Abstract
Important antibiotics in human medicine have been used for many decades in animal agriculture for growth promotion and disease treatment. Several publications have linked antibiotic resistance development and spread with animal production. Aquaculture, the newest and fastest growing food production sector, may promote similar or new resistance mechanisms. This review of 650+ papers from diverse sources examines parallels and differences between land-based agriculture of swine, beef, and poultry and aquaculture. Among three key findings was, first, that of 51 antibiotics commonly used in aquaculture and agriculture, 39 (or 76%) are also of importance in human medicine; furthermore, six classes of antibiotics commonly used in both agriculture and aquaculture are also included on the World Health Organization’s (WHO) list of critically important/highly important/important antimicrobials. Second, various zoonotic pathogens isolated from meat and seafood were observed to feature resistance to multiple antibiotics on the WHO list, irrespective of their origin in either agriculture or aquaculture. Third, the data show that resistant bacteria isolated from both aquaculture and agriculture share the same resistance mechanisms, indicating that aquaculture is contributing to the same resistance issues established by terrestrial agriculture. More transparency in data collection and reporting is needed so the risks and benefits of antibiotic usage can be adequately assessed.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-015-9722-z) contains supplementary material, which is available to authorized users.
KEY WORDS: agriculture, antibiotic resistance, aquaculture, resistance mechanisms
INTRODUCTION
Antibiotics are arguably the most successful and important family of drugs developed for the protection of human health. Since the discovery of penicillin in 1928, over 100 antibiotics have been discovered and used, with the majority of these being introduced before 1970 (1). With the unveiling of each new antibiotic class, resistant bacterial strains were soon identified thereafter, and treatment of some is now a major medical challenge. Today, approximately 70% of characterized nosocomial infections are resistant to at least one clinically relevant antibiotic (2). Moreover, many strains have been discovered that exhibit multidrug resistance (MDR) to nearly all commonly available classes of antibiotics (3). Coded by antibiotic resistance genes (ARGs), resistance mechanisms such as efflux pumps have made many zoonotic pathogens extremely difficult to treat, forcing doctors to use antibiotics of last resort, for example, the fluoroquinolone ciprofloxacin, to treat pathogenic Escherichia coli strains (4).
Usage of antibiotics in the production of food animals to sustain and nurture the world’s continually increasing human population has contributed to the development of antibiotic resistance (5). In agriculture—referred to in this review as the farming of swine, poultry, and cattle—uses of antibiotics include disease prevention, treatment, control, and application as growth-promoting antibiotics (GPA) in order to improve feed utilization and production (5). The jurisdictions for specific antibiotics are allowed and their usage in agriculture varies depending on the location; for example, in the European Union (EU), the use of antibiotics for growth promotion is not allowed (6). In aquaculture—referred to in this review as the production of aquatic seafood in captivity but excluding plants—application of antibiotics is regulated sparingly, differing greatly from country to country with little to no enforcement in many of the countries that produce the majority of the world’s aquaculture products (7). Usage purposes are the same as those in agriculture, with the exception that in aquaculture, prophylactic treatment is more common (8). Previous research has linked agricultural antibiotic usage practices with antibiotic resistance development, resulting in calls for more judicious usage of antibiotics (5,9). Many studies have found drug-resistant bacterial strains in agricultural facilities, whether originating in the meat itself (10–12) or in the surrounding environment (13–15). The same has been shown for aquaculture (16–18), triggering repeated calls for improved regulation and enforcement (7). Efforts to document resistance have increased in recent years, a notable one being the National Antimicrobial Resistance Monitoring System (NARMS) that was established in 1996 as a collaboration between the US Food and Drug Administration (FDA) Center for Veterinary Medicine (19), the US Department of Agriculture (USDA), and the Centers for Disease Control and Prevention (CDC). However, the role of antibiotic usage in agriculture and aquaculture in the development of resistance and dissemination of ARGs is still poorly understood.
Acknowledging the recent growth of aquaculture as a major agricultural sector, this review explores similarities and differences between antibiotic resistance risks associated with agriculture and aquaculture. Specifically, we address whether the recent rise of aquaculture is creating new resistance issues or whether it is simply exacerbating the same ones already established for agriculture. To answer this question, we first discuss how antibiotics have been traditionally used in these industries around the world. We then focus on peer-reviewed academic literature contributions containing data on resistance development in foodborne pathogens. And finally, we use the USA as a case study to discuss in more detail specific issues identified in the global analysis.
METHODOLOGY
A systematic review was conducted of over 650 reports extracted from the peer-reviewed academic literature, non-government organizations (NGOs), industry, and government (see Supplemental Information for a full list of documents reviewed). Initial searches started with Web of Science and Google Scholar using key terms “antibiotics,” “livestock,” “agriculture,” “aquaculture,” and “food production.” Additional articles were identified using each article’s reference section and further searches were conducted depending on the topic section. Information was also obtained through conversations with food production experts. When possible, the most recent peer-reviewed academic literature was used as the cited reference. A total of 98 key sources are cited in text to illustrate key issues, show novel data or ways of analysis, and highlight key research gaps still awaiting attention in future studies. A full list of references is available as supplemental information.
Animal Farming and Antibiotic Usage
In addition to the search terms above, various country/region names were searched alongside (European Union, Brazil, China, etc.). Each jurisdiction’s official government website was further surveyed to collect relevant data. Non-government documents such as ones from the Food and Agricultural Organization (FAO) were also extensively reviewed in this section.
Foodborne Pathogens and Antibiotic Resistance Mechanisms
A separate search was conducted to analyze the link between antibiotic resistance and animal production. The initial search of literature on Web of Science started with the search terms “antibiotics, resistance, and agriculture” and “antibiotics, resistance, and aquaculture/seafood” (see supplemental information). These results were then filtered based on title to exclude topics that are not covered in this review (see exclusion criteria in supplemental information). Further literature searches were conducted as needed using terms such as “drug resistance, seafood, and antimicrobials” in order to find articles not captured in the primary search.
US Agriculture and Aquaculture
Much of these data were collected from governmental websites and through personal communications with personnel from various organizations such as the National Oceanic and Atmospheric Administration (NOAA) and the National Resources Defense Council (NRDC).
The cutoff date for the literature search was September 1, 2014. Information from the 2007 US Agriculture Census, kindly provided by the Food and Water Watch in raw and processed data formats, served to create the composite geographic information system (GIS) illustrations in Fig. 5. Whenever possible, an update to currently reported data is provided.
Fig. 5.
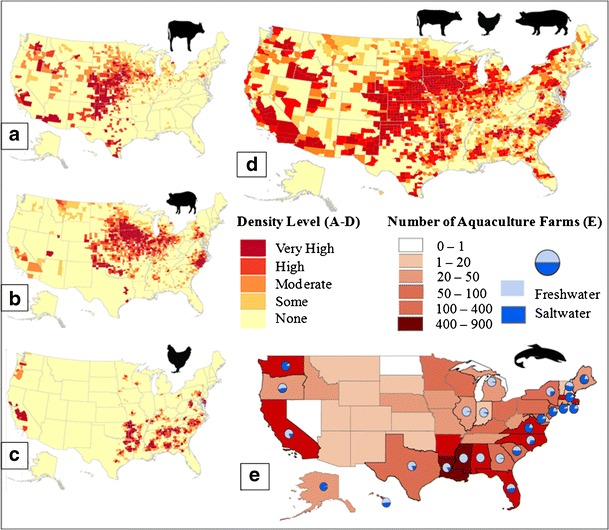
2007 density maps of cattle, swine, poultry, and combined values of production and 2005 number of aquaculture farms in the USA. 2007 US density of a cattle, b swine, c poultry, and d combined production. a–c Animal density by county. a Cattle density level per area indicated: very high ≥ 17,400; high = 7300–17,400; moderate = 2175–7299; some ≤ 2175; none = 0. b Swine density level: very high ≥ 48,500; high = 19,000–48,500; moderate = 9500–18,999; some ≤ 9500; none = 0. c Poultry density level: very high ≥ 2.75 million; high = 1–2.75 million; moderate = 350,000–999,000; some ≤ 350,000; none = 0. d Combined production, the total number of livestock across different animal types was calculated using the US Department of Agriculture definition of a livestock unit, which is 1000 lbs (454 kg) of live weight. County density level (in livestock units per area indicated): very high ≥ 13,200; high = 5200–13,200; moderate = 2000–5199; some ≤ 2000; none = 0. e 2005 US density of aquaculture production by number of reported farms, with percentage of farm being freshwater or saltwater indicated in blue pie charts. States without a pie chart contain fully freshwater operations (74,75)
The use of terminology in the field of drug resistance is not always consistent. In this paper, we define prophylaxis as the precautionary administration of antibiotics at levels predetermined to be therapeutic in the absence of disease (sometimes also termed “disease prevention”). “Sub/non-therapeutic” usage of antibiotics refers to the usage of these compounds for growth promotion at concentrations lower than the dosages required to effectively inhibit the growth of harmful bacteria.
AGRICULTURE VS. AQUACULTURE
Animal Farming and Antibiotic Usage
Over the last 60 years, worldwide production of swine, poultry, and cattle has grown continuously, with poultry outpacing the others (Fig. 1a). World aquaculture production only became a major animal production industry around 1985 (Fig. 1b). Before then, it was a largely non-commercial affair, representing a traditional way of life for centuries and often providing the sole reliable source of nourishment for its producers (23). Reasons for the recent growth of aquaculture include an increased demand for what is now recognized as a healthy protein choice, advances in seafood feed production, depleted wild fish stocks, and improvements in farming facilities enabling high-density farming (16,23). Total seafood production is now almost evenly split between wild-caught and farmed with the former steadily becoming stagnant in volume for the past two decades.
Fig. 1.
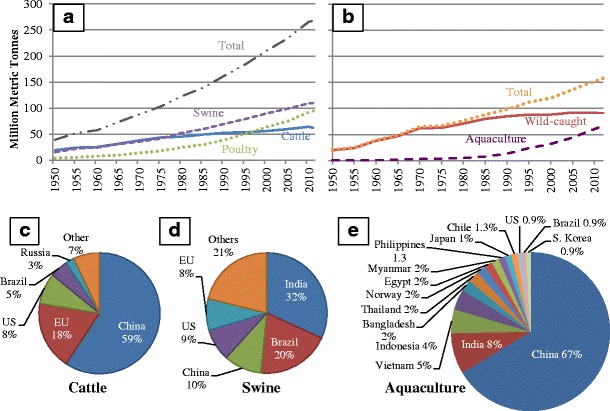
Animal production values in 1950–2011 and top producing countries of cattle, swine, and aquaculture. a 1950–2011 world production of swine (purple), cattle (blue), poultry (green), and total for all three (gray). b 1950–2011 world production of total seafood (orange), wild-caught seafood (red), and aquacultured seafood (purple). c Top 5 cattle-producing countries in 2013, counting only beginning stocks by head. d Top 5 swine-producing countries in 2013, counting only beginning stocks by head. e Top 15 aquaculture-producing countries in 2010 by percentage of total world production (20–22)
Figure 1c–e shows the top countries that produce cattle, swine, and aquacultured seafood. Perhaps the most important detail here is that the majority (>90%) of aquaculture occurs in Asia whereas agriculture’s concentrated animal feeding operations (CAFOs) that confine large populations of animals in buildings or feedlots (9) can be found distributed across several regions. Aquaculture facilities vary in design, with some keeping animals contained in ocean nets and others in secluded ponds or reservoirs. In Asia, aquaculture often links to the natural water environment (24). Many of these freshwater farms irrigate or flow through ponds that often tie with water reservoirs, lakes, and rivers (25). Brackish water aquaculture has more than doubled over the past decade and is primarily producing shrimp in coastal ponds and tanks (25).
Data regarding the classes and amounts of antibiotics used for agriculture and aquaculture depends on the region. For example, in 2003, salmon aquaculture in Chile used about 0.5 kg of antibiotic for each kg of salmon produced, whereas the amount in Norway was 0.002 kg (26). Figure 2 shows the most recent data available regarding antibiotic sales in the USA and the EU (25 countries). It is important to keep in mind that antibiotic sales do not equate to antibiotic usage, and usage information is not readily available or even reported in most cases. In both regions, the tetracycline class is the largest class of antibiotics sold, comprising about 40% of total sales. Similar reliable data from other regions of the world proved to be unavailable. Antibiotic sales and usage in India are not regulated (29,30). In China, two different reports of antibiotic usage were found, one stating the annual usage in animal feeds as 6000 tons (31) and the other stating over 8000 tons were used annually in animal husbandry (32). In Brazil, it has been reported that the most commonly used antibiotic classes are fluoroquinolones (34% of total antibiotics), ionophores (20%), and macrolides (10%) (33). Overall, worldwide usage of antibiotics in both animal production and human medicine has increased in recent decades; agriculture accounts for the majority of drugs used, and the mass of antibiotics used for the production of terrestrial food animals is estimated to exceed the amount of drugs used in aquaculture (34).
Fig. 2.
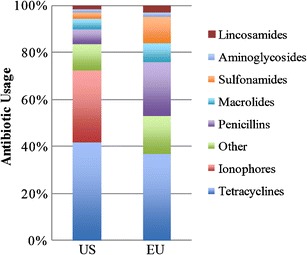
Antibiotic classes sold annually for use by animal production industries in the USA and EU (25 countries) in 2011. Total sold in the USA is approximately 13.5 million kg. Total volume sold in the EU is approximately 8.4 million kg (27,28)
How the antibiotics are used depends on the location and is not typically reported. Global trends in agriculture, aquaculture, and human medicine point to a steady increase in the usage of antibiotics. The most important delineation in usage is whether antibiotics are used for growth promotion. Among the top five cattle- and swine-producing countries (see Fig. 1c, d), only the EU has a confirmed ban on use of GPAs (6). In the USA, ionophores are used only in animals for growth promotion, a usage which is probably true in Brazil as well where ionophores are also reported to be commonly used (33). It should be noted that ionophores are typically reserved for animal usage and not for human usage, unlike the other antibiotic classes (35). These drugs can alter the stomach microorganisms in livestock to increase feed efficiency and energy extraction in the conversion of feeds (36). As Fig. 2 shows, ionophores are absent from EU antibiotic sales because of the 2006 ban on usage of GPAs in food animals (6,37). Although there is no law against GPA usage in the USA, the FDA has recently issued formal guidance to industry strongly urging drug companies to withdraw their GPAs and/or convert their usage guidelines to “therapeutic only” (38). In China and Russia, antibiotic usage in animals is restricted to using only non-human medicine drugs (39) and since 2003, several reforms have been attempted in China to improve food safety (40). However, reports of medically important antibiotics such as tetracyclines being used (41) and detections of illegal veterinary antibiotics like chloramphenicol in Chinese waters suggest that enforcement of the regulation is lax (32,42). Today, unlike in the EU (37), no veterinary prescriptions are required in China for the use of antibiotics in animals (37). One of the first steps that can be taken to ensure better monitoring of antibiotic usage is to require veterinary prescriptions when antibiotics are used in animals (5,8,37). This approach is being favored in India, as reported in 2011 in a national policy document outlining details to contain antibiotic resistance (30). Whereas data on actual implementation of such measures are scarce, the current trend in published papers indicates that many countries are taking steps to better regulate and report antibiotic usage.
The data presented above is for all antibiotics used in animal production, which includes aquaculture. Specific data for antibiotic usage patterns in aquaculture is available mostly in non-academic literature from the FAO and reports based on surveys as to what antibiotics are commonly used. In 2008, a review article identified three antibiotics to be in common use in aquaculture: oxytetracycline, oxolinic acid, and chloramphenicol (16). A more recent survey conducted by the FAO of 21 countries engaging in aquaculture confirmed the continued use of oxytetracycline as the top antibiotic applied in the treatment of disease in all major seafood species (43). Florfenicol and trimethoprim/sulfadiazine were next in line with respect to usage frequency. Oxytetracycline was also reported as the most widely used antibiotic for prophylactic treatment. A total of 24 countries were surveyed, including 11 of the top 15 aquaculture producers; the four countries missing from the survey were Egypt, Japan, South Korea, and Myanmar.
To assess the similarities and differences in antibiotics used for agriculture, aquaculture, and human health, the 2011 World Health Organization (WHO) list of important antimicrobials was compared to the above data (44). The WHO list is a categorization system of 260 antimicrobials created in an effort to contain antimicrobial resistance development and spread, and to reserve key drugs for human medicine (45). This list was intended for public health and animal health authorities as a reference for prioritizing risk assessment with respect to antibiotic resistance development. Two criteria are considered for inclusion on this list: first, the antibiotic must be the sole or one of a few limited available therapies to treat serious human diseases and second, it must be used to treat diseases caused either by (a) organisms that may be transmitted to humans from non-human sources or (b) human diseases caused by organisms that may acquire resistance genes from non-human sources. “Critically important” antimicrobials (n = 162) meet both criteria. “Highly important” antimicrobials (n = 88) meet one of the two criteria, and “important” antimicrobials (n = 10) meet neither criterion but are still recognized as drugs of importance in human medicine. In this paper, antibiotics from all three classes were screened for usage similarity with results shown in Fig. 3 (excluding antibiotics listed for veterinary use only). Six common classes of antibiotics (aminoglycosides, macrolides, penicillins, quinolones, sulfonamides, tetracyclines) on the WHO list are regularly used in agriculture and aquaculture. Of the 51 antibiotics reported to be used by the top agriculture and aquaculture countries, 39 are on the WHO list. Of these 39 antibiotics, only 2 are listed as important; the other 37 are either critically important or highly important. These numbers indicate that there is extreme crossover of antibiotic usage in human medicine and animal food production. It is important to note that data provided in Fig. 3 most likely underestimate the antibiotics actually used as this information is not reported and recorded systematically. The most important message from these data is that several of the same classes of antibiotics are used for both human medicine and animal production. This parallel antibiotic usage may be promoting similar resistance issues in both aquaculture and agriculture.
Fig. 3.
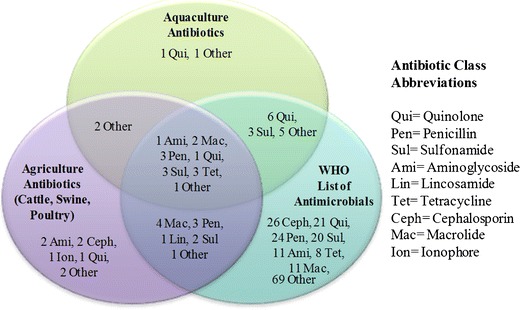
Common antibiotics used in aquaculture and agriculture and included in the 2011 WHO antimicrobials list. Diagram is displayed as number of antibiotics followed by antibiotic class. Aquaculture antibiotics include the ones reported to be used by top 15 aquaculture-producing countries. Agricultural antibiotics include the ones used in cattle, swine, and poultry farming. WHO antibiotics are the ones on the antimicrobial list in all three labels: “critically important,” “highly important,” and “important” (16,39,44,46–48). Aquaculture: qui—sarafloxacin; other—miloxacin. WHO: excludes antibiotics used solely for veterinary use. See reference (44) for full list. Agriculture: ami—apramycin*, neomycin; ceph—cefquinome*, ceftiofur*; ion—monensin; qui—marbofloxacin*; other—virginiamycin*, narasin. Agriculture and Aquaculture: other—tiamulin, ormetoprim. Agriculture and WHO: mac—kanamycin, oleandomycin, spectinomycin, streptomycin; pen—cloxacillin, dicloxacillin, oxacillin; lin—lincomycin; sul—sulfamethazine, sulfathiazole; other—tylosin. Aquaculture and WHO: qui—norfloxacin, ciprofloxacin, pefloxacin, oxolinic acid, nalidixic acid, flumequine; sul—sulfadiazine, sulfamerazine, sulfamethoxazole; other—chloramphenicol, colistin, florfenicol, furazolidone, thiamphenicol. Aquaculture, Agriculture, and WHO: ami—gentamicin; mac—spiramycin, erythromycin; pen—amoxicillin, ampicillin, penicillin G; qui—enrofloxacin; sul—sulfadimethoxine, sulfadimidine, sulfapyridine; tet—chlortetracycline, oxytetracycline, tetracycline; other—trimethoprim. *These agriculture antibiotics are included in the WHO list but are reserved for veterinary use only
Foodborne Pathogens and Antibiotic Resistance Mechanisms
As shown in the previous section, the antibiotics used in agriculture and aquaculture span many of the same antibiotic classes. Thus, as agriculture has been using antibiotics for much longer than aquaculture has, we ask whether the same resistance mechanisms exist in both or if the latter is promoting the development of new ones. In this section, we identified reported bacterial pathogens from meat and seafood, characterized how resistance may develop, and looked for resistance development pathways in agriculture and aquaculture. To relate the isolated strains to human health risks, we focused our identified strains on zoonotic foodborne pathogens.
The most prevalent and serious emerging pathogens in agricultural meat products are Campylobacter jejuni, Salmonella enterica serovar Typhimurium DT104, and E. coli O157:H7 (49). Often, these products are contaminated during handling and processing in the CAFOs where the animals are slaughtered. Pathogens present in feces and/or animal hides often are transferred to edible fractions or spread as aerosols produced during dehiding, evisceration, and carcass splitting (49). In aquaculture, foodborne diseases are not as well documented, but the literature shows that Salmonella and Vibrio spp. are likely to be the most common pathogens detected in seafood, with Listeria monocytogenes, Aeromonas, and Clostridium spp. becoming emerging threats (50–52). Cases of human infections from seafood most often arise from handling, such as contact with the wash water or through processing in the food industry, and by oral consumption of infected fish or related products (53).
Aside from the potential to cause infections in the people that are exposed, these bacteria, along with others that are less often found, are capable of developing and spreading antibiotic resistance. In both agriculture and aquaculture, development/persistence of resistance can occur when these bacteria are exposed to sub-therapeutic concentrations of antibiotics (54). In terrestrial agriculture, this exposure occurs when antibiotics used for growth promotion are added as a CAFO feed additive over a period of time for fattening and for increased feed efficiency (55). In the USA, about 55% of all antibiotic usage in cattle is during the feedlot stage of cattle production (56). The feedlot stage is when the animals weigh in between 700 and 1200 lbs, with average antibiotic dosages estimated at 80 mg/animal/day for about 120 days (56). This means that these cattle are subject to sub-therapeutic antibiotic concentrations for almost one-third of a year.
The commonly cited rationale behind using GPAs is an economic benefit, with average increases in animal mass reported in the range of 4 to 8% (57). Other advantages reported in the literature include an improvement of animal health, decreases of bacterial contamination in animal products, a reduction of adverse environmental impacts such as greenhouse gas emissions, and prevention of water eutrophication (46). However, an economic analysis of using antibiotics in commercial broiler chickens for growth promotion showed that the net economic effect of using GPAs is negative, with an estimated lost value of $0.0093 per chicken or about 0.45% of the total cost; the positive production changes associated with antibiotic use reportedly were insufficient to offset the cost of more expensive feed (58). The latter study did not consider the potential benefits of GPA removal in terms of preventing external costs from medical and public health burdens resulting from antibiotic-resistance infections. Considering such would further increase the cost incurred by the use of antibiotics. No other such analysis is available in the literature, and more are needed to assess the economic impact of using GPAs.
In aquaculture, sub-therapeutic exposure concentrations are mostly encountered after the prophylactic use of antibiotics. Unconsumed fish feed and feces can contain residues that persist in the surrounding environment (8), allowing for bacteria to be exposed to low concentrations that can select for resistance. The exposed bacteria then can spread ARGs to the natural microbiota in nearby ecosystems, which may pose a greater threat than low levels of residues, as resistance genes may persist for decades due to the marginal impact of gene maintenance on fitness (7). As previous studies suggest that the environment already harbors ARGs (59), the mixing of residues that is made easier via the water pathway make aquaculture more likely to spread contaminants compared to agriculture. In many cases, these compounds are only slightly transformed, or even unchanged and conjugated to polar molecules, allowing for easier dispersion in water (47). The added potential impacts on the environment include direct antibiotic toxicity in natural microbiota, flora, and fauna have been voiced in the literature (24,60). However, not all detected antibiotic concentrations are environmentally relevant enough to negatively impact invertebrates and fish (61,62). These reports in the literature indicate that the risks associated with antibiotic residues in aquaculture may vary depending on the situation and that there is a gap in knowledge regarding residues and their effects on resistance development. It must be noted that the usage of antibiotics in animal production has provided many benefits as well. Antibiotics have allowed for animal health to be improved, increasing economic gain for the farmers, as pathogens are significantly reduced when antibiotics are utilized (46,55). However, despite these benefits, we cannot ignore the risks and potential negative human health and environmental impacts.
To compare the potential for agriculture and aquaculture to be developing the same mechanisms of antibiotic resistance, we reviewed reports in the literature of bacterial isolates resistant to commonly used antibiotics in these food production industries. In agriculture, four common resistance mechanisms have been identified (Fig. 4). These categories are presented very broadly to be more inclusive; “altered intracellular target” can mean any mutation that allows for ribosomal active site changes or an RNA polymerase mutation that leads to reduced binding of the antibiotic (63). Antibiotics in many classes can be ineffective against these mechanisms; both macrolides and penicillins can be pumped out of the bacterial cell by efflux pumps, for example. In other words, co-resistance can occur with any of these mechanisms. The zoonotic pathogens of concern listed in Fig. 4 are typical examples of bacteria exhibiting the common resistance mechanisms. For example, Pseudomonas aeruginosa is well known for expressing MDR efflux pumps (64). Examples of these pathogens isolated from agriculture that have been molecularly shown to harbor each resistance mechanism’s ARGs are also shown in Fig. 4. Many are resistant to several antibiotics, but ones commonly used in agriculture are noted.
Fig. 4.
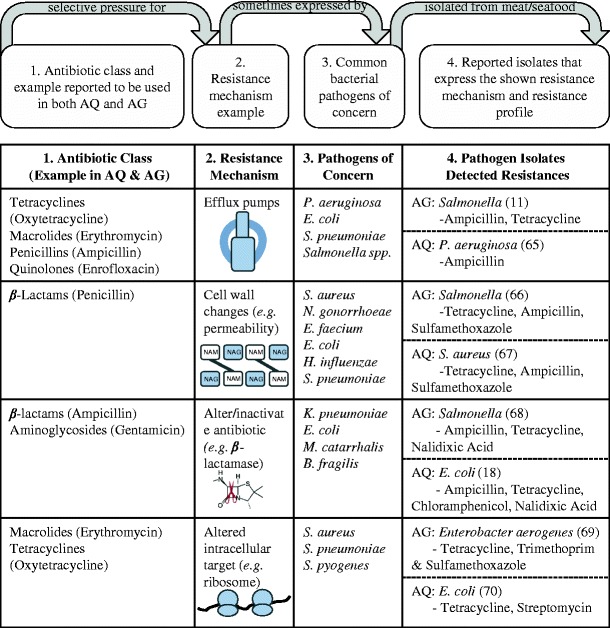
Resistance mechanism development in agriculture and aquaculture. Top panel explains how each row exhibits a resistance mechanism. Each row in chart is an example via a different resistance mechanism. Each resistance mechanism can allow bacteria to be resistant to many classes of antibiotics. Antibiotics reported to be used in agriculture and aquaculture (column 1) can select for resistance mechanisms (column 2) that are sometimes expressed by common pathogens (column three), such as ones listed here as examples. Column 4 shows bacterial isolates reported in the literature that are resistant to the stated antibiotics and have been genetically shown to express the resistance mechanism in that row. AG indicates isolates from terrestrial agriculture, AQ indicates isolates from aquaculture. Reference numbers for the publications are noted with the bacterial strain. Strain genera are as follows: P, Pseudomonas; E, Escherichia; S, Streptococcus pneumoniae/pyogenes or Staphylococcus aureus; N, Neisseria; E, Enterococcus; H, Haemophilus; K, Klebsiella; M, Moraxella; and B, Bacillus. Resistance mechanisms from Giedraitiene et al., 2011 (63)
The same four mechanisms were also found to be associated with aquaculture. Zoonotic pathogens resistant to aquaculture antibiotics have been isolated from seafood containing all of the four resistance mechanisms (18,65–70). Some of these microbes are relevant pathogens in agricultural products as well (i.e., Salmonella). Tetracycline resistance is the most commonly seen resistance among bacterial isolates from aquaculture; a recent study showed that as the number of resistance reports increased, so did the incidence of tetracycline resistance (71). Among 23 publications on drug-resistant bacteria isolated from seafood for human consumption, 21 reported resistance to at least one antibiotic belonging to the class of tetracyclines. This previous study only reported publications from 2003 to 2013 and limited the search to bacterial strains from seafood products only (excluding aquaculture facilities, the surrounding water, etc.). If the exclusions were not applied, the number of resistant strains isolated would most likely increase. The major issue with detections of specific resistance determinants such as efflux pumps is the ability of these genes to be spread via horizontal gene transfer, possibly to bacteria that are even more pathogenic to humans. In both aquaculture and agriculture, native environmental bacteria are mixed with zoonotic bacteria, providing a situation where resistance can develop, spread, and linger among them. The biggest human health risk is coming into contact with pathogenic bacteria that are also resistant to multiple antibiotics, especially ones from different classes. As noted above, several such cross-resistant isolates have already been found in terrestrial agriculture and aquaculture. These data suggest that identical resistance mechanisms are being promoted and developed in both agriculture and aquaculture. Alarmingly, some of the same pathogens have been isolated from both seafood and meat. Different strains of MDR Salmonella were isolated containing the same resistance genes from both shellfish and pork (68). Similarly, E. coli strains isolated from pork, beef, poultry, and fish were resistant to several tetracyclines (72). This review only focuses on human health risks posed by edible animal products themselves, but it should be noted that additional risks result from the processing and handling of all materials involved, such as the disposal of animal feces containing resistant bacteria (73). The studies available and examined for this work show that the same resistance mechanisms are being promoted in agricultural and aquacultural environments (including processing and handling), thereby allowing for resistance to develop and spread via food and the environment, resulting in significant human health threats.
CASE STUDY: US AGRICULTURE AND AQUACULTURE
Animal Farming and Antibiotic Usage
The USA is one of the largest producers of agriculture in the world, ranking (counting beginning year stock numbers) fourth in 2013 cattle production at approximately 89 million heads and third in swine production at approximately 66 million heads (20). As seen in Fig. 5, the cattle and swine industries dominate over the poultry industry, with much higher densities reported for many of the US counties and states shown. These data (Fig. 5a–d) are from the 2007 USDA Agricultural Census, which conducts a new survey every 5 years (the 2012 report is expected to be released within the next year). Shown at the county level, the majority of the US cattle, swine, and poultry farming is done in the Great Plains states and along the west border of the Mississippi river. These geographic locations differ, as one would expect, from the locales of aquaculture, which are largely situated near the ocean and along the Gulf of Mexico (Fig. 5e).
Aquaculture can be divided into freshwater and saltwater culture (Fig. 5e). By value of production, saltwater and freshwater aquaculture in the USA contributed approximately $800 and $550 million, respectively, in 2011 (76). About two thirds by value of saltwater (or marine) aquaculture consists of mollusks such as oysters, clams, and mussels (77). This type of aquaculture takes place in cages that are located on the ocean floor or suspended in water column (78). The majority of this farming is done in the northwest region of the USA (see Fig. 5e for blue pie chart inserts) and in WA and OR. Freshwater aquaculture is predominated by trout, catfish, and tilapia (76). Figure 5 only shows the density of aquaculture farms contained in each state based on the 2005 Agricultural Census, but these numbers do not necessarily reflect the amount of production. The top 5 aquaculture states by value in 2005 were as follows: MS, AR, AL, LA, and WA, together producing about a half a billion dollars worth of products, which is about half of the total US value produced (79).
As production of cattle, poultry, and swine expanded to large-scale productions over the last half-century, the usage of antibiotics in agriculture has also become the norm and has greatly increased. Based off of FDA reports, we calculated that in 2011, 80% of the antibiotics sold by weight were designated for animal usage (27,80). This percentage was calculated from the annual FDA released summary report on antimicrobials sold/distributed for food-producing animals (13.5 million kg) and from the FDA drug use review, where sales numbers for human medicine usage (3.29 million kg) were obtained (27). Similar numbers have previously been reported by several other NGOs, including the Natural Resources Defense Council (81,82), the UCS, and the Center for Science in the Public Interest, among others (Table I). These organizations primarily based their estimates on annual FDA summary reports for antimicrobials. However, the numbers reported by the Animal Health Institute (AHI) are much different, resembling those reported by the US Farmers and Ranchers Alliance, another entity representing the industry. The AHI estimates that only about 35% of antibiotics in the USA is used in animals for food production (56).
Table I.
Total Reported US Antibiotic Usage (in Million kg) by Animal Industry and for Human Health
| Reporting source | Year reporteda | Total amt. sold for food production animals (million kg) |
Reported sub-therapeutic usageb, million kg (% of total animal amt.) | Total human usage (million kg) | % of total AB sold for animals | Reference |
|---|---|---|---|---|---|---|
| AHI | 2001 | 8.1 | 1.4 (18%) | 14.6 | 35% | (56) |
| UCS | 2001 | 12.5 | 11 (88%) | 3 | 70% | (56) |
| USFRA | 2007 | NR | (13%) | NR | NR | (83) |
| FDA; Rep. Slaughter | 2009 | 13.1 | NR | 3.3 | 80% | (84,85) |
| CSPI, NRDC, this review | 2011 | 13.5 | NR | 3.3 | 80% | (27,80–82) |
NR not reported in publication, amt amount, AB antibiotic, AHI Animal Health Institute, UCS Union of Concerned Scientists, USFRA US Farmers and Ranchers Association, FDA Food and Drug Administration, CSPI Center for Science in the Public Interest, NRDC National Resources Defense Council
aYear reported does not always correspond to year data that was collected/formulated
bReported sub-therapeutic usage, does not differentiate between amounts of antibiotics used for prophylaxis, metaphylaxis, growth promotion, or feed efficiency
A second data discrepancy requiring more transparency is what antibiotics are annually used in animal production as well as their frequency of usage. This reporting is difficult in part not only because animal producers are not required to report this information but also because “non-therapeutic” or “sub-therapeutic” usage of antibiotics can mean different things. As the FDA allows antibiotics to be used for growth promotion, feed efficiency, disease, and metaphylaxis, it is hard to specifically enumerate the amount of antibiotics used in each of these categories (86). Thus, it must be noted that the numbers reported in Table I column “Reported Sub-Therapeutic Usage” are only estimates by a few organizations and that these numbers may not reflect the situation accurately. As the FDA is now required to report antimicrobial usage numbers, the next step would be to report what the antibiotics are used for. Recent FDA/CVM guidance now provides recommendations for industry to voluntarily align their products with FDA #209 (87). This guidance includes two principles: (1) limiting medically important antimicrobials to uses in food-producing animals that are considered necessary for assuring animal health and (2) limiting these usages to only those with veterinary oversight or consultation (87). These guidelines encourage better documentation and usage practices.
With regard to aquaculture production, the USA produces a relatively low amount compared to other countries. This is partly due to the fact that China provides close to 70% of total aquaculture products, as well as the fact that the USA imports about 90% of its seafood. There is a major effort in place to expand the aquaculture industry in the USA so that the reliance on imported fish is reduced. The USA is a leading global consumer of fish and fishery products, and yet only about 5–7% of the national supply comes from its aquaculture industry (78). It has been estimated that up to 433,000 lbs (approximately 196,000 kg) of antibiotics were used in 2002 in US aquaculture (88). These data indicates that the vast majority (approximately 80%) of animal antibiotics used in the USA are used in agricultural animal production (see Table I). Antibiotics do not improve growth or feed efficiency in fish like they have been reported to do in certain livestock (89). The usage of vaccines has also greatly limited antibiotic usage in the USA, and at present, only three antibiotics are registered and sold for disease control in fish: oxytetracycline, florfenicol, and sulfadimethoxine/ormetoprim (90). Thus, it can be assumed that the majority of the antibiotics used for food-producing animals in the USA is in livestock, which is most likely the case in other countries as well (34).
Foodborne Pathogens and Detected Resistance
In the USA, foodborne pathogens of concern in agricultural meats are E. coli, Salmonella, and Campylobacter. The NARMS Retail Meat Annual Report of 2011 identifies E. coli as the most commonly detected bacterium in all retail meat products (19). Out of 1920 retail meats tested in 2011, 55.7% were found to be culture positive for E. coli. Although no isolates were resistant to ciprofloxacin, some isolates were shown to be resistant to third-generation cephalosporins, and co-resistances to other β-lactam compounds were reported. For Salmonella, the three serotypes most commonly detected were Typhimurium, Kentucky, and Heidelberg. Resistance to ampicillin rose from 17% of isolates in 2002 to 41% in 2011. A similar trend was seen for third-generation cephalosporins (from 10 to 34%). Most concerning is the fact that 45% of retail chicken harbored isolates featuring resistance to three or more classes of antimicrobials. Approximately 27% showed resistance to at least five classes. With regard to Campylobacter, the species jejuni and coli were most commonly detected. The majority of the isolates (90%) were from retail chicken. Although macrolide resistance has remained low, tetracycline resistance increased by about 10% of isolates for both species from 2010 to 2011. MDR was low in Campylobacter, as only nine out of 634 isolates were resistant to at least three antimicrobial classes. Enterococcus (faecalis and faecium) is used as a sentinel for antibiotic selection pressures by anti-gram-positive antibiotics. Vancomycin resistance was not detected, and streptogramin resistance has significantly decreased in retail chicken from 56% of isolates in 2002 to 27% in 2011. Overall, it seems that most of the risk is from gram-negative bacteria and gram-positive bacteria pose a lesser risk to humans in retail meats. In reference to Fig. 4’s resistance pipelines, these data support the notion that feeding food production animals with antibiotics like ampicillin and tetracycline may contribute to the increased drug resistances observed in the USA as shown in NARMS data (19).
In US aquaculture, as most of the seafood is imported, foodborne pathogens of concern are often ones that are considered food safety risks overseas as well. In 2004, it was reported that eating contaminated seafood resulted in about 15% of the reported foodborne outbreaks in the USA. This is a greater percentage than was found for either meat or poultry, which are consumed at volumes eight and six times higher than those of seafood (91). Our literature search shows that Vibrio spp. and Salmonella are the most commonly isolated zoonotic pathogens from seafood. Specifically, Vibrio vulnificus, followed by parahaemolyticus, are the two most important Vibrio spp. noted, causing gastroenteritis that may lead to septicemia (92). Vibrio spp. are a natural inhabitant of many aquatic organisms and are the leading cause of seafood-related deaths in the USA (93). Mostly a concern in oysters, Vibrio spp. have been isolated and characterized in several studies (94–96). Antibiotic residue in bivalves is not a significant concern because they are not fed feed as they are filter feeders that survive on particles in the water (89). Salmonella are an issue in almost all types of seafood, and species distribution is broad, with frequently reported serotypes including Weltevreden, Senftenberg, Lexington, and Paratyphi-B (97). Mostly of human origin, Salmonella also causes gastroenteritis, and primarily contaminates seafood during processing (98). This is similar to agricultural meat products, where Salmonella is also an important foodborne pathogen. Recent seafood outbreaks include three in 2011 where a total of 168 cases resulted in 48 hospitalizations and 1 death (82). The Salmonella isolated in the latter study were all resistant to ampicillin, tetracycline, and amoxicillin/clavulanic acid, all of which are on the WHO list. These data suggest that resistance in zoonotic pathogens isolated from commonly eaten meats and seafoods is prevalent and a growing concern for the food industry.
CONCLUSIONS
Swine, cattle, and poultry agriculture all have relied on antibiotic usage for over half a century, promoting the development and spread of antibiotic resistance. As aquaculture continues to grow, the knowledge gap regarding how antibiotic usage, development of resistance mechanisms, and human health risks connect with each other must be filled with scientific research and results. Here, we present data showing that agriculture and aquaculture share many similarities, from the antibiotics used to the resistance mechanisms shared by the zoonotic pathogens corresponding to these two important food production sectors. The bacteria isolated from both meat and seafood have been reported to display resistance to antibiotics commonly applied in animal production. From the data gathered here, it is concluded that the recent growth of aquaculture is contributing to the development of the same resistance mechanisms also seen in agricultural production. The usage of antibiotics provides selective pressure that can accelerate ARG development and spread. As zoonotic pathogens have been isolated exhibiting resistance mechanisms known to be effective against multiple antibiotics, co-resistance is increasingly becoming a major concern. The lack of data and discrepancies in existing data regarding antibiotic usage contribute to the fact that it is challenging at present to accurately determine the magnitude of influence both aquaculture and agriculture has on resistance development. However, as water provides a constant and facile mechanism for dispersal of drug residues, microbial pathogens, and resistance genes, aquaculture will continue to pose a threat that may increase as the demand for seafood increases.
ELECTRONIC SUPPLEMENTARY MATERIAL
(DOCX 206 kb)
ACKNOWLEDGMENTS
The authors would like to thank Patty Lovera of the Food and Water Watch for graciously letting us use the density maps for US agriculture of cattle, swine, and poultry. We would also like to thank Diane Windham, Kevin Amos, and Barbara Seekins of NOAA for providing resources and email correspondences regarding US aquaculture antibiotic usage and census data. Thanks to Mae Wu of the NRDC, Margaret Mellon (Science Policy Consultant), David Love (Johns Hopkins University), Keeve Nachman (Johns Hopkins University), and Steve Roach (Food Animals Concerns Trust) for their communications and help with antibiotics usage data and references. This study was supported in part by the Piper Charitable Trust and by the National Institute of Environmental Health Sciences grants R01ES015445, R01ES020889 and their respective supplements. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH).
REFERENCES
- 1.Davies J. Where have all the antibiotics gone? Can J Infect Dis Med Microbiol. 2006;17(5):287. doi: 10.1155/2006/707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Kinkelaar D, Huang Y, Li Y, Li X, Wang HH. Acquired antibiotic resistance: are we born with it? Appl Environ Microbiol. 2011;77(89):7134–41. doi: 10.1128/AEM.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–46. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Antimicrobial Resistance—Global Report on Surveillance. World Health Organization. 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf. Accessed 24 Nov 2014.
- 5.Mathew AG, Cissell R, Liamthong S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog Dis. 2007;4(89):115–33. doi: 10.1089/fpd.2006.0066. [DOI] [PubMed] [Google Scholar]
- 6.EU. Ban on antibiotics as growth promoters in animal feed enters into effect. European Union. 2005. http://europa.eu/rapid/press-release_IP-05-1687_en.htm. Accessed 24 Nov 2014.
- 7.Pruden A, Larsson DGJ, Amezquita A, Collignon P, Brandt KK, Graham DW, et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect. 2013;121(8):878–85. doi: 10.1289/ehp.1206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabello FC. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol. 2006;8(7):1137–44. doi: 10.1111/j.1462-2920.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 9.Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–69. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 10.Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo. 2014;56(89):341–6. doi: 10.1590/S0036-46652014000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ta YT, Nguyen TT, To PB, Pham DX, Le HTH, Thi GN, et al. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam. J Food Prot. 2014;77(89):57–66. doi: 10.4315/0362-028X.JFP-13-221. [DOI] [PubMed] [Google Scholar]
- 12.Asadpour L. Antibacterial drug resistance patterns in poultry isolated enterococci. Afr J Microbiol Res. 2012;6(29):5857–61. [Google Scholar]
- 13.Hsu JT, Chen CY, Young CW, Chao WL, Li MH, Liu YH, et al. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J Hazard Mater. 2014;277:34–43. doi: 10.1016/j.jhazmat.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Sun J, Liu BT, Zhao DH, Ma J, Deng H, et al. Quantification of lincomycin resistance genes associated with lincomycin residues in waters and soils adjacent to representative swine farms in China. Front Microbiol. 2013;4:364. doi: 10.3389/fmicb.2013.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp CW, Dolfing J, Ehlert PAI, Graham DW. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ Sci Technol. 2010;44(89):580–7. doi: 10.1021/es901221x. [DOI] [PubMed] [Google Scholar]
- 16.Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, et al. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int. 2008;34(8):1215–26. doi: 10.1016/j.envint.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Shah SQA, Cabello FC, L'Abee-Lund TM, Tomova A, Godfrey HP, Buschmann AH, et al. Antimicrobial resistance and antimicrobial resistance genes in marine bacteria from salmon aquaculture and non-aquaculture sites. Environ Microbiol. 2014;16(5):1310–20. doi: 10.1111/1462-2920.12421. [DOI] [PubMed] [Google Scholar]
- 18.Ryu SH, Park SG, Choi SM, Hwang YO, Ham HJ, Kim SU, et al. Antimicrobial resistance and resistance genes in Escherichia coli strains isolated from commercial fish and seafood. Int J Food Microbiol. 2012;152(1–2):14–8. doi: 10.1016/j.ijfoodmicro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.CVM. 2011 Retail Meat Report. National Antimicrobial Resistance Monitoring System. Center for Veterinary Medicine, Food and Drug Administration. 2011. http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm059103.htm. Accessed 24 Nov 2014.
- 20.USDA. Production, supply and distribution online. United States Department of Agriculture. Foreign Agricultural Service. 2014. http://apps.fas.usda.gov/psdonline/. Accessed 4 August 2014.
- 21.FAO. FAOSTAT. Food and Agricultural Organization of the United Nations. 2014. http://www.faostat.fao.org. Accessed 24 Nov 2014.
- 22.FAO. The state of world fisheries and aquaculture, 2012. Food and Agriculture Organization of the United Nations. Rome, Italy. 2012. http://www.fao.org/docrep/016/i2727e/i2727e.pdf. Accessed 24 Nov 2014.
- 23.Cole DW, Cole R, Gaydos SJ, Gray J, Hyland G, Jacques ML, et al. Aquaculture: environmental, toxicological, and health issues. Int J Hyg Environ Health. 2009;212(89):369–77. doi: 10.1016/j.ijheh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Rico A, Satapornvanit K, Haque MM, Min J, Nguyen PT, Telfer TC, et al. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev Aquac. 2012;4(89):75–93. doi: 10.1111/j.1753-5131.2012.01062.x. [DOI] [Google Scholar]
- 25.FAO. FishStat fishery statistical collections: aquaculture production (1950–2008). Food and Agricultural Organization of the United Nations, Rome. 2010. http://www.fao.org/fishery/topic/16073/en. Accessed 24 Nov 2014.
- 26.Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henriquez L. Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manag. 2009;52(5):243–9. doi: 10.1016/j.ocecoaman.2009.03.002. [DOI] [Google Scholar]
- 27.FDA. 2011 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Center for Veterinary Medicine. 2011. http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM338170.pdf. Accessed 24 Nov 2014.
- 28.EMA. European Medicine Agency. European surveillance of veterinary antimicrobial consumption, 2013. “Sales of Veterinary Antimicrobials in 25 EU/EEA Countries in 2011” (EMA/236501/2013). 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/10/WC500152311.pdf. Accessed 24 Nov 2014.
- 29.Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JPS, Gupta U, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134(3):281–94. [PMC free article] [PubMed] [Google Scholar]
- 30.NICD. National Policy for Containment of Antimicrobial Resistance India 2011. National Centre for Disease Control, Directorate General of Health Services, Ministry of Health and Family Welfare Nirman Bhawan, New Delhi. 2011. http://nicd.nic.in/ab_policy.pdf. Accessed 24 Nov 2014.
- 31.Zhao L, Dong YH, Wang H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci Total Environ. 2010;408(5):1069–75. doi: 10.1016/j.scitotenv.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Chen YS, Zhang HB, Luo YM, Song J. Occurrence and dissipation of veterinary antibiotics in two typical swine wastewater treatment systems in east China. Environ Monit Assess. 2012;184(89):2205–17. doi: 10.1007/s10661-011-2110-y. [DOI] [PubMed] [Google Scholar]
- 33.Regitano JB, Leal RMP. Performance and environmental impact of antibiotics in animal production in Brazil. Rev Bras Cienc Solo. 2010;34(3):601–16. doi: 10.1590/S0100-06832010000300002. [DOI] [Google Scholar]
- 34.Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24(89):718–33. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman HD, Jeffers TK, Williams RB. Forty years of monensin for the control of coccidiosis in poultry. Poult Sci. 2010;89(9):1788–801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- 36.Coffman J. The use of drugs in food animals: benefits and risks: CAB International; 1999. [PubMed]
- 37.Maron DF, Smith TJS, Nachman KE. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization and Health. 2013;9(48). [DOI] [PMC free article] [PubMed]
- 38.FDA. #213 Guidance for Industry. US Department of Health and Human Services Food and Drug Administration Center for Veterinary Medicine. 2013. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf. Accessed 25 Sep 2014 .
- 39.Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65(5):725–59. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Broughton EI, Walker DG. Policies and practices for aquaculture food safety in China. Food Policy. 2010;35(5):471–8. doi: 10.1016/j.foodpol.2010.05.007. [DOI] [Google Scholar]
- 41.Jin S. Regulation, realities and recommendation on antimicrobial use in food animal production in China. In: the Medical Impact of the Use of Antimicrobials in Food Animals. WHO, Geneva (Section 2.3.4). 1997.
- 42.Hu XG, Zhou QX, Luo Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut. 2010;158(9):2992–8. doi: 10.1016/j.envpol.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Alday-Sanz V, Corsin F, Irde E, Bondad-Reantaso MG. Survey on the use of veterinary medicines in aquaculture. In M.G. Bondad-Reantaso, J.R. Arthur & R.P. Subasinghe, eds. Improving biosecurity through prudent and responsible use of veterinary medicines in aquatic food production, pp. 29–44. FAO Fisheries and Aquaculture Technical Paper No. 547. Rome, FAO, 207 pp. 2012.
- 44.WHO. Critically important antimicrobials for human medicine. 3rd Revision 2011. World Health Organization. Geneva, Switzerland. 2012. http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf. Accessed 26 Sep 2014.
- 45.WHO. Critically important antimicrobials for human medicine: categorization for the development of risk management strategies to contain antimicrobial resistance due to non-human antimicrobial use. Report of the Second WHO Expert Meeting. 2007;Copenhage, 29–31 May 2007. http://www.who.int/foodborne_disease/resistance/antimicrobials_human.pdf. Accessed 24 Nov 2014.
- 46.Hao HH, Cheng GY, Iqbal Z, Ai XH, Hussain HI, Huang LL, et al. Benefits and risks of antimicrobial use in food-producing animals. Frontiers in Microbiology. 2014;5(288). [DOI] [PMC free article] [PubMed]
- 47.Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic. 2008;8(89):1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- 48.Yuan X, Chen W. Use of Veterinary Medicines in Chinese Aquaculture: Current Status. In M.G. Bondad-Reantaso, J.R. Arthur and R.P. Subasinghe, eds. Improving Biosecurity Through Prudent and Responsible Use of Veterinary Medicines in Aquatic Food Production, pp. 51–67. FAO Fisheries and Aquaculture Technical Paper No 547. 2012 (Rome, FAO. 207 pp.).
- 49.Mor-Mur M, Yuste J. Emerging bacterial pathogens in meat and poultry: an overview. Food Bioproc Technol. 2010;3(89):24–35. doi: 10.1007/s11947-009-0189-8. [DOI] [Google Scholar]
- 50.Feldhusen F. The role of seafood in bacterial foodborne diseases. Microb Infect. 2000;2(13):1651–60. doi: 10.1016/S1286-4579(00)01321-6. [DOI] [PubMed] [Google Scholar]
- 51.Herrera FC, Santos JA, Otero A, Garcia-Lopez ML. Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain. J Appl Microbiol. 2006;100(3):527–36. doi: 10.1111/j.1365-2672.2005.02848.x. [DOI] [PubMed] [Google Scholar]
- 52.Normanno G, Parisi A, Addante N, Quaglia NC, Dambrosio A, Montagna C, et al. Vibrio parahaemolyticus, Vibrio vulnificus and microorganisms of fecal origin in mussels (Mytilus galloprovincialis) sold in the Puglia region (Italy) Int J Food Microbiol. 2006;106(89):219–22. doi: 10.1016/j.ijfoodmicro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Novotny L, Dvorska L, Lorencova A, Beran V, Pavlik I. Fish: a potential source of bacterial pathogens for human beings. Vet Med. 2004;49(9):343–58. [Google Scholar]
- 54.Sapkota AR, Lefferts LY, McKenzie S, Walker P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ Health Perspect. 2007;115(5):663–70. doi: 10.1289/ehp.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, et al. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J Antimicrob Chemother. 2004;53(89):28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- 56.Mellon M, Benbrook C, Benbrook KL. Hogging It. Estimates of Antimicrobial Abuse in Livestock. Union of Concerned Scientists Cambridge, MA. 2001. http://www.ucsusa.org/assets/documents/food_and_agriculture/hog_front.pdf. Accessed 4 August 2014.
- 57.Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev. 2003;16(89):175–88. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham JP, Boland JJ, Silbergeld E. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep. 2007;122(89):79–87. doi: 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marti E, Variatza E, Balcazar JL. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014;22(89):36–41. doi: 10.1016/j.tim.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Baquero F, Martinez JL, Canton R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19(3):260–5. doi: 10.1016/j.copbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Zounkova R, Kliemesova Z, Nepejchalova L, Hilscherova K, Blaha L. Complex evaluation of toxicity and genotoxicity of antimicrobials oxytetracycline and flumequine used in aquaculture. Environ Toxicol Chem. 2011;30(5):1184–9. doi: 10.1002/etc.486. [DOI] [PubMed] [Google Scholar]
- 62.Park S, Choi K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology. 2008;17(6):526–38. doi: 10.1007/s10646-008-0209-x. [DOI] [PubMed] [Google Scholar]
- 63.Giedraitiene A, Vitkauskiene A, Naginiene R, Pavilonis A. Antibiotic resistance mechanisms of clinically important bacteria. Med Lith. 2011;47(3):137–46. [PubMed] [Google Scholar]
- 64.Nikaido H, Pages J-M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2012;36(89):340–63. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uddin GMN, Larsen MH, Guardabassi L, Dalsgaard A. Bacterial flora and antimicrobial resistance in raw frozen cultured seafood imported to Denmark. J Food Prot. 2013;76(3):490–9. doi: 10.4315/0362-028X.JFP-12-402. [DOI] [PubMed] [Google Scholar]
- 66.Chen S, Zhao SH, White DG, Schroeder CM, Lu R, Yang HC, et al. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl Environ Microbiol. 2004;70(1):1–7. doi: 10.1128/AEM.70.1.1-7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng HC, Zhang ZG, Chen MR, Su YY, Li L, Miyoshi S, et al. Characterization and horizontal transfer of class 1 integrons in Salmonella strains isolated from food products of animal origin. Int J Food Microbiol. 2011;149(3):274–7. doi: 10.1016/j.ijfoodmicro.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Van TTH, Moutafis G, Tran LT, Coloe PJ. Antibiotic resistance in food-borne bacterial contaminants in Vietnam. Appl Environ Microbiol. 2007;73(24):7906–11. doi: 10.1128/AEM.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang XB, Shi L. Distribution of tetracycline and trimethoprim/sulfamethoxazole resistance genes in aerobic bacteria isolated from cooked meat products in Guangzhou, China. Food Control. 2013;30(1):30–4. doi: 10.1016/j.foodcont.2012.06.042. [DOI] [Google Scholar]
- 70.Nawaz M, Khan SA, Tran Q, Sung K, Khan AA, Adamu I, et al. Isolation and characterization of multidrug-resistant Klebsiella spp. isolated from shrimp imported from Thailand. Int J Food Microbiol. 2012;155(3):179–84. doi: 10.1016/j.ijfoodmicro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Done HY, Halden RU. Reconnaissance of 47 antibiotics and associated microbial risks in seafood sold in the United States. J Hazard Mater. 2015;282:10–7. doi: 10.1016/j.jhazmat.2014.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koo HJ, Woo GJ. Distribution and transferability of tetracycline resistance determinants in Escherichia coli isolated from meat and meat products. Int J Food Microbiol. 2011;145(2–3):407–13. doi: 10.1016/j.ijfoodmicro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Tadesse DA, Bahnson PB, Funk JA, Morrow WEM, Abley MJ, Ponte VA, et al. Yersinia enterocolitica of porcine origin: carriage of virulence genes and genotypic diversity. Foodborne Pathog Dis. 2013;10(89):80–6. doi: 10.1089/fpd.2011.1120. [DOI] [PubMed] [Google Scholar]
- 74.Food and Water Watch. Factory Farm Map. Food and Water Watch. 2007. http://www.factoryfarmmap.org. Accessed 24 Nov 2014.
- 75.Department of Agriculture. Census of Aquaculture Publication. “Freshwater and Saltwater Acres Used for Aquaculture Production, by State and United States: 2005 and 1998”. United States Department of Agriculture. 2005. http://www.agcensus.usda.gov/Publications/2002/Aquaculture/. Accessed 24 Nov 2014.
- 76.NOAA. U.S. Commercial Fishery Landings. Commercial Fisheries Statistics. National Oceanic and Atmospheric Administration. 2012. http://www.st.nmfs.noaa.gov/commercial-fisheries/fus/fus12/. Accessed 24 Nov 2014.
- 77.NOAA. Aquaculture in the United States. National Oceanic and Atmospheric Administration Fisheries. 2014. http://www.nmfs.noaa.gov/aquaculture/aquaculture_in_us.html. Accessed 24 Nov 2014.
- 78.NOAA. In the U.S. FishWatch U.S. Seafood Facts. National Oceanic and Atmospheric Administration. 2014. http://www.fishwatch.gov/farmed_seafood/in_the_us.htm. Accessed 24 Nov 2014.
- 79.USDA. Top 10 States. 2005 Census of Aquaculture. US Department of Agriculture, the Census of Agriculture. Last updated 2007. 2005. http://www.agcensus.usda.gov/Publications/2002/Aquaculture/index4.asp. Accessed 24 Nov 2014.
- 80.FDA. Drug Use Review. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology. April 5, 2012. http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM338170.pdf. Accessed 24 Nov 2014.
- 81.NRDC. Food, Farm animals, and Drugs. Natural Resources Defense Council. 2014. http://www.nrdc.org/food/saving-antibiotics.asp. Accessed 4 Aug 2014.
- 82.DeWaal CG, Grooters SV. Antibiotic Resistance Foodborne Pathogens. Center for Science in the Public Interest White Paper. Washington, DC. 2013. http://cspinet.org/new/pdf/outbreaks_antibiotic_resistance_in_foodborne_pathogens_2013.pdf. Accessed 24 Nov 2014.
- 83.USFRA. Food Source: Antibiotics. The Food Dialogues. U.S. Farmers and Ranchers Association. 2007. http://www.fooddialogues.com/foodsource/antibiotics. Accessed 24 Nov 2014.
- 84.FDA. 2009 Summary Report on antimicrobials sold or distributed for use in food-producing animals Food and Drug Administration, Department of Health and Human Services: Center for Veterinary Medicine. 2010. http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM231851.pdf. Accessed 24 Nov 2014.
- 85.Slaughter L. Confirmed: 80 Percent of all Antibacterial Drugs Used on Animals, Endangering Human Health. Congresswoman Louise M Slaughter. 2011. http://louise.house.gov/press-releases/confirmed-80-percent-of-all-antibacterial-drugs-used-on-animals-endangering-human-health/. Accessed 24 Nov 2014.
- 86.MacDonald JM, Wang SL. Foregoing sub-therapeutic antibiotics: the impact on broiler grow-out operations. Appl Econ Perspect Policy. 2011;33(89):79–98. doi: 10.1093/aepp/ppq030. [DOI] [Google Scholar]
- 87.FDA. #209. Guidance for Industry. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals. US Department of Health and Human Services Food and Drug Administration Center for Veterinary Medicine. 2012. http://www.fda.gov/downloads/animalveterinary/guidancecomplianceenforcement/guidanceforindustry/ucm216936.pdf. Accessed 25 Sep 2014.
- 88.Benbrook CM. Antibiotic Drug Use in US Aquaculture. Northwest Science and Environmental Policy Center Sandpoint, Idaho. 2002. http://www.iatp.org/documents/antibiotic-drug-use-in-us-aquaculture-1. Accessed 24 November 2014.
- 89.NOAA. Feeds for Aquaculture. National Oceanic and Atmospheric Administration Fisheries. 2014. http://www.nmfs.noaa.gov/aquaculture/faqs/faq_feeds.html. Accessed 24 Nov 2014.
- 90.FDA. Approved Drugs. United States Food and Drug Administration US Department of Health and Human Services. 2014. http://www.fda.gov/animalveterinary/developmentapprovalprocess/aquaculture/ucm132954.htm. Accessed 24 Nov 2014.
- 91.Rakowski KT. Thermal inactivation of Escherichia coil O157:H7 and Salmonella on catfish and tilapia. Food Microbiol. 2012;30(89):427–31. doi: 10.1016/j.fm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 92.Powell JL. Vibrio species. Clin Lab Med. 1999;19(3):537–52. [PubMed] [Google Scholar]
- 93.Williams TC, Ayrapetyan M, Oliver JD. Implications of chitin attachment for the environmental persistence and clinical nature of the human pathogen Vibrio vulnificus. Appl Environ Microbiol. 2014;80(5):1580–7. doi: 10.1128/AEM.03811-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reynaud Y, Pitchford S, De Decker S, Wikfors GH, Brown CL. Molecular Typing of Environmental and Clinical Strains of Vibrio vulnificus Isolated in the Northeastern USA. Plos One. 2013;8(12). [DOI] [PMC free article] [PubMed]
- 95.Turner JW, Paranjpye RN, Landis ED, Biryukov SV, Gonzalez-Escalona N, Nilsson WB, et al. Population Structure of Clinical and Environmental Vibrio parahaemolyticus from the Pacific Northwest Coast of the United States. Plos One. 2013;8(89). [DOI] [PMC free article] [PubMed]
- 96.Givens CE, Bowers JC, DePaola A, Hollibaugh JT, Jones JL. Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus—potential roles for fish, oyster, sediment and water. Lett Appl Microbiol. 2014;58(6):503–10. doi: 10.1111/lam.12226. [DOI] [PubMed] [Google Scholar]
- 97.Heinitz ML, Ruble RD, Wagner DE, Tatini SR. Incidence of Salmonella in fish and seafood. J Food Prot. 2000;63(5):579–92. doi: 10.4315/0362-028x-63.5.579. [DOI] [PubMed] [Google Scholar]
- 98.Amagliani G, Brandi G, Schiavano GF. Incidence and role of Salmonella in seafood safety. Food Res Int. 2012;45(89):780–8. doi: 10.1016/j.foodres.2011.06.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 206 kb)


