| Mayonnaise (g/day) | 1.7 ± 3 | 2.1 ± 3.7 | 0.28 | 0.3 |
| Total energy intake (kcal/day) | 2528 ± 783 | 2533 ± 916 | 0.9 | 0.79 |
| Protein (g/day) | 88 ± 28.2 | 90 ± 37.6 | 0.6 | 0.36 |
| Carbohydrate (g/day) | 333.8 ± 110 | 338 ± 125.8 | 0.7 | 0.65 |
| Total fat (g/day) | 101.5 ± 44.8 | 100.4 ± 47.3 | 0.8 | 0.66 |
| Dietary fiber (g/day) | 41 ± 18.8 | 44 ± 22.4 | 0.16 | 0.09 |
| Saturated fatty acids (g/day) | 26.3 ± 10 | 25.4 ± 9.8 | 0.4 | 0.28 |
| Monounsaturated fatty acids (g/day) | 34.8 ± 17.2 | 33.5 ± 17 | 0.5 | 0.29 |
| Polyunsaturated fatty acids (g/day) | 25.1 ± 14.4 | 25 ± 15.8 | 0.9 | 0.86 |
Data are mean ± SD
aData are unadjusted
bData are adjusted for age, gender, physical activity and total energy intake
As shown in Table 3, no significant difference was observed in the average of anthropometric parameters of the two group genotypes. Although, the mean weight, BMI and waist circumference were higher in the CC group than the TT + TC group, but these differences were not statistically significant. Besides, after adjusting for age, gender, tobacco smoking, physical activity and total energy intake, no significant differences were observed.
Table 3.
Association between the APOA2 polymorphism and anthropometric variables
| APOA2 genotype | p valuea | p valueb | ||
|---|---|---|---|---|
| TT + TC (n = 645) | CC (n = 92) | |||
| Mean ± SD | Mean ± SD | |||
| Weight (kg) | 74.8 ± 11.6 | 75.8 ± 12.3 | 0.5 | 0.3 |
| Height (cm) | 161.3 ± 9.1 | 160 ± 8.8 | 0.2 | 0.3 |
| BMI (kg/m2) | 28.8 ± 3.8 | 29.3 ± 4.1 | 0.3 | 0.2 |
| Waist circumference (cm) | 90.9 ± 8.9 | 92.3 ± 9.3 | 0.2 | 0.2 |
Data are mean ± SD
aData are unadjusted
bData are adjusted for age, gender, tobacco smoking, physical activity and total energy intake
Table 4 describes the mean anthropometric measurements by APOA2 genotypes and SFA intake. In T allele carriers, no significant association was found by means of weight, BMI and waist circumference between two categories of SFA intake (<22 and ≥22 g/day). In contrast, CC genotype with SFA intake ≥22 g/day was associated with greater weight (p = 0.01).
Table 4.
Association of APOA2 polymorphism with anthropometric variables in different saturated fatty acids intake categories
| APOA2 genotype | ||||||
|---|---|---|---|---|---|---|
| TT + TC | p value | CC | p value | |||
| SFA <22 g/day (n = 240) | SFA ≥22 g/day (n = 405) | SFA <22 g/day (n = 39) | SFA ≥22 g/day (n = 53) | |||
| Mean ± SD | Mean ± SD | |||||
| Weight (kg) | 74.2 ± 12.7 | 75.2 ± 10.8 | 0.2 | 72.3 ± 12.9 | 78.5 ± 11.1 | 0.01 |
| BMI (kg/m2) | 29 ± 4.4 | 28.6 ± 3.4 | 0.3 | 28.5 ± 4.4 | 29.9 ± 3.9 | 0.1 |
| Waist circumference (cm) | 91.2 ± 9.9 | 90.7 ± 8.2 | 0.6 | 90.2 ± 9.2 | 93.9 ± 9.1 | 0.06 |
Data are mean ± SD
Based on the results of Fig. 1, after adjusting for age, gender, tobacco smoking, physical activity and total energy intake, BMI and weight were not different in all subjects (CC and TC + TT group) with low SFA intake (<22 g/day). However, in patients with high SFA intake (≥22 g/day), a significant relation between APOA2 genotype with weight (p = 0.031) and BMI (p = 0.022) were found. In fact, CC subjects with high saturated fatty acids intake had higher weight and BMI than T allele carriers who consumed the same amount of SFA.
Fig. 1.
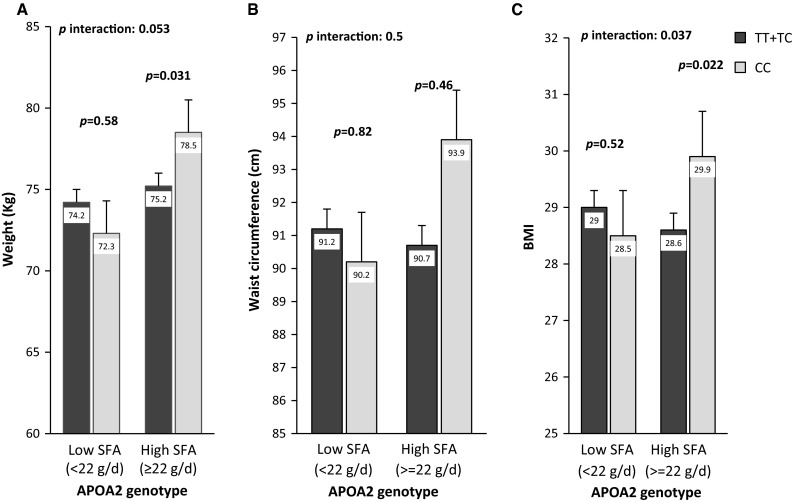
Interaction between the APOA2 polymorphism and saturated fatty acids intake with regard to weight (a), waist circumference (b) and BMI (c). Means are adjusted for age, gender, tobacco smoking, physical activity, total energy intake (a, c) and body mass index (b). p interactions are obtained with the multivariate interaction model containing fat intake as a categorical variable and the APOA2 polymorphism and additional control for the covariates indicated above. Bars indicate mean ± SD
After adjusting for confounding factors, there was no significant interaction between APOA2 genotypes and SFA intake in relation to waist circumference.
Table 5 shows that ghrelin concentration was significantly higher in the CC group than the TT + TC group (p = 0.03), while after adjusting for confounding variables (age, sex, tobacco smoking, physical activity, BMI and total energy intake), the serum leptin level was not significant between the two groups. In this study, based on the SFAs intake, no significant difference was found between the mean hormone levels of participants and APOA2 genotypes (data not shown).
Table 5.
Association between the APOA2 polymorphism and serum leptin and ghrelin values
| APOA2 genotype | p valuea | ||
|---|---|---|---|
| TT + TC (n = 169) | CC (n = 89) | ||
| Mean ± SD | |||
| Serum leptin (ng/ml) | 23.2 ± 13.9 | 24.8 ± 12.8 | 0.4 |
| Serum ghrelin (ng/ml) | 2.3 ± 1 | 2.4 ± 1.7 | 0.03 |
Data are mean ± SD
aData are adjusted for age, gender, body mass index, tobacco smoking, physical activity and total energy intake
Discussion
We found a significant interaction between saturated fatty acid intake and the −256T>C APOA2 genotypes for BMI. Our results are consistent with the findings of previous studies conducted in the three American populations (Corella et al. 2009), Mediterranean population, Chinese and Asian Indian subjects (Corella et al. 2011) who participated in a Singapore National Health Survey. These studies showed the association of high SFAs intake with higher BMI and obesity in CC genotype.
In our population, the frequency of homozygous minor allele carriers (CC) was 12.5 % while the distribution of CC genotype differed in various populations (1–16 %; Corella et al. 2009, 2011; Smith et al. 2011). In FOS (16 %; Corella et al. 2009), GOLDEN study (15 %; Corella et al. 2009) and Mediterranean population (15 %; Corella et al. 2011), the prevalence of CC subjects was higher than our and other population, whereas the frequency of CC genotype was the same of our study (12 %) in Spanish population (Smith et al. 2011).
In the current study, we divided the SFAs intake into two groups (low and high) according to previous studies (Corella et al. 2009). In subjects with lower SFAs intake, after adjusting for age, gender, physical activity and total energy intake, there was no significant association between the mean weight or BMI and APOA2 genotypes. However, these relations were significantly different in patients with high SFAs intake. After adjusting for age, gender, physical activity, total energy intake and BMI, in both high and low SFAs intake groups, a significant interaction between the waist circumference and two groups of APOA2 genotypes was not found.
The mechanism by which −256T>C APOA2 SNP affects fat metabolism is still unknown. So far, researchers have been described that APOA2 transcription is controlled by an array of A to N regulatory elements in the promoter region of the gene. The −265T>C polymorphism is located in the middle of the element D (Chambaz et al. 1991). This element binds several different nuclear factors (Cardot et al. 1994). It is suggested that the nuclear-binding factors in the presence of C allele were associated with lower APOA2 expression in liver cells that results in reduced APOA2 plasma concentration (Van’t Hooft et al. 2001), while the results of this study indicate that the probable difference in APOA2 plasma concentration had no effect on anthropometric variables, but lifestyle changes, such as high intake of SFAs, may change the unknown metabolic pathways which lead to increase susceptibility to weight gain and obesity.
This is the reason that individuals with CC genotypes, who consume SFAs <22 g/day, have a lower prevalence of overweight. We could conclude that the CC group with high SFAs intake is more prone to obesity and overweight, compared to CC individuals with low SFAs intake.
According to our data, there was no significant difference between the serum leptin levels of TT + TC and CC groups, but the latter group showed significantly higher ghrelin concentration. Based on SFAs intake of these groups, no significant difference was found in the serum levels of these hormones. So far, Smith et al. study has been the only published report about the relation of these hormones and TT + TC or CC groups. Based on their research, plasma ghrelin of the CC group with low SFA intake was lower than the CC group with high SFA intake. It was also lower in TT + TC subjects with low SFA intake than TT + TC subjects with high SFA intake. However, regarding SFA intake, they found no significant difference between the serum leptin of CC group and T allele carriers (Smith et al. 2011). Since ghrelin is an orexigenic and appetite hormone, it seems natural that the serum level of ghrelin in C allele carriers to be higher than T allele carriers. This suggests a new role for APOA2 as a satiety signal. Due to the higher level of serum ghrelin in CC group, it was expected that these subjects had more energy intake, compared to T allele carriers. However, since all the subjects of our study were diabetic patients, who followed dietary advises for controlling their disease, then we did not find a significant difference of energy intake between CC and TT + TC groups.
Regarding the limitation of our research, there could be some measurement errors, like underestimation or overestimation of some food items in a food frequency questionnaire. We also did not measure the plasma concentration of APOA2. Despite all these limitations, this current study was the singular attempt to investigate the relation between anthropometric variables and SFA intake with APOA2 polymorphism in type 2 diabetic patients. Our data could help to detect the high-risk genotypes, to design the screening programs and to provide dietary suggestions for the prevention or treatment of overweight and obesity.
In conclusion, we have investigated a gene–diet interaction between the APOA2 SNP and saturated fatty acids intake for BMI in Iranian patients with type 2 diabetes mellitus. In fact, individuals with the CC genotype consuming a high SFA diet appear to be more prone to obesity and overweight than T allele carriers. The APOA2 −256T>C polymorphism was associated with elevated levels of serum ghrelin, but there were no significant APOA2-saturated fat interactions with regard to hormonal variables. Based on these findings, genotype-based personalized dietary recommendations for SFAs intake might be useful in the prevention of obesity.
Acknowledgments
This study is supported by a grant from the Research Deputy of Tehran University of Medical Science (90-03-27-15060). The authors would like to thank all who participated in or collaborated with current study.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- APOA2
Apolipoprotein A2
- BMI
Body mass index
- CDV
Cardiovascular disease
- HDL
High-density lipoprotein
- MET
Metabolic equivalent task
- SFA
Saturated fatty acid
- SNP
Single-nucleotide polymorphism
References
- Aadalh M, Jorgensen T. Validation of a new self-repot instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35:1196–1202. doi: 10.1249/01.MSS.0000074446.02192.14. [DOI] [PubMed] [Google Scholar]
- Alvandi E, Koohdani F. Zip nucleic acid: a new reliable method to increase the melting temperature of real-time PCR probes. JDMD. 2014;13:26. doi: 10.1186/2251-6581-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN. Cancer prevention and diet: help from single nucleotide polymorphisms. Proc Natl Acad Sci USA. 1999;96:12216–12218. doi: 10.1073/pnas.96.22.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Vaca F, Escola-Gil JC, Martin-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res. 2001;42:1727–1739. [PubMed] [Google Scholar]
- Cardot P, Pastier D, Lacorte J, Mangeney M, Zannis V, Chambaz J. Purification and characterization of nuclear factors binding to the negative regulatory element D of human apolipoprotein A-II promoter: a negative regulatory effect is reversed by GABP, an Ets-related protein. Biochemistry. 1994;33:12139–12148. doi: 10.1021/bi00206a017. [DOI] [PubMed] [Google Scholar]
- Chambaz J, Cardot P, Pastier D, Zannis V, Cladaras C. Promoter elements and factors required for hepatic transcription of the human apoA-II gene. J Biol Chem. 1991;266:11676–11685. [PubMed] [Google Scholar]
- Corella D, Arnett DK, Tsai MY. The 256 T_C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53:1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- Corella D, Peloso G, Arnett DK, Demissie S, Cupples LA, Tucker K, Lai CQ, Parnell LD, Coltell O, Lee YC, Ordovas JM. APOA2, dietary Fat, and body mass index replication of a gene-diet interaction in 3 independent populations. Arch Intern Med. 2009;169:1897–1906. doi: 10.1001/archinternmed.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella D, Tai ES, Sorli JV, Kai Chew S, Coltell O, Sotos Prieto M, Garcia Rios A, Estruch R, Ordovas JM. Association between the APOA2 promoter polymorphism and body weight in Mediterranean and Asian populations: replication of a gene-saturated fat interaction. Int J Obes. 2011;35:666–675. doi: 10.1038/ijo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth D, Coady S, Chen W, Srinivasan S, Boerwinkle E, Berenson G. Interactive effects between polymorphisms in the β-adrenergic receptors and longitudinal changes in obesity. Obes Res. 2005;13:519–526. doi: 10.1038/oby.2005.55. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: a favorable association in Tehranian adults. Eur J Clin Nutr. 2004;59:353–362. doi: 10.1038/sj.ejcn.1602080. [DOI] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Taylor SL, Stengard JH, Salomaa V, Boerwinkle E, Nickerson DA. Sequence polymorphism at the human apolipoprotein AII gene (APOA2): unexpected deficit of variation in an African-American sample. Hum Genet. 2002;111:75–87. doi: 10.1007/s00439-002-0763-x. [DOI] [PubMed] [Google Scholar]
- Ghaffarpour M, Houshyar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of food. Tehran: Keshavarzi press; 1999. [Google Scholar]
- Hill J. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Rabiee K, Khosravi A, Famori F, Sadeghi M, Roohafza H, Shirani S. Assessment of physical activity in adolescents of Isfahan. J Shahrekord Univ Med Sci. 2004;3:55–65. [Google Scholar]
- Klein S, Wadden T, Sugerman H. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Champaign: Human Kinetics; 1988. pp. 28–80. [Google Scholar]
- Maggio C, Pi-Sunyer F. The prevention and treatment of obesity: application to type 2 diabetes. Diabetes Care. 1997;20:1744–1766. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Parra MD, Santos JL, Moreno-Aliaga MJ, Marti A, Martinez-Gonzalez MA. Genotype-dependent response to energy-restricted diets in obese subjects: towards personalized nutrition. Asia Pac J Clin Nutr. 2008;117:119–122. [PubMed] [Google Scholar]
- Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadnejad M, Pourshams A, Malekzadeh R. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322–2324. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C, Carroll M, Curtin L. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ordovas J, Corella D. Nutritional genomics. Annu Rev Genomics Hum Genet. 2004;5:71–118. doi: 10.1146/annurev.genom.5.061903.180008. [DOI] [PubMed] [Google Scholar]
- Prentice A. Early influences on human energy regulation: thrifty genotypes and thrifty phenotypes. Physiol Behav. 2005;86:640–645. doi: 10.1016/j.physbeh.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Bouchard C. Human genomics and obesity: finding appropriate drug targets. Eur J Pharmacol. 2000;410:131–145. doi: 10.1016/S0014-2999(00)00811-6. [DOI] [PubMed] [Google Scholar]
- Sam S, Haffner S. Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Ordovas J, Sanchez-Moreno C. Apolipoprotein A-II polymorphism: relationships to behavioural and hormonal mediators of obesity. Int J Obes. 2011;36:130–136. doi: 10.1038/ijo.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. Obesity: the integrated roles of environment and genetics. J Nutr. 2004;134:2090–2105. doi: 10.1093/jn/134.8.2090S. [DOI] [PubMed] [Google Scholar]
- Van’t Hooft F, Ruotolo G, Boquist S, de Faire U, Eggertsen G, Hamsten A. Human evidence that the apolipoprotein A-II gene is implicated in visceral fat accumulation and metabolism of triglyceride-rich lipoproteins. Circulation. 2001;104:1223–1228. doi: 10.1161/hc3601.095709. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun M. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]


