Abstract
In Canada, as many as 20 pharmaceutically active compounds (PhACs) have been detected in samples of treated drinking water. The presence of these PhACs in drinking water raises important questions as to the human health risk posed by their potential appearance in drinking water supplies and the extent to which they indicate that other PhACs are present but have not been detected using current analytical methods. Therefore, the goal of the current investigation was to conduct a screening-level assessment of the human health risks posed by the aquatic release of an evaluation set of 335 selected PhACs. Predicted and measured concentrations were used to estimate the exposure of Canadians to each PhAC in the evaluation set. Risk evaluations based on measurements could only be performed for 17 PhACs and, of these, all were found to pose a negligible risk to human health when considered individually. The same approach to risk evaluation, but based on predicted rather than measured environmental concentrations, suggested that 322 PhACs of the evaluation set, when considered individually, are expected to pose a negligible risk to human health due to their potential presence in drinking waters. However, the following 14 PhACs should be prioritized for further study: triiodothyronine, thyroxine, ramipril and its metabolite ramiprilat, candesartan, lisinopril, atorvastatin, lorazepam, fentanyl, atenolol, metformin, enalaprilat, morphine, and irbesartan. Finally, the currently available monitoring data for PhACs in Canadian surface and drinking waters was found to be lacking, irrespective of whether their suitability was assessed based on risk posed, predicted exposure concentrations, or potency.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-015-9729-5) contains supplementary material, which is available to authorized users.
KEY WORDS: drinking water, hospitals, human health, pharmaceuticals, risk assessment
INTRODUCTION
The presence of a number of pharmaceutically active compounds (PhACs) has been confirmed in treated drinking waters (1). This typically raises concerns since, due to their very nature, PhACs are expected to be pharmacologically active upon human exposure. These concerns are further heightened by the fact that drinking water standards have been developed for only a handful of PhACs (2,3) and, thus, they are not being regularly monitored in drinking waters nor are their human health impacts widely understood by most water utility personnel, regulators, scientists, and the general public.
The presence of PhACs in drinking water supplies largely results from aquatic releases of wastewaters from sewage treatment plants (STPs) (4). PhACs are typically found in wastewaters from STPs due to their use and subsequent release by the respective upstream populations (1,4). Agricultural use and the endogenous (i.e., originating or produced within an organism) excretion of these compounds by animals could also be an important source (5). PhACs that result from agricultural sources are not necessarily routed directly to the environment, but are more likely to be released in a diffuse and attenuated manner either through the losses of animal manure during storage or through application to agricultural land.
PhACs in STP influents and, hence, effluents originate from a number of human sources including among others: the natural production and excretion of the PhAC (6); the release of the PhAC from facilities where it is manufactured (7); its clinical use within the community (8,9) and within hospitals (10); the disposal of the PhAC into landfills and its resulting presence in landfill leachates (11); the illicit use of the PhAC (12,13); and the clinical and/or illicit use of other drugs/chemicals that are metabolized to the PhAC upon consumption by humans (12–14).
To date, only a few studies, relative to other environmental pollutants, have been performed to evaluate the human health relevance of the aquatic release of PhACs (4,15–24) and these were primarily focused on the situation in the USA (4,15–22) and Europe (4,17,23,24). As a result, the human health relevance of PhACs releases to Canadian surface waters and, in turn, their potential presence in treated Canadian drinking waters, remains to be evaluated. Besides this obvious knowledge gap, a number of other considerations also drive the need for a Canadian specific human health risk assessment and prioritization study. For instance, among Organization for Economic Co-operation and Development (OECD) countries, the per capita drug expenditure of the Canadian population is second only to that of the USA (25). In addition, the use, and by extension the aquatic release, of certain PhACs is known to vary significantly from one national population to another. For example, consider that the per capita consumption of codeine in Canada is orders of magnitude higher than the levels with which the drug is used by certain European populations (12). Or, consider that the per capita use of rabeprazole in Canada is nearly 500 times greater than the level with which it is used in Sweden (26–28). A comparison with the situation in the USA also reveals some noteworthy differences. For example, meprobamate, which is a PhAC that was found at the highest level in finished USA drinking waters (29), is only used in negligible quantities in Canada. Consider that, in 2006, the per capita Canadian consumption of meprobamate only amounted to 1.5 ng/capita × day (27,28), while in the USA the consumption of meprobamate amounted to nearly 12,000 ng/capita × day (30). Furthermore, the drug carisoprodol, which is metabolized by humans to meprobamate, has been discontinued in Canada since 2003 (31), even though it remains in use in the USA. Overall, an evaluation performed for one national population may not directly translate well to another unstudied national population.
The exposure of Canadians to PhACs was evaluated in this study through the use of predicted and, where available, measured data. When estimating the predicted exposure concentrations of selected PhACs, an attempt was made to account for a number of anthropogenic sources that could lead to the release of these compounds to the environment. In total, for each PhAC studied, up to seven contributions were considered, as follows: (i) the use of the PhAC in the general population; (ii) the use of the PhAC in hospitals; (iii) the general population’s use of other drugs/chemicals that are metabolized by humans to the PhAC; (iv) the hospital-based use of other drugs/chemicals that are metabolized by humans to the PhAC; (v) the endogenous excretion of the PhAC; (vi) the illicit use of the PhAC; and (vii) the illicit use of other drugs/chemicals that are metabolized by humans to the PhAC. A number of previous studies only accounted for the release of a given PhAC due to its particular use in the general population (e.g., 15,21). Consequently, in these earlier studies, the actual environmental load of a number of PhACs is unlikely to have been fully quantified. As an added example, consider that no human health risk assessment study to date has considered that morphine can be released to the aquatic environment through all of the following sources: its endogenous excretion (32), its clinical use in the general population, its use in hospitals, the use of a number of precursors (i.e., codeine, ethylmorphine, nicomorphine, and pholcodine) in the general population and in hospitals, the illicit use of heroin, and, finally, the general population’s consumption of poppy seeds (12). Furthermore, such broad source considerations can become important when conducting an assessment for a specific geographic region, as is demonstrated by the fact that almost the entire environmental load of morphine results from the use of heroin in a number of European countries, while in North America the predominant sources are the clinical use of morphine and its precursors (12).
Among the sources listed above, hospitals are intuitively considered to be an important source of PhACs to the environment (33). However, it often remains unclear as to which PhACs are predominantly used in or sourced through hospitals and, by extension, what fraction of a given load of a PhAC originates in hospitals. Such information is of importance since it can serve to indicate whether hospital wastewaters should be pre-treated for the presence of PhACs. More specifically, in Canada, the relative importance of hospitals as a source for PhACs has only been evaluated for nine PhACs to date (34). Therefore, there is much room to advance our understanding about the importance of Canadian hospitals as point sources for the clinical use and/or dispensing of PhACs. Even outside of Canada, only a handful of analyses of sufficient scale have been performed, evaluating the importance of hospitals as a point source of PhACs (33,35–37).
With respect to determining the environmental levels of a PhAC that may trigger a human health effect, in the absence of established acceptable daily intake values (ADIs), most previous studies have resorted to using the lowest oral therapeutic dose (LOTD) as the point of departure to arrive at screening-level ADIs for the PhAC under consideration (3,4,15,18,20,23). Even though this is a fairly pragmatic approach (15), alternative points of departure have been suggested. For example, occupational exposure limits (OELs) developed by pharmaceutical manufacturers to protect their workforce, may represent a more suitable point of departure to arrive at screening-level ADIs for a given PhAC (38). Such an assertion stems from the recognition that OELs are limits that have specifically been developed to protect human health. In contrast, by definition, LOTD is a point of departure that is known to elicit varied responses in humans and not necessarily a health impact (15,18). To date, a broad scale evaluation using OELs and other points of departure to arrive at screening-level acceptable daily intake (ADI) values has yet to be performed. Furthermore, no approach has been used that integrates these various methods to identify screening-level ADIs for individual PhACs.
Therefore, given the above, the objectives of the current work were to:
Develop a list of PhACs for Canada whose human health relevance were to be evaluated;
Estimate the environmental loading of each selected PhAC by considering all seven potential sources described above;
Evaluate the importance of hospitals as a source of PhACs;
Translate the estimated environmental loading of each of the PhACs into corresponding estimates of predicted exposure concentrations;
Compile measured data from all literature sources reporting on the presence of PhACs in finished (i.e., treated) Canadian drinking waters and use the data to evaluate whether predicted concentrations can be used as conservative estimates for measured data when such data are not currently available;
Develop an approach that integrates the various points of departure to estimate a conservative screening-level ADI for each selected PhAC;
Evaluate human health relevance of each selected PhAC by considering both predicted and measured exposure concentrations; and
Develop a prioritized sub-set of PhACs that warrant further evaluation with respect to their human health relevance.
In carrying out the health risk assessment of PhACs in the Canadian context, as described above, the intent was to develop a comprehensive approach that can be generally applied to any geographic region and updated over time as additional information becomes available.
MATERIALS AND METHODS
Selection of PhACs for Evaluation
In total, 335 PhACs were selected for evaluation. PhACs were specifically selected if:
They were among the top 100 dispensed drugs (units/year) in Canada (27,28), however natural salts and metals among this list were not considered further;
Their presence had been confirmed in finished Canadian drinking waters (39–45);
Their presence had been reported in Canadian surface waters (note: see Appendix A for a full list of 34 references used to perform an evaluation with this criteria);
They had been prioritized or studied in previous comparable studies from geographical locations other than Canada (4,15–20,23,24);
They were reported to have been illicitly used in Canada (46);
They are an antibiotic that is used in Canada;
They are among antineoplastic drugs that are widely used (e.g., fluorouracil); and/or
They are among hormonal drugs that are widely used (e.g., cyproterone; tamoxifen).
The selected evaluation set is listed in its entirety in Appendix B.
To facilitate analysis and discussion, the evaluation set was classified according to the World Health Organization’s Anatomical Therapeutic Chemical (ATC) Classification System (47). When a given PhAC was found to be prescribed under more than one ATC code, the code associated with the predominant use of that PhAC was assumed. Following a classification according to the ATC system, the evaluation set was composed of the following: 26 Class A (alimentary tract and metabolism) drugs; 4 Class B (blood and blood forming organs) drugs; 55 Class C (cardiovascular system); 10 Class D (dermatologicals) drugs; 17 Class G (genito-urinary system and sex hormones) drugs; 7 Class H (systemic hormonal preparations, excluding sex hormones and insulins) drugs; 52 Class J (antiinfectives for systemic use) drugs; 31 Code L (antineoplastic and immunomodulating agents) drugs; 21 Class M (musculo-skeletal system) drugs; 75 Class N (nervous system) drugs; 4 Class P (antiparasitic products, insecticides and repellents) drugs; 17 Class R (respiratory system) drugs; 5 Class S (sensory organs) drugs, and 4 Class V (various) drugs. The remaining 7 PhACs did not belong to any ATC classification code. These PhACs included the two androgens, androstenedione and dihydrotestosterone, and five illicit drugs including cocaine, heroin, methamphetamine, 3,4-methylenedioxy-N-methylamphetamine (commonly known as ecstasy) and tetrahydrocannabinol. Overall, PhACs considered for evaluation covered a very broad range of pharmacological activities.
Exposure Assessment
Exposure of the population to each of the compounds in the evaluation set via drinking water was estimated by considering both predicted and measured exposure concentrations. In order to arrive at predicted exposure concentrations for each PhAC, it was also necessary to estimate the total environmental loading for each. When doing so, only anthropogenic sources were considered and, therefore, the potential agricultural contributions to the environmental load of a PhAC were not considered. However, the implications of this are discussed below. Further, only exposure through drinking water was considered, while exposure through food (e.g., fish, agricultural produce and breast milk) was not. The reason for this was that the PhACs under consideration were found to cover a large range of ionic states and for most such states models for their transfer into food are currently not available (48,49).
Total Environmental Load
The total environmental load, MT (μg/year), for each PhAC was estimated using the following equation, in which the seven possible contributing sources for a PhAC of interest are summed:
| 1 |
where: MCG is the clinical use of the PhAC in the general population; MCH is the clinical use of the PhAC in hospitals; MOC is the clinical use among the general population of drugs/chemicals that are metabolized to the PhAC; MOH is the clinical use in hospitals of other drugs/chemical that are metabolized to the PhAC; Mendo is the endogenous production and excretion of the PhAC; MIDU is the illicit use of the PhAC; and MOID is the use of other drugs/chemicals that are metabolized to the PhAC. The manner in which each of these contributions was quantified for a given PhAC is described below.
The clinical use of the PhAC, MCG (μg/year), was estimated using the following equation:
| 1a |
where: I is the total number of individual routes of administration of clinical preparations, i, with which the PhAC is administered when dispensed by community pharmacies (dimensionless); Ni,c is the total number of prescriptions dispensed of the PhAC via community pharmacies for a given route of administration i (number of prescription items/year); Si,c are the prescription strengths for Ni,c (μg/prescription item); Uex,i is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the urinary route for a given route of administration i (dimensionless); and Fex,i is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the fecal route for a given route of administration i (dimensionless). With the exception of acetaminophen, acetylsalicylic acid, clotrimazole and ibuprofen, the Ni,c values used were those extracted from the Canadian Compuscript Audit database (27) of IMS Brogan. Similarly, with the exception of acetaminophen, acetylsalicylic acid, clotrimazole, and ibuprofen, Si,c values were also reported in the same database but, in certain instances, a cross reference to the Health Canada’s Drug Product Database (DPD) (31) was required to arrive at accurate estimates for Si,c. To estimate Ni,cSi,c values for acetaminophen, acetylsalicylic acid, clotrimazole, and ibuprofen, methods and data sources detailed in Appendix C were utilized. A database of Uex,i + Fex,i values for each of the PhACs in the evaluation set was developed by compiling information from more than 500 literature sources. The database is presented in its entirety in Appendix D. In this database, primary literature references are used to estimate conservative excretion factors for each PhAC that also account for the effect of route of administration on the metabolic disposition of a given PhAC.
The clinical use of each PhAC in hospital, MCH (μg/year), was estimated using the following equation:
| 1b |
where: J is the total number of individual routes of administration, j, with which a PhAC is administered when dispensed by and/or at hospital (dimensionless); Nj,H is the total number of prescriptions dispensed of a given a PhAC via hospitals for a given route of administration j (number of prescription items/year); Sj,H are the prescription strengths for Nj,H (μg/prescription item); Uex,j is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the urinary route for a given route of administration j (dimensionless); and Fex,j is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the fecal route for a given route of administration j (dimensionless). The Nj,H values used were those reported in the Canadian Drug Store and Hospital Purchases Audit database (28). Sj,H values were also drawn from the same database but, in certain instances, a cross reference to the Health Canada’s DPD database (31) was required to arrive at accurate estimates for Sj,H. Uex,j + Fex,j values were estimated from the database described above and are presented in Appendix D.
The clinical use among the general population of drugs/chemicals that are metabolized to the PhAC, MOC (μg/year), was estimated using the following equation:
| 1c |
where: L is the total number of community pharmacy-dispensed drugs, l, that are metabolized to the PhAC upon human consumption (dimensionless); K is the total number of individual routes of administration, k, with which drug l is administered (dimensionless); Nl,k,c is the total number of prescriptions dispensed of drug l for a given route of administration k (number of prescription items/year); Sl,k,c are the prescription strengths for Nl,k,c (μg/prescription item); Uex,l,k is the fraction of drug l that is excreted via the urinary route as the PhAC for a given route of administration k (dimensionless); Fex,l.k is the fraction of the drug l that is excreted via the fecal route as the PhAC when administered via the route of administration k (dimensionless); MwPHAC is the molecular weight of the PhAC (g/mol); and Mwl is the molecular weight of the drug l (g/mol). L was established by developing source models for all PhACs of the evaluation set that result from the metabolism of other drugs dispensed at community pharmacies. All source models developed are presented in Appendix E. The Nex,l,k values used were those reported in the Canadian Compuscript Audit database (27). Sex,l,k values were also reported in this same database but, in certain instances, a cross reference to the Health Canada’s DPD database (31) was required to arrive at accurate estimates for Sex,l,k. Uex,l,k + Fex,l,k were estimated by compiling data from relevant literature sources, as presented in Appendix E.
The clinical use in hospitals of other drugs/chemical that are metabolized to the PhAC, MOH (μg/year), was estimated using the following equation:
| 1d |
where: M is the total number of other hospital-dispensed drugs, m, that are metabolized to the PhAC upon human consumption (dimensionless); O is the total number of individual routes of administration, o, with which drug m is administered (dimensionless); Nm,o,h is the total number of prescriptions dispensed of drug m for a given route of administration o (number of prescription items/year); Sm,o,h are the prescription strengths for Nm,o,h (μg/prescription item); Uex,m,o is the fraction of drug m that is excreted via the urinary route as the PhAC for a given route of administration o (dimensionless); Fex,m.o is the fraction of the drug m that is excreted via the fecal route as the PhAC for a given route of administration o (dimensionless); MwPHAC is the molecular weight of the PhAC (g/mol).; and Mwm is the molecular weight of the drug m (g/mol). M was established by developing source models for all PhACs of the evaluation set that result from the metabolism of other hospital-dispensed drugs. All source models are presented in Appendix E. The Nm,o,h values used were those reported in the Canadian Drug Store and Hospital Purchases Audit database (28). Sm,o,h values were also reported in the same database but, in certain instances, a cross reference to Health Canada DPD database (31) was required to arrive at accurate estimates for Sm,o,h. Uex,m,o + Fex,m,o were estimated by compiling data from relevant literature sources, as presented in Appendix E.
The endogenous excretion of the PhAC, Mendo (μg/year), was estimated using the following equation:
| 1e |
where PT is total population (capita) and Eendo is the daily per capita endogenous release of a PhAC (μg/capita × year). Canadian population data circa 2006 was used in all calculations. PT was estimated using data from Statistics Canada (50). Eendo was estimated using various methods and models presented in Appendix F.
The illicit use of the PhAC, MIDU (μg/year), was estimated using the following equation:
| 1f |
where: CIDU is the net mass of the PhAC that is illicitly used (μg/year); P is the total number of individual routes of administration, p, with the PhAC is illicitly administered (dimensionless); fp is the mass fraction of CIDU that is administered via route p (dimensionless); Uex,i,p is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the urinary route when illicitly administered via route p (dimensionless); and Fex,i,p is the fraction of the PhAC that is excreted unchanged and as its glucuronide and sulfate conjugates via the fecal route when illicitly administered via route p (dimensionless). CIDU was estimated using methods and models presented in Appendix G. fp values were estimated using trends reported in Canadian Centre on Substance Abuse (CCSA) (51), Khan and Nicell (12,13) and Royal Canadian Mounted Police (RCMP) (52). Uex,i,p + Fex,i,p values were estimated from the database described earlier and presented in Appendix D.
The illicit use of other drugs/chemicals that are metabolized to the PhAC, MOID (μg/year), was estimated using the following equation:
| 1g |
where: R is the total number of illicit drugs, r, that are metabolized to the PhAC upon human consumption (dimensionless); Q is the total number of individual routes of administration, q, via which drug r is administered (dimensionless); COID,r,q is the net mass of drug r which is administered via route q (μg/year); fq,r is the mass fraction of COID,r,q that is administered via route q (dimensionless); Uex,r,q is the fraction of drug r that is excreted via the urinary route as the PhAC for route of administration q (dimensionless); and Fex,r.q is the fraction of the drug r that is excreted via the fecal route as the PhAC for route of administration q (dimensionless); MwPHAC is the molecular weight of the PhAC (g/mol); and Mwr is the molecular weight of the drug r (g/mol). R was established by developing source models for all PhACs of the evaluation set that result from the metabolism of other illicit drugs. All source models developed are presented in Appendix E. COID,r,q was estimated using methods and models summarized in Appendix G. fq,r were estimated using trends reported in CCSA (51), Khan and Nicell (12,13) and RCMP (52). Uex,r,q + Fex,r,q were estimated by compiling data from relevant literature sources, as presented in Appendix E.
When estimating values of MCG, MCH, MOC, MOH, MIDU, and MOID, it was conservatively assumed that the total mass dispensed of a PhAC/drug and the mass demand for an illicit drug end up being used. Therefore, for each source chemical, the mass that is purchased/acquired but then not used was unaccounted for.
Predicted Exposure Concentrations
The predicted exposure concentrations (PEC) (ng/L) for each PhAC of the evaluation set were estimated using the following equation:
| 2 |
where: MT is total load of the PhAC to the environment (μg/year), Qww is median level of wastewater generated on a per capita basis (L/capita × day); DF is a conservative level of dilution of a wastewater upon its immediate release to the environment (dimensionless); fo, f1, and f1,2 are the fractions of wastewaters that undergo no treatment, primary treatment, and a secondary level of treatment (dimensionless), respectively; and R1 and R1,2 are the levels with which the PhAC is removed in primary and secondary treatment plants (dimensionless), respectively. MT was estimated using Eq. 1. Qww was estimated to be 504 L/capita × day by analyzing data reported for nearly 1000 sewage treatment plants in Environment Canada’s Municipal Water and Wastewater Survey (53) (see Appendix H). DF was conservatively assumed to be 10 based on a recent report co-authored by the authors of this study to Health Canada (54), in which immediate dilution factors for nearly 900 Canadian sewage treatment plants were estimated for average and low flow conditions. fo, f1, and f1,2 values were as those suggested by Environment Canada (55). R1 and R1,2 were those compiled in an in-house database of removal rates reported in the literature for PhACs in sewage treatment plants. For cases where literature data was not available, the Simple Treat model (56) was used along with appropriate quantitative structure–activity relationship models (57,58) to estimate removal rates. Overall, the database aimed to conservatively account for the removal of PhACs in sewage treatment plants. The database, not included here, will be provided upon request.
Equation 2 was used to calculate three estimates for the predicted exposure concentrations, ranging from the most to least conservative; namely, PEC1, PEC2, and PEC3. PEC1 values were estimated assuming no metabolism or removal in treatment plants of the PhACs. PEC2 estimates accounted for some metabolism of the PhACs, but it was assumed that the PhACs were not removed in sewage treatment plants. Estimates for PEC3 accounted both for metabolism and for removal in sewage treatment plants. Note that PEC3, even though it is the least conservative of the three estimates furnished, still represents a conservative estimate for the concentration at which the population could be exposed to individual PhACs via drinking water. Consider that PEC3 is akin to the population continuously drinking treated wastewaters that had been minimally diluted. Therefore, estimates for PEC3 as furnished here, do not account for the added attenuation (dilution, degradation, photolysis, or sorption) of the minimally diluted load before its arrival at the intake of a drinking water treatment plant and any subsequent removal in the plant before supply to the community. However, in arid areas where little to no dilution of wastewater effluents in receiving waters may occur, exposure concentrations can approach PEC3 values. This is also expected to be the case for water reuse situations. PEC1 and PEC2 are also of value since a risk evaluation with these concentrations, as opposed to PEC3, reveals those PhACs for which the risk outcome resides in assumptions made about metabolic conversion and removal via sewage treatment plants. Overall, an evaluation performed with any of the three PEC estimates is expected to be conservative and, perhaps most importantly for the overall objective of the current study, aims to ensure that false negative classifications (i.e., suggesting an insignificant risk when some risk might actually exist) are minimized.
Measured Exposure Concentrations
Measured exposure concentrations (MEC) for the evaluation set were compiled from literature sources reporting on the presence of PhACs in finished (i.e., treated) drinking waters in Canada. Concentrations for samples in which the PhAC was not detected were conservatively entered into the database as a value equal to the limit of detection. In total, 5813 MECs were compiled from all relevant literature sources (39–45).
Estimation of Acceptable Daily Intake
The acceptable daily intake (ADI) of a substance is defined as the maximum quantity that can be ingested on a daily basis and not result in undesirable effects on human health (18). Here, consistent with previous work (4), any effect is considered undesirable. Since ADIs for most PhACs have not been established by regulatory bodies, multiple lines of evidence were considered to arrive at a conservative estimate of the ADI for each PhAC of the evaluation set. The overall approach taken is summarized in Fig. 1 and discussed below.
Fig. 1.
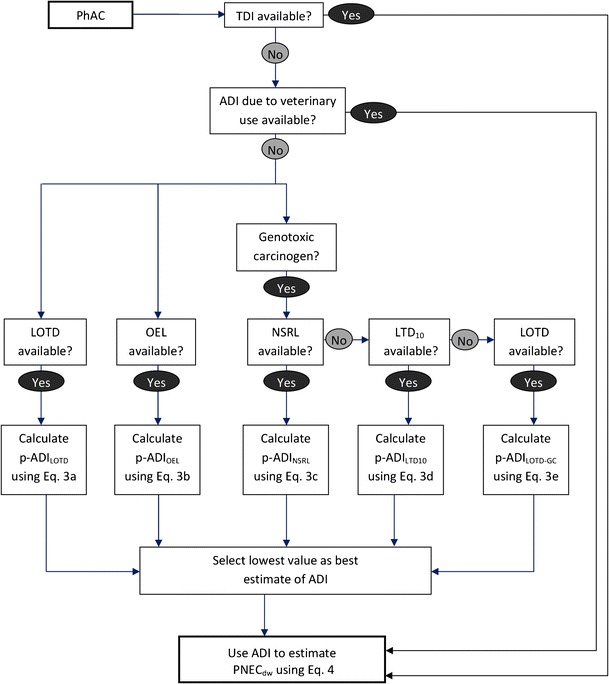
Approach used to estimate the acceptable daily intake for each PhAC of the evaluation set. ADI acceptable daily intake; TDI tolerable daily intake; LOTD lowest oral therapeutic dose, LTD 10 lower 95% confidence limit of the dose required to induce tumors in 105 of exposed animals; NSRL no significant risk level; OEL occupational exposure limit
When available, tolerable daily intake (TDI) values, as derived by Health Canada, were directly adopted as the ADI for the compound of interest. In the absence of such data, ADIs derived for the potential veterinary use of the PhAC by such agencies as the Joint FAO/WHO Expert Committee on Food Additives and the European Medicines Agency Veterinary Medicines and Inspections were used. However, for the vast majority of the PhACs in the evaluation set, TDI values were not available and, furthermore, these compounds have not been used for veterinary medicine purposes. Therefore, ADI values for most PhACs of the evaluation set remain to be established. In the absence of government/international agency-derived benchmarks for a PhAC, a number of approaches were considered to estimate a variety of provisional ADIs (p-ADIs) for each PhAC of the evaluation set. Following such evaluations, the lowest of the derived p-ADIs was selected as the ADI for the PhAC under consideration (see Fig. 1).
One point of departure typically used to derive a p-ADI for a PhAC is its lowest oral therapeutic dose, LOTD (15,23). In recognition of this, the following equation was used to estimate the p-ADI for a PhAC from its LOTD, when available:
| 3a |
where LOTD is the lowest oral therapeutic dose of the PhAC in adults and SF is a safety factor, the purpose of which is to extrapolate LOTD to levels which can be considered safe for continuous human exposure. LOTDs were estimated from drug monographs available through Health Canada’s Drug Product Database (31) and by consulting dosage schedules presented in Lexi-Comp’s Drug Information Handbook (59) and those presented through UptoDate.com (60). LOTD values for adults were typically used here, since not all drugs of the evaluation set are used pediatrically. Typically, a default SF of 1000 was used (15). However, an additional factor of 10 (i.e., an SF of 10,000) was used if the PhAC was found to possess at least one of the following attributes: it is a cytotoxic drug, it is a hormone, it is a pregnancy Class D or Class X drug, or it has been categorized as a hazardous drug by National Institute for Occupational Safety and Health (61).
An alternative to the LOTD approach described above has been suggested by Straub and Flückiger (38). Specifically, they suggested that occupational exposure limits derived by pharmaceutical manufacturers for their workers can be used with appropriate extrapolation factors to arrive at a p-ADI for a given PhAC. Their proposed approach can be expressed as follows:
| 3b |
where: OEL is the 8-h time-weighted average occupational exposure limit derived by pharmaceutical manufacturers to protect their workers; Qair is the volume of air breathed by a worker over duration of 8 h; and EF is extrapolation factor used to extrapolate a human health benchmark derived for those who work in pharmaceutical manufacturing operations to the general population. OELs are typically reported in material safety data sheets (MSDS) developed for each PhAC by it various manufacturers. Therefore, OELs were compiled from MSDS from various manufacturers of pharmaceuticals. In the cases were multiple OELs were available for a given PhAC, the lowest was adopted. For Qair the default value of 10 m3 was used (62). EF was assumed to be 100 following the work of Straub and Flückiger (38).
For genotoxic carcinogenic PhACs, additional benchmarks that are protective of an added lifetime cancer risk must also be considered. Conservatively, the “one hit” model of carcinogenesis was used (63) in which it is assumed that a cancer cell can result from a single genetic change in a normal cell and, therefore, a carcinogen presents a risk of cancer at any dose.
When available, no significant risk levels (NSRL) as derived by California EPA (64) were used to estimate a p-ADI for a genotoxic carcinogenic PhAC of the evaluation set, as follows:
| 3c |
where NSRL is the daily intake as estimated by California EPA and BW is the average weight of an adult. The NSRL values used here were those reported in a California EPA compilation (64) and the value of BWNSRL-adult used was the average weight of an adult assumed by California EPA in their estimations of NSRL levels.
In the event a NSRL level of a given genotoxic carcinogen PhAC of the evaluation set was not available, the following approach was used:
| 3d |
where: LTD10 is the lower 95% confidence limit of the dose required to induce tumors in 10% of exposed animals and 105 is the factor that linearly and therefore conservatively extrapolates LTD10 to an acceptable added lifetime cancer risk of 10−6. The LTD10 values used here were those reported in the Carcinogenic Potency Database (65).
In cases where neither a NSRL nor a LTD10 were available for a genotoxic carcinogen, the following equation was used:
| 3e |
where, as before, LOTD is the lowest oral therapeutic dose for an adult and 105 is the factor that linearly and, therefore, conservatively extrapolates LOTD to an acceptable added lifetime cancer risk of 10−6 (63).
Predicted No Effect Concentration for Exposure of Humans via Drinking Water
Predicted no effect concentrations (PNEC) for the exposure of humans to each PhAC via drinking water (PNECdw) were estimated using the following equation:
| 4 |
where: ADI is the acceptable daily intake for the PhAC; BW is the body weight for the most sensitive human receptor; and IRdw is the average daily ingestion rate of drinking water by the most sensitive human receptor. ADI was estimated using the methods described in “Estimation of Acceptable Daily Intake” section and summarized in Fig. 1. Of the five receptors considered by Health Canada—infants (0 to 6 months), toddlers (7 months to 4 years), children (5 to 11 years), adolescents (12 to 19 years) and adults (20 years+)—the combination of the BW and IRdw of infants is such that they are determined to be the most sensitive receptor to contaminants in drinking water. Therefore, BW and IRdw values were assumed to be those of a Canadian infant as defined by Health Canada (66).
Risk Assessment
The risk associated with each PhAC of the evaluation set was quantified by calculating margins of exposure (MOE) for each. The lower the magnitude of MOE, the higher is the risk to human health. The MOEs were estimated using both predicted (PEC) and, where available, measured exposure concentrations (MECs). It is important to recognize that MECs, though often considered to be a gold standard for assessment purposes, may have been deduced from samples that have not have been collected under reasonably anticipated worst-case exposure conditions (e.g., low flow conditions). Therefore, even for PhACs for which MECs were available, concurrent evaluations using their respective PECs were also performed.
The predicted margins of exposure (MOEP) for each PhAC of the evaluation set were estimated using the following equation:
| 5 |
Where possible, the margins of exposure using measured exposure concentrations (MOEM) for PhACs were estimated using the following equation:
| 6 |
Typically, an MOE of less than 1.0 is considered to be indicative of a possible human health risk. Recognizing the highly conservative nature with which PNECdw and PEC values were estimated here, an MOEp of less than 1.0 was interpreted as an indicator of possible risk. However, an MOE of >1 and <10 was used for the added goal of developing a list of priority PhACs for further evaluation. An MOE > 10 was interpreted as suggesting negligible risk.
Note that while the evaluation of mixtures represents a very important issue that must eventually be addressed, this was beyond the scope of the current investigation.
RESULTS AND DISCUSSION
Sources of PhACs to the Environment
As discussed above, seven distinct source terms were considered when estimating the total environmental load (MT) of each PhAC. Figure 2 summarizes the relevance and the significance of each source term in terms of their contribution to the net environmental load of the 335 PhACs selected for analysis. Appendix I further elaborates on the data summarized in Fig. 2 by listing the details of these source terms for each PhAC.
Fig. 2.
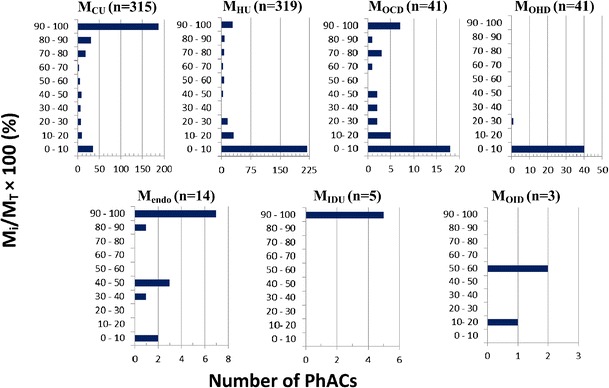
Significance of each source term M i (i.e., M CU , M HU , M OCD , M OHD , M endo , M IDU, or M OID) with respect to the net load of each PhAC (M T) of the evaluation set of 335 PhACs. n is the total number of PhACs for which a given source term was found to be relevant
The overall relevance of each source term for the entire evaluation set is indicated in Fig. 2 by the variable n. The use of a PhAC in the general population (MCU) and in hospitals (MHU) was found to be relevant for 315 and 319 PhACs, respectively. The reason that the relevance number for MHU was higher than that for MCU was that certain drugs such as cytarabine, daunorubicin, ifosfamide, cisplatin, and idarubicin are only clinically dispensed in hospitals and, as such, are not dispensed in the general public. For 41 PhACs, the use of other drugs/chemicals in the general population (MOCD) and in hospitals (MOHD) was found to be relevant sources of these specific compounds. Another 14 PhACs were established to be endogenously excreted (Mendo), whereas a handful of PhACs were either illicitly used (MIDU) or resulted from the illicit use of other drugs (MOID).
Figure 2 further suggests that, depending on the PhAC being considered, each of the source terms could account for, at the very least, a significant fraction of a PhACs net environmental load. For example, consider that the data summarized in Fig. 2 suggest that the individual source terms MCU, MHU, MOCD, Mendo, and MIDU account for greater than 90% of the net environmental load for 188, 28, 7, 7, and 5 PhACs, respectively. The two other contributions, MOHD and MOID, were found to be significant for a select few cases. From the perspective of the entire evaluation set, not surprisingly, the use of the PhAC in the general population (MCU) was found to be the single most important source, followed by the use of the PhACs in hospitals (MHU). Overall, since each of the seven contributions can be significant for the release of a given PhAC, it is suggested that all seven source terms must be considered when estimating the net environmental loading of a PhAC. To neglect some of these source terms might result in a significant underestimation of the environmental burden of a given PhAC.
Due to particular concerns associated with hospitals that can serve as potential “hotspots” for the use and the release of certain PhACs (33), the contributions of hospitals is evaluated further in Fig. 3. Overall, hospitals were found to be a relevant source for 320 PhACs (i.e., MHU + MOHD) of the evaluation set (see Appendix I). Figure 3(a) suggests that hospitals loads of the PhACs of the evaluation set can vary over several orders of magnitude. The median hospital load for the 320 PhACs was established to be 180 μg/bed × day. Figure 3(b) suggests that, when the evaluation set is considered in its entirety, drugs dispensed through hospitals account for less than 10% of the environment load of most PhACs. However, there are a significant number of PhACs that appear to be predominantly or almost entirely sourced through hospitals. Consider that for 41 PhACs more than 70% of their net environmental load was sourced through hospitals (see Appendix I). Overall, the hospital contribution to the net environmental load of a PhAC can vary significantly from one case to another. Note that the overall trend suggested by Fig. 3(b) is not entirely unexpected since a dominant selection criterion for the PhACs in the evaluation set was the inclusion of the top 100 highly dispensed drugs in Canada and the vast majority of these are community-based drugs.
Fig. 3.
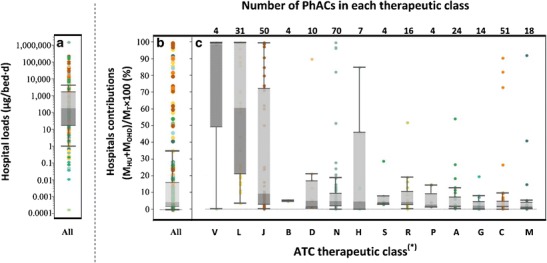
Hospitals as a source of PhACs: a Net load of all 320 PhACs for which hospitals are significant sources; b Overall significance of hospitals as a source (M HU + M OHD) relative to the net environmental load (M T) of each PhAC for the 320 PhACs; c Significance of hospitals as a source of PhACs grouped according to therapeutic classes as defined by the Anatomical Therapeutic Chemical (ATC) Classification System. Colors are coordinated across all the three graphs and are indicative of therapeutic classes shown in (c). *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
To better grasp which types of PhACs are predominantly used in hospitals and which are not, Fig. 3(c) segregates that data presented in Fig. 3(b) by the broad therapeutic classes defined by the World Health Organization (47). From this, trends begin to emerge. Figure 3(c) suggests that PhACs belonging to most drug classes are only used at relatively minor level in hospitals; however exceptions were found to exist for most such drug classes. The two drug classes that were found to be primarily used in hospitals were Class L (antineoplastic and immunomodulating agents) and Class V (various). Thirty-one PhACs of the evaluation set were Class L drugs and, of these, 19 were cytotoxic drugs that are primarily administered in and/or made available through hospitals (67). The median hospital contribution for all Class L drugs was 61%, with estimates for this class of drugs ranging from 4% for azathioprine to 100% for a number of cytotoxic drugs. Only four PhACs of the evaluation set were Class V drugs; therefore, the sample size upon which a judgment is being made is rather small. Nevertheless, Class V contains diagnostic agents and contrast media and these drugs are also known to be primarily administered in hospitals (67).
Hospitals were also found to be a minor but still significant source for Class J (antiinfectives) drugs. Of the 50 Class J drugs for which hospital contributions were found to be relevant, 44 were antibiotics. The median hospital contribution for all Class J drugs was 9.3% (see Fig. 3c), with values ranging from 0.1% for spiramycin to 99.8% for penicillin G. Hospitals accounted for greater than 95% of the net environmental load of 8 Class J drugs, all of which were antibiotics. Specifically, these were: cefazolin, ceftriaxone, cefuroxime, ceftazidime, piperacillin, penicillin G, tazobactam, and ticarcillin. All eight aforementioned PhACs are administered in Canada through injections, which might be a reason for their predominant use in hospitals. Overall, hospitals appear to be a major source for only some antibiotics.
It is worth noting that the values reflected in Fig. 3 do not account for the fact that a fraction of these hospital-dispensed drugs are issued and administered to outpatients and are, therefore, in actual fact excreted in the general population. For example, Weissbrodt et al. (67) reported that, at a Swiss hospital, 70% of the studied cytotoxics and 50% of the studied iodinated contrast media were administered to outpatients and were therefore destined to be excreted in the general population rather than in the hospital. Therefore, the fractions plotted in Fig. 3 likely overestimate the significance of hospitals as a specific geographical source of PhACs into the environment but can be considered accurate representations of the proportion of PhACs that are sourced through hospitals. Overall, trends observed here for the contribution of Canadian hospitals to the net environmental loads of PhACs agree with trends reported for Dutch and Danish hospitals (35,36) in that hospitals appear to be a minor source for most PhACs but in certain specific instances can be a significant or dominant source of the environmental load of a given PhAC. The broader implications of this observation is that, even if PhACs in the wastewaters of hospitals are successfully treated before capture by municipal sewer systems, the majority of the mass load of most PhACs will still be released into sewer systems for subsequent treatment and/or disposal in the environment.
The discussion above has focused on the significance of the various source terms to the overall selected set of 335 PhACs. An equally valuable evaluation concerns how many source terms are relevant for a given PhAC. The number of source terms that are relevant for individual PhACs ranged from 0 to 6 (see Appendix I). Specifically, none of the seven sources accounted for 1 PhAC (as explained below), a single source accounted for 11 PhACs, 2 sources accounted for 280 PhACs, and 3, 4, 5 and 6 source terms lead to the release of 12, 25, 5, and 1 PhACs, respectively. The data suggests that the vast majority of the environmental loads of PhACs in the evaluation set resulted from 2 source terms. Not unexpectedly, the reason for this was that most of the PhACs were only clinically used within the general population and in hospitals (see Appendix I). However, for 43 PhACs, more than 2 source terms were found to be relevant (see Appendix I). In the case of morphine, as many as six source terms (and seven contributions) had to be considered in order to arrive at an estimate for its load to the Canadian environment (see Appendix J). The single PhAC for which none of the seven source terms was found to be relevant was roxithromycin, since this PhAC has yet to be approved for sale in Canada or in the USA (31,68). Despite this, roxithromycin has been detected in Canadian surface waters and treated drinking waters (42). At present, the origin of this PhAC in Canadian waters remains uncertain but it is possible that the detected roxithromycin is a residue arising from the degradation of another chemical. Note that the latter point highlights a potential limitation of the present analysis in that the possible environmental generation of a PhAC has not been accounted for since such information is typically not available.
It is also of interest to examine the importance of the net environmental load of a PhAC that results from endogenous excretion. Fourteen PhACs of the evaluation set were found to be released via endogenous excretions (see Appendix K). As expected, most of the PhACs for which endogenous excretion was found to be significant are naturally produced hormones. For example, similar to estimates furnished for the USA (69,70), the hormones estrone, estradiol and estriol were found to predominantly or almost entirely result from endogenous excretions. However, it was surprising to note that other compounds that are usually associated with clinical use such as morphine and codeine can also be endogenously produced. Overall, more than 90% of the environmental loads for eight of the PhACs were found to result from endogenous excretions (see Appendix K). A consequence of this is that if these PhACs are found to pose an unacceptable human health and/or eco-toxicological risk then one has no choice but to facilitate their removal from sewage and drinking water through process upgrades in treatment plants. Conversely, if a PhAC almost entirely results from the exogenous use of relevant pharmaceutical preparations (e.g., codeine) and is found to pose unacceptable human health and/or eco-toxicological risk, the possibility of managing the risk through limiting or substituting the use of this PhAC with alternative pharmaceutical preparations remains available.
Similar to the interest in the fraction of the environmental load that results from endogenous excretions, there is also interest in the fraction that results from illicit sources. Again, this interest arises from the consideration that should a PhAC that predominantly results from illicit sources pose an unacceptable human health and/or eco-toxicological risk one may have no choice but to facilitate its removal in sewage and/or drinking water treatment plants. Illicit contributions were found to be relevant for the following six PhACs: amphetamine, cocaine, heroin, methamphetamine, MDMA, and morphine. Of these, MDMA and heroin are not clinically used in Canada (31) and, therefore, they are only released to Canadian aquatic environment through the illicit use of these drugs. In contrast, amphetamine, cocaine, methamphetamine, and morphine, can all be released to the Canadian aquatic environment through licit and illicit sources. As shown in Appendix J, a model accounting for the numerous sources of morphine is quite complex, with nearly 13.5% of its net environmental load resulting from the population’s use of illicit heroin. Cocaine, in addition to being an important illicit drug, is also used clinically in Canada (31); however, only 0.3% of its environmental load is estimated to result from its clinical use in hospitals (see Appendix I). In addition to its clinical sources, amphetamine can also result from the illicit use of methamphetamine (71); with as much as 56% of its environmental load accounted for by the illicit use of methamphetamine (see Appendix I). Note that methamphetamine itself can result from the clinical use of the precursor drug selegiline and its own illicit use (see Appendix E). More specifically, the illicit use of methamphetamine accounts for 99.7% of the PhAC’s load to the Canadian aquatic environment (see Appendix I). Overall, of the six PhACs for which illicit contributions were found to be relevant, five were established to largely or almost entirely result from illicit sources. Therefore, if these five PhACs are found to pose an unacceptable human health or eco-toxicological risk, one would have little choice but to consider process modifications to facilitate their removal in wastewater and/or drinking water treatment plants.
Predicted Exposure Concentrations
Three successively less conservative values for the predicted exposure concentrations, denoted as PEC1, PEC2, and PEC3 as described above, are plotted in Appendix L for the set of selected PhACs. Individual PEC estimates for each PhAC are also tabulated in Appendix L. PEC3 estimates have also been plotted in Fig. 4 where the data has been categorized by ATC classes to facilitate interpretation and discussion. Since three PhACs of the evaluation set were not in use in Canada for the study year of 2006, PEC estimates could only be furnished for 332 PhACs of the 335 in the evaluation set. The three exceptions are fenoprofen which was discontinued from use in Canada circa 2002, roxithromycin which has yet to be approved for use in Canada, and buprenorphine which was only approved for sale in Canada in 2007 (31).
Fig. 4.
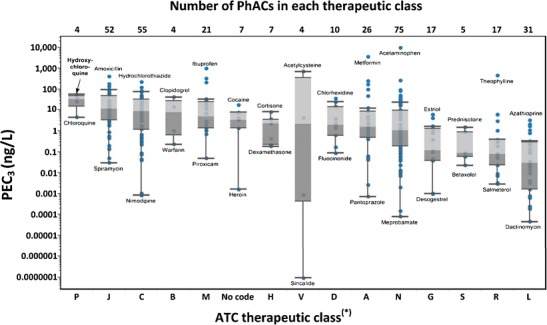
PEC3 estimates grouped according to therapeutic classes as defined by the Anatomical Therapeutic Chemical (ATC) Classification System. *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
Overall, when the metabolic conversion of individual compounds are accounted for (i.e., estimates of PEC2 relative to PEC1), an estimated median reduction of 56% of the environmental load is predicted. When accounting for their removal in sewage treatment plants over and above metabolism (i.e., estimated of PEC3 relative to PEC2), an additional 22% median reduction in environmental load is predicted (see Appendix L).
The top 50 PhACs ranked according to their estimate PEC3 values are tabulated in Table I. PEC3 values ranged from 33 ng/L for sotalol to approximately 10,000 ng/L for acetaminophen. Class J (antiinfectives for systemic use) drugs were particularly well represented with 14 of the 52 drugs from this class in this list and, of these, 13 were antibiotics. The median PEC3 for all 52 Class J drugs was 12 ng/L, with estimates ranging from 0.03 ng/L for spiramycin to 424 ng/L for amoxicillin (see Fig. 4). Of all classes, the median PEC3 for Class J drugs was the second highest and lower only than that of Class P drugs, for which only four drugs were included in the evaluation set. Class C (cardiovascular) drugs were also well represented in the top 50 list with 12 of the 55 evaluation set drugs from this class making the list (Table I). The median PEC3 for Class C drugs was the third highest at 9 ng/L, with specific estimates for this class of drugs varying from 0.0008 ng/L for nimodipine to 229 ng/L for hydrochlorothiazide (see Fig. 4). Even though 31 Class L (antineoplastic and immunomodulating) drugs were included in the evaluation set, not one was among the top 50 list (Table I). The highest PEC3 for a Class L drug was estimated to be 3.4 ng/L for azathioprine, an immunosuppressant drug, and the highest estimated PEC3 for a cytotoxic drug was estimated to be 2.1 ng/L for fluorouracil. The median level PEC3 for all 31 Class L drugs was estimated to be 0.03 ng/L, with estimates ranging from 0.00005 ng/L for dactinomycin to 3.4 ng/L for azathrioprine (see Fig. 4). Furthermore, the median PEC3 of 0.03 ng/L for all Class L drugs was the lowest of all drug classes considered (see Fig. 4). The second and third lowest median PEC3 values were for Class S and Class R drugs. Most Class S and Class R drugs included in the evaluation set are administered locally into the eye, the ear, the nose or the throat and this may be the likely reason for the low median PEC3 estimated for these drug classes. Not one of the Class S drugs and only acetylcysteine of the 17 Class R drugs were in the top 50 list. However, clinical use in the general population and in hospitals is a minor contributor to the net environmental load of acetylcysteine, since its load to Canadian environment almost entirely results from endogenous excretions (see Appendix K). Similar to Class L drugs, not one of the 17 Class G (genito-urinary system and sex hormones) drugs included in the evaluation set was in the top 50. PEC3 estimates for Class G drugs ranged from 0.001 ng/L for desogestrel to 6.2 ng/L for estriol and the median level PEC3 for all 16 Class G drug was estimated to be 0.1 ng/L. Even though it is clear that most Class L and Class G drugs are only expected to be present in the Canadian aquatic environments at low nanogram per liter to sub nanogram per liter levels, these levels by themselves cannot be taken to be indicative of the lack of risk posed by such drugs since Class L and G drugs are expected to be highly potent in their action (15).
Table I.
Fifty Top Ranked PhACs According to Estimates of Predicted Environmental Concentrations (PEC3) and the Reported Number of Canadian Drinking Water and Surface Water Samples that Have Been Analyzed for Their Presence
| PhAC | ATC Class | PEC3 | Number of samples that have been analyzed for their presence | |
|---|---|---|---|---|
| Surface watera | Treated drinking waterb | |||
| Acetaminophen | N | 10,000 | 212 | 176 |
| Metformin | A | 3500 | –c | – |
| Ibuprofen | M | 1000 | 496 | 219 |
| Acetylcysteine | V | 714 | – | – |
| Theophylline | R | 459 | – | – |
| Amoxicillin | J | 424 | – | – |
| Naproxen | M | 333 | 514 | 219 |
| Gabapentin | N | 270 | – | – |
| Docusate | A | 255 | – | – |
| Hydrochlorothiazide | C | 229 | 9 | – |
| Etidronic acid | M | 219 | – | – |
| Cephalexin | J | 178 | – | – |
| Aminosalicylic acid | A | 169 | – | – |
| Penicillin G | J | 140 | 26 | 32 |
| Irbesartan | C | 126 | – | – |
| Atenolol | C | 122 | 19 | – |
| Sulfasalazine | A | 101 | – | – |
| Clarithromycin | J | 98 | 12 | 3 |
| Codeine | N | 96 | 9 | – |
| Penicillin V | J | 96 | – | – |
| Ciprofloxacin | J | 91 | 160 | 159 |
| Valsartan | C | 85 | – | – |
| Atorvastatin | C | 81 | 27 | – |
| Furosemide | C | 76 | – | – |
| Cefazolin | J | 67 | – | – |
| Piperacillin | J | 63 | – | – |
| Carbamazepine | N | 61 | 453 | 221 |
| Hydroxychloroquine | P | 58 | – | – |
| Sulfamethoxazole | J | 54 | 364 | 221 |
| Cefprozil | J | 52 | – | – |
| Tetracycline | J | 52 | 214 | 214 |
| Acetylsalicylic acid | N | 51 | – | – |
| Acyclovir | J | 51 | – | – |
| Ceftazidime | J | 51 | – | – |
| Diltiazem | C | 50 | – | – |
| Cimetidine | A | 49 | – | – |
| Metoprolol | C | 46 | 16 | – |
| Valproic acid | N | 46 | – | – |
| Acebutolol | C | 45 | – | – |
| Clopidogrel | B | 43 | – | – |
| Losartan | N | 41 | – | – |
| Metronidazole | P | 41 | – | – |
| Trimethoprim | J | 38 | 369 | 221 |
| Chlorhexidine | D | 37 | – | – |
| Methyldopa | C | 37 | – | – |
| Lamotrigine | N | 36 | – | – |
| Eprosartan | C | 35 | – | – |
| Phenytoin | N | 35 | – | – |
| Allopurinol | M | 34 | – | – |
| Sotalol | C | 33 | 7 | – |
As expected, among the top 10 PhACs were acetaminophen and ibuprofen, two pain killers that are available over-the-counter and are widely used in Canada (27,28) and elsewhere (23). The prescription pain killer naproxen was also among the top 10 PhACs. Amoxicillin, a widely used antibiotic, was estimated to have the fifth highest PEC3. Also among the top 10 were acetylcysteine and theophylline, two PhACs that are almost entirely released to Canadian surface waters due to sources other than their clinical use (see Appendix K). Almost 99% of theophylline’s environmental load results from the Canadian population’s ingestion of caffeine; since theophylline, in addition to being a clinical drug itself, is also a metabolite of caffeine (see Appendix E). Metformin, gabapentin, and hydrochlorothiazide ranked 2nd, 8th, and 10th, respectively, and despite their high use in Canada and elsewhere these drugs are only occasionally studied for their aquatic presence and relevance (72). The PEC3 for docusate was likely an underestimate since this PhAC, in addition to be a common excipient in pharmaceutical preparations, is also available over-the-counter in Canada for use as a laxative (31).
Compilation of Measured Exposure Concentrations
In total, 5813 measurements of exposure concentrations were compiled from studies (39–45) reporting on the presence of PhACs in finished (i.e. treated) Canadian drinking waters. Of the 5813 analyses performed, 170 yielded a positive detection for a PhAC. Therefore, to date, PhACs have been detected within finished Canadian drinking water with an overall detection frequency of 2.9% in samples taken. Despite the fact that PhACs are only rarely detected in finished Canadian drinking waters, concentrations as high as 601 and 155 ng/L have been reported for carbamazepine and erythromycin, respectively (see Table II).
Table II.
PhACs Monitored and Detected in Canadian Drinking Water Samples Following Treatment and Their Predicted Environmental Concentrations Based on Three Levels of Conservative Assumptions
| PhAC | ATC class | Measureda | Predictedb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n c | Detected | FODd | MECmax | Reference for MECmax | PEC1 | PEC2 | PEC3 | ||
| % | ng/L | ng/L | |||||||
| Ofloxacin | J | 4 | 2 | 50 | 1.6 | (33) | 5 | 4 | 2 |
| Ibuprofen | N | 219 | 47 | 22 | 75 | (36) | 7702 | 2619 | 1030 |
| Carbamazepine | N | 221 | 42 | 19 | 601 | (35) | 357 | 89 | 89 |
| Gemfibrozil | C | 218 | 26 | 12 | 4 | (35) | 73 | 29 | 15 |
| Erythromycin | J | 214 | 13 | 6.1 | 155 | (35) | 75 | 21 | 16.3 |
| Roxithromycin | J | 214 | 10 | 4.7 | 41 | (35) | –e | ||
| Acetaminophen | N | 176 | 6 | 3.4 | 29 | (36) | 32,642 | 30,357 | 10,117 |
| Tetracycline | J | 214 | 5 | 2.3 | 15 | (35) | 116 | 116 | 52 |
| Fluoxetine | N | 65 | 1 | 1.5 | 6 | (36) | 20 | 7 | 6 |
| Bezafibrate | C | 218 | 3 | 1.4 | 2.9 | (38) | 29 | 22 | 11 |
| Ketoprofen | M | 218 | 3 | 1.4 | 1 | (38) | 6 | 5 | 3 |
| Sulfamethoxazole | J | 221 | 3 | 1.4 | 2 | (35) | 280 | 84 | 54 |
| Clofibric acid | C | 219 | 2 | 0.9 | 1.1 | (33) | –f | ||
| Ciprofloxacin | J | 159 | 1 | 0.6 | 7 | (38) | 65 | 32 | 32 |
| Equilin | 159 | 1 | 0.6 | n.r.g | (35) | –h | |||
| Norfloxacin | J | 159 | 1 | 0.6 | n.r. | (35) | 23 | 9 | 9 |
| Diclofenac | M | 186 | 1 | 0.5 | n.r. | (35) | 151 | 44 | 31 |
| Naproxen | M | 219 | 1 | 0.5 | 26 | (36) | 878 | 659 | 333 |
| Trimethoprim | J | 221 | 1 | 0.5 | 15 | (35) | 61 | 48 | 38 |
| Estrone | G | 237 | 1 | 0.4 | 1 | (38) | 5 | 3 | 2 |
Additional PhACs monitored but not detected in Canadian drinking water samples: estradiol (n =237), estriol (n = 236), ethinylestradiol (n = 236), indomethacin (n = 218), chloramphenicol (n = 155), doxycycline (n = 155), progesterone (n = 155), warfarin (n = 155), testosterone (n = 68), fenoprofen (n = 63), pentoxifylline (n = 63), fenofibrate (n = 59), norethisterone (n = 32), penicillin G (n = 32), sulfadiazine (n = 32), cyclophosphamide (n = 7), sulfacetamide (n = 4), sulfapyridine (n = 4), sulfisoxazole (n = 4), clarithromycin (n = 3), enalapril (n = 3), methotrexate (n = 3), bupropion (n = 1), citalopram (n = 1), paroxetine (n = 1), sertraline (n = 1) and venlafaxine (n = 1). Not included in the list above are PhACs that are only used veterinary purposes
aBased on data compiled from the following sources: (39–45)
bEstimated using Eq. 2
c n = number of samples analyzed
dFOD = frequency of detection
eNot approved for sale in Canada or the USA (31,68) and, therefore the source of the PhAC to Canadian aquatic environment currently remains unclear
fParent drug was discontinued in 2006 (31)
g n.r. not reported by the reference in question
hKnowledge gaps prevent the generation of reliable predictions for this PhAC (70)
The compiled data covers the analysis of only 47 PhACs and of those 20 have been detected in at least one finished Canadian drinking water sample (Table II). The number of samples analyzed for each PhAC ranged from 1 for a number of antidepressants (bupropion, citalopram, paroxetine, sertraline, and venlafaxine) to 237 for the estrogens estradiol and estrone. Of the 47 PhACs monitored, extensive data (n > 100) was available for 26 of them. Of these, ibuprofen and carbamazepine are the two that are most commonly detected, with overall detection frequencies of 22 and 19%, respectively. In contrast, extensive monitoring data in Canada for the presence of estradiol (n = 237), estriol (n = 236), ethinylestradiol (n = 236), indomethacin (n = 218), chloramphenicol (n = 155), doxycycline (n = 155), progesterone (n = 155), and warfarin (n = 155) are still to yield a single positive detection. If the list of PhACs monitored is analyzed by therapeutic classes, it is clear that antibiotics are well represented and typically well monitored, while only limited to no monitoring data are available for some other classes. For example, Class L drugs are only covered by two drugs (cyclophosphamide and methotrexate) and even then, for these two drugs only 10 analyses have been reported.
It is worth noting that the MECs for enalapril, equilin, erythromycin, estradiol, estrone, estriol, ketoprofen, penicillin G, pentoxifylline, progesterone, testosterone, tetracycline, and trimethoprim also result from the release of these drugs from agricultural sources since these PhACs are either endogenously excreted by animals (e.g., estrone) and/or are exogenously administered or fed to animals in Canada (e.g., erythromycin) (31).
PEC as a Conservative Estimate for MEC
As established in the preceding section, MECs are only available for a handful of PhACs. Therefore, for cases where such data was not available, it was of interest to establish whether PEC3 estimates can be used as conservative substitutes for measurements. The degree to which PEC3 estimates are conservative can be assessed by comparing MEC data and their respective PEC3 estimates for all PhACs that have been positively detected in finished Canadian drinking waters. However, the question remains as to which MEC value should the respective PEC3 estimate be compared? Typical practice in risk assessment is to use 95th percentile MEC (MEC95) value or, in other words, an MEC value that is only exceeded by 5% of the values in the data set (73). However, MEC95 values for 15 of the 20 detected PhACs were below the detection limit as their presence in drinking waters has only yielded positive detections in less than 5% of the samples analyzed (Table II). Because of this, a two-step approach was taken to evaluate the degree to which PEC3 estimates were conservative. Firstly, and in a more conservative fashion than typical risk assessment practice, the highest MEC (MECmax) values for all detected PhACs were compared to their respective PEC3 estimates. Then, only for those PhACs for which the PEC3 estimates were found to be lower than their respective MECmax values was a second evaluation performed with MEC95 values.
Table II suggests that, with the exceptions of carbamazepine and erythromycin, PEC3 estimates are higher and hence more conservative than their respective MECmax values. Evaluations for diclofenac, equilin, and norfloxacin could not be performed since the study that reported the single positive detections for these PhACs (42) failed to report the levels at which they were detected. Nevertheless, 99.5% of the MECs for these PhAC were below the detection limit.
For the cases of carbamazepine and erythromycin, a more elaborate assessment using MEC95 values was necessary. The analysis summarized in Appendix M suggests that MEC95 for carbamazepine in finished Canadian drinking water samples was 9.8 ng/L, while the PEC3 was estimated to be 82 ng/L. The approach used in Appendix M further suggests that the PEC3 estimate of 82 ng/L for carbamazepine was higher (i.e., more conservative) than 98% of MECs reported. The MEC95 for erythromycin was estimated to be between 3 and 10 ng/L (35,36,38), which was lower than the PEC3 estimate furnished for this PhAC of 16 ng/L. Overall, it appears that the estimates of PEC3 values as furnished here are more conservative than the vast majority of the MECs measured for its presence in finished Canadian drinking waters. This is not entirely unexpected given the number of conservative assumptions made in estimating PEC3 values.
Acceptable Daily Intake Values
The approached summarized in Fig. 1 was used to estimate the ADI for each PhAC of the evaluation set.
A TDI derived by Health Canada was only available for the PhAC chloral hydrate. For another 31 PhACs, ADIs were available due to the veterinary use of these compounds in Canada and/or elsewhere. These 31 PhACs included 19 antibiotics, 5 glucocorticoid steroids, 4 painkillers, 1 androgen, 1 estrogen, and 1 diuretic. For the remaining 303 PhACs, provisional ADI (p-ADI) values were developed following the procedure summarized in Fig. 1.
LOTDs were available for 289 of the 303 PhACs for which p-ADIs were to be established. The remaining 14 PhACs were either not orally administered (e.g., levobunolol) or were not clinically used and, therefore, generally lacked a dosing schedule (e.g., clofibric acid). While performing p-ADI evaluations with LOTD as the point of departure, LOTDs for adults were typically used. The reason this was done was that a large fraction of the PhACs being considered did not have an established pediatric use and therefore lacked corresponding dosing schedules. To assess the suitability of using adult LOTDs, an evaluation was performed for all 224 PhACs of the evaluation set for which adults as well as pediatric dosage regimens were both found to exist. The results of the evaluation, which are summarized in Appendix N, suggested that for 199 PhACs, or 88% of the cases, LOTDs for adults were lower, and hence more conservative, than their pediatric counterparts. Without performing a comprehensive assessment as the one performed here, others (18) have also alluded to the fact that, for most PhACs, the LOTD for adults are lower than their pediatric counterparts. Overall, for the 199 PhACs for which adults LOTDs were found to be more conservative, adult LOTDs were used. For the 25 exceptions for which pediatric LOTDs were found to be lower, pediatric LOTDs were used. For all remaining PhACs, which lacked pediatric dosing schedules, adult LOTDs were used. The use of adult LOTDs for PhACs that lacked pediatric dosing schedules, though considered to be a pragmatic choice made in light of an unavoidable information gap, should also be viewed with some degree of reservation. The reason a pediatric dosing schedule does not exist for some PhACs is that they are, or can potentially be, hazardous to children at levels that approach the LOTDs of adults. Similarly, reservations should also be expressed regarding the use of adults LOTDs to protect those with impaired metabolism or with hypersensitivity to medication.
OELs were available for 234 of the 303 PhACs for which p-ADIs were to be estimated. For the remaining compounds, either OELs had not been developed by the respective manufacturers or, despite an extensive data search conducted during this study; no suitable material safety data sheets containing such information could be located. NSRL and LTD10 as shown in Fig. 1 were only relevant for genotoxic carcinogenic PhACs of the evaluation set.
Ultimately, following the procedure in Fig. 1, estimated ADI values for 133, 164, 3, and 3 PhACs were based on LOTD, OEL, NSRL, and LTD10 values, respectively. Appendix O presents a summary for each PhAC of the data inputs that were available and, of these, which one was ultimately selected following the procedure of Fig. 1 to arrive at an ADI for each of the 335 PhAC of the evaluation set.
For 220 of the PhACs, both OEL and LOTD values were available and in 70% of these cases, or 149 PhACs, p-ADIs derived from the OEL point of departure were more conservative than those derived using the LOTD approach. To date, OEL limits have only been used in the development of ADI for only four PhACs (16,38)
Predicted No Effect Concentrations for Exposure via Drinking Water
Predicted no effect concentrations for exposure via drinking water (PNECdw) were estimated for each PhAC from their respective ADI values using Eq. 4. Of the receptors defined by Health Canada, infants were selected since they are the most sensitive to drinking water contaminants given that it has been reported that infants in Canada drink more water on a per weight basis than toddlers, children, teens, and adults (66). Therefore, conservatively, PNECdw estimates furnished and discussed here are specific for exposure of infants. Note that Canadian exposure factors (66) are such that the respective PNECdw for teens, adults, children and toddlers were found to be 2.2, 1.7, 1.5, and 1.01 times higher than those derived for infants.
PNECdw values for the entire evaluation set are plotted in Fig. 5 where the data has been categorized by ATC classes to facilitate interpretation. The estimated PNECdw values ranged from a minimum of 0.03 ng/L for the anticancer drug dactinomycin (a Class L drug) to a maximum of nearly 9 × 106 ng/L for the contrast media diatrizoate (a Class V drug). Among the most potent (i.e. lowest median PNECdw) drug classes were Class G, Class L, those for which an ATC class code has not been assigned (i.e., “No Code”), and Class H (see Fig. 5). These classes cover such sub-classes as anticancer drugs, sex hormones, and other hormones, drugs used for endocrine therapy, illicit drugs and immunosuppressants, all of which are sub-classes of drugs that would be expected to be highly potent in their potential to yield effects on human health.
Fig. 5.
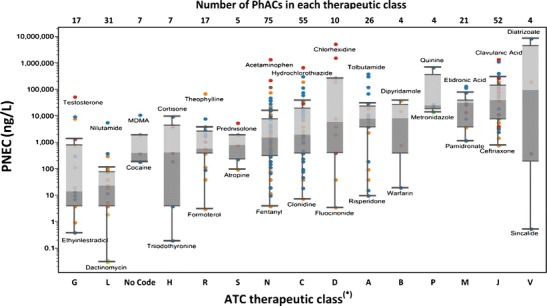
PNEC estimates grouped according to therapeutic classes as defined by the Anatomical Therapeutic Chemical (ATC) Classification System. PNEC estimates have been color coded according to the data input ultimately used in the derivation of ADIs following the procedure of Figure 1 as follows: ADIvet ( ), LOTD (
), LOTD ( ), LTD10 (
), LTD10 ( ), NSRL (
), NSRL ( ), OEL (
), OEL ( ), TDI (
), TDI ( ). *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
). *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
The most potent drug class was Class G with a median PNECdw of 14 ng/L. Class L drugs were also found to be particularly potent in terms of their potential to cause human health effects where the median PNECdw for this class of drugs was estimated to be 23 ng/L. Of the 31 Class L drugs, 19 were anticancer drugs and another 11 were endocrine therapy drugs. The median PNECdw for all 19 anticancer drugs was 12 ng/L with individual estimates ranging from 0.03 ng/L for dactinomycin to 390 ng/L for capecitabine. The median PNECdw for all 11 endocrine therapy drugs was 39 ng/L with individual estimates ranging from 2 ng/L for leuprolide to 6000 ng/L for nilutamide. The PhACs classified as “No Code” included five illicit drugs and two endogenous androgens. The PNECdw for the five illicit drugs ranged from 187 ng/L for cocaine to nearly 11,000 ng/L for MDMA. The PNECdw for the two androgens included in the “No Code” class were 390 ng/L for dihydrotestosterone and approximately 2000 ng/L for androstenedione. Particularly potent among the Class H drugs were the thyroid hormones triiodothyronine and thyroxine with PNECdw values of 0.2 and 3.9 ng/L, respectively. Triiodothyronine and thyroxine are almost entirely released to the Canadian environment due to their endogenous excretion (see Appendix K)
Drug classes that were found to lack potency in potentially affecting human health included Class J and Class M drugs. Consider that the median PNECdw for all 52 Class J drugs, of which 43 were antibiotics, was approximately 39,000 ng/L. The PNECdw estimates for class J drugs ranged from nearly 800 ng/L for ceftriaxone to 1.4 × 106 ng/L for clavulanic acid (see Fig. 5). The median PNECdw for all 21 Class M drugs, of which 14 were anti-inflammatory drugs, was 31,000 ng/L. Specific estimates for Class M drugs ranged from 1200 ng/L for pamidronate to 137,000 ng/L for etidronic acid (Fig. 5). For the 14 anti-inflammatory drugs, the median PNECdw was estimated to be 35,000 ng/L with individual estimates ranging from 4000 ng/L for indomethacin to 117,000 ng/L for mefenamic acid. Included among the 14 anti-inflammatory drugs was ibuprofen, the one PhAC that is most commonly detected in finished Canadian drinking water (see Table II). The PNECdw for ibuprofen was estimated to] [Fig6] be nearly 8000 ng/L.
Risk Assessment Using Measured Exposure Concentrations
A risk assessment using measured exposure concentrations was performed in a conservative manner by estimating the margin of exposure (MOE) for the highest MEC (MECmax) reported for each PhAC that has been detected in at least one finished Canadian drinking water sample (see Table III). The resulting MOEm-max estimates ranged from 4 for estrone to 137,500 for sulfamethoxazole. Therefore, not a single MOEm-max value was estimated to be below the risk trigger limit of 1.0. MOEm-max values below 100 were only observed for 2 PhACs; namely, estrone and carbamazepine. The MOEm-max for estrone was estimated to be 4 based a single positive detection reported by Tabe et al. (45). However, to date, 237 drinking water samples have been analyzed in Canada for the presence of estrone and only 1 of these samples has yielded a positive detection. Therefore, 99.6% of the MECs for estrone in finished Canadian drinking water were below detection limits. In contrast, carbamazepine is one of the most commonly detected PhACs in finished Canadian drinking waters (see Table III). The MOEm-max for carbamazepine was estimated to be 13. However, if the evaluation is performed with MEC95 instead, which is in line with typical risk assessment practice, the MOE for exposure to carbamazepine increases to 802 (PNECdw of 7860 ng/L divided by MEC95 of 9.8 ng/L). Overall, a risk assessment with MECs reported thus far for PhACs in finished Canadian drinking waters suggest that they pose a negligible risk to human health.
Table III.
Risk Assessment of PhACs Using Maximum Environmental Concentrations, MECmax, Reported in Finished Canadian Drinking Water Samples
| PhAC | Number | FODa | MECmax | Reference for MECmax | PNECdw b | MOEm-max = MECmax/PNEC |
|---|---|---|---|---|---|---|
| % | ng/L | ng/L | ||||
| Estrone | 237 | 0.4 | 1 | (38) | 4 | 4 |
| Carbamazepine | 221 | 19 | 601 | (35) | 7860 | 13 |
| Ibuprofen | 219 | 22 | 75 | (36) | 7860 | 105 |
| Fluoxetine | 65 | 1.5 | 6 | (36) | 1960 | 327 |
| Ciprofloxacin | 159 | 0.6 | 7 | (38) | 4130 | 590 |
| Erythromycin | 214 | 6.1 | 155 | (35) | 138,000 | 890 |
| Roxithromycin | 214 | 4.7 | 41 | (35) | 58,900 | 1437 |
| Naproxen | 219 | 0.5 | 26 | (36) | 39,300 | 1512 |
| Tetracycline | 214 | 2.3 | 15 | (35) | 82,500 | 5500 |
| Trimethoprim | 221 | 0.5 | 15 | (35) | 110,000 | 7333 |
| Gemfibrozil | 218 | 12 | 4 | (35) | 39,300 | 9825 |
| Bezafibrate | 218 | 1.4 | 2.9 | (38) | 39,300 | 13,552 |
| Ketoprofen | 218 | 1.4 | 1 | (38) | 27,500 | 27,500 |
| Clofibric Acid | 219 | 0.9 | 1.1 | (33) | 31,400 | 28,545 |
| Acetaminophen | 176 | 3.4 | 29 | (36) | 1,375,000 | 47,414 |
| Ofloxacin | 4 | 50 | 1.6 | (33) | 157,000 | 98,125 |
| Sulfamethoxazole | 221 | 1.4 | 2 | (35) | 275,000 | 137,500 |
Risk Assessment Using Predicted Exposure Concentrations
Since extensive monitoring data of finished Canadian drinking waters was only available for 27 PhACs (n > 100; see Table I), a conservative risk assessment of the evaluation set with PEC estimates was also performed. An estimated MOEp of less than 1.0 was taken to be indicative of “possible risk” and that the PhAC in question should be considered for further study and prioritization. A value of greater than 1.0 but less than 10 was taken to suggest that the PhAC in question represented a “low risk” but still required further consideration for prioritization, and a value greater than 10 was taken to indicate “negligible risk”.
Margins of exposure based on PEC3 estimates (MOEp3) are plotted by ATC Classes in Fig. 6. Specific MOEp3 estimates for each PhAC are listed in Appendix P with individual values ranging from virtually infinity for clofibrate, ethynodiol, famciclovir, sildenafil, and rabeprazole to 0.08 for triiodothyronine. Of the entire evaluation set only two PhACs, estriol (a Class G drug) and triiodothyronine (a Class H drug), were predicted to have MOEp3 values below the trigger limit for possible risk of 1.0. MOEp3 estimates for an additional 14 PhACs were predicted to fall between 1 and 10 (Fig. 6). The remaining 319 PhACs were found to pose a negligible risk since their respective MOEp3 estimates were found to be greater than 10 (Fig. 6). Specifically, for these 319 PhACs, 44, 95, 93, 47, and 40 PhACs were estimated to have an MOEp3 of greater than 10 but less than 100, greater than 100 but less than 1000, greater than 1000 but less than 10,000, greater than 10,000 but less than 100,000, and greater than 100,000, respectively (Appendix Q).
Fig. 6.
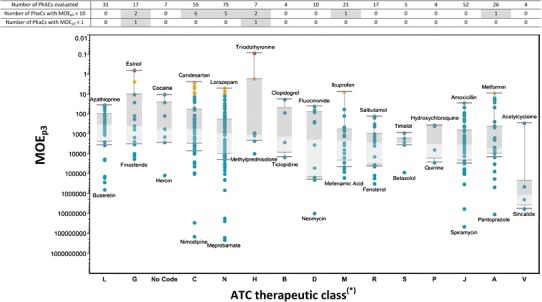
Margin of exposure, MOEp3 (PNECdw divided by PEC3), estimates grouped according to therapeutic classes as defined by the Anatomical Therapeutic Chemical (ATC) Classification System. MOEp3 estimates have been color coded with respect to their estimated risk to human health as follows: Possible risk ( ); Low risk, but should be considered for further prioritization (
); Low risk, but should be considered for further prioritization ( ); Negligible risk (
); Negligible risk ( ). *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
). *Classifications according to the World Health Organization ATC classification system (47) where abbreviations used on the y-axis represent the following classes of drugs: A alimentary tract and metabolism; B blood and blood forming organs; C cardiovascular system; D dermatologicals, G genito-urinary system and sex hormones; H systemic hormonal preparations; J antiinfectives; L antineoplastic and immunomodulating agents; M musculo-skeletal system; N nervous system; P antiparasitic products; R respiratory system; S sensory organs; V various
If MOEp3 estimates are evaluated according to therapeutic class, Class L drugs were found to present the highest level of relative risk with a median MOEp3 of 330, with specific estimates for this class of drugs ranging from 621 × 103 for buserelin to 34 for azathioprine. Therefore, every one of the 31 Class L drugs evaluated was found to pose a negligible risk to human health (see Fig. 6). Similar to Class L drugs, all drugs of the evaluation set belonging to ATC Classes B, D, R, S, P, J, and V were found to pose a negligible risk to human health. All PhACs for which an ATC class could not be assigned (represented by “No Code”) were also found to pose a negligible risk to human health. After Class L drugs, Class G drugs were found to have the second lowest median MOEp3 of 395, with specific estimates for this class of drugs ranging from nearly 18 × 103 for finasteride to 0.63 for estriol. In addition to estriol, estrone was the other Class G drug for which an MOEp3 of <10 was predicted, all 15 other Class G drugs were found to pose a negligible risk. Also noteworthy was the fact that as many as 7 and 3 PhACs belonging to Classes C and N, respectively, were predicted to have MOEp3 values between 1 and 10 (see Fig. 6 and Table IV). Of the seven Class H drugs evaluated only the two thyroid hormones were found to have MOEp3 estimates of less than 10 (see Fig. 6 and Table IV). Specifically, an MOEp3 of 1.7 was estimated for thyroxine and 0.08 for triiodothyronine. Of all 21 Class M drugs evaluated, only ibuprofen was predicted to have an MOEp3 estimate of less than 10 (see Fig. 6). Similarly, of all 26 Class A drugs evaluated, only metformin was predicted to have an MOEp3 estimate of less than 10 (see Fig. 6). All genotoxic carcinogenic PhACs of the evaluation set were also predicted to present a negligible risk.
Table IV.
Prioritization of PhACs for Future Monitoring Studies Based on Predicted and Measured Environmental Concentrations
| PhAC | Predicted | Measured in drinking water | Potential to transport to drinking water intakes | Prioritize? | |||
|---|---|---|---|---|---|---|---|
| PEC3 a | MOEp3 b | Detects/analyzedc | MOEm-max = PNECd/MECmax c | Sorptione | Degradatione | ||
| ng/L | |||||||
| Triiodothyronine | 2.4 | 0.08 | n.m.f | Sorption could not be modelled. Log D (@ pH 7.4) = 2.50 |
t 1/2 = 60 days est. (g) | Yes | |
| Estriol | 6.2 | 0.64 | 0/236 | n.d. | Log K oc-sediment = 2.99 | t 1/2 = 0.12 days | No. Extensive Canadian monitoring data is available without a single positive detection. Rapidly degrades once released into the aquatic environment |
| Thyroxine | 2.3 | 1.7 | n.m. | Sorption could not be modelled. Log D (@ pH 7.4) = 2.89 |
t 1/2 = 180 days est. | Yes | |
| Estrone | 1.7 | 2.3 | 1/237 | 4 | Log K oc-sediment = 3.44 | t 1/2 = 2.3 days | No. Extensive Canadian monitoring data is available with only a single positive detection. A photolysis product, lumiestrone, warrants further monitoring since it has been suggested that it is both estrogenic and persistent (69) |
| Ramipril | 20 | 2.4 | n.m. | Sorption could not be modelled. Log D (@ pH 7) = −0.16 |
t
1/2 = 38 days est. 20–50% degradation of the compound seen after 28 days in an OECD 301 test. |
Yes | |
| Candesartan | 15 | 2.6 | n.m. | K d-sediment = 2–4 L/kg | t 1/2 = 95–222 days | Yes | |
| Lisinopril | 25 | 3.9 | n.m. | Sorption could not be modelled. Log D (@ pH 7.4) = −1.81 |
t
1/2 = 15 days est. 0% degradation of the compound seen after 28 days in an OECD 301 test. |
Yes | |
| Atorvastatin | 81 | 4.8 | n.m. | Log K oc-sediment = 3.20 |
t
1/2 = 6.6 days (Half-life observed in a microcosm) |
Yes | |
| Lorazepam | 3.9 | 5.0 | n.m. | Sorption could not be modelled. Log D (@ pH 7.4) = −1.81 |
t
1/2 = <1 day (Photolysis half-lives) |
Yes | |
| Fentanyl | 0.6 | 7.0 | n.m. | Log K oc = 3.20 (est.) |
t
1/2 = 15 days est. Unstable in raw sewage. |
Yes | |
| Ibuprofen | 1030 | 7.6 | 47/227 | 105 | Log K oc-sediment = 2.08 | t 1/2 = 2.5–5 days | No. Extensive Canadian monitoring for the PhAC suggests negligible risk to human health. Although it has potential for use as an indicator for other PhACs |
| Atenolol | 122 | 8.0 | n.m. | Log K oc-sediment = 1.9–2.1 | t 1/2 = 2.3–30 days | Yes | |
| Metformin | 3540 | 8.8 | n.m. | Log K oc = 1.5 | t 1/2 = 8–9 days | Yes | |
| Enalaprilat | 11 | 8.9 | n.m. | Sorption could not be modelled. Log D (@ pH 7) = 2.50 |
t
1/2 = <1 day (Photolysis half-lives) |
Yes | |
| Morphine | 22 | 9.0 | n.m. | Log K oc-sediment = 2.6–2.7 | t 1/2 = 0.3 days | Yes | |
| Irbesartan | 126 | 9.3 | n.m. | Log K oc = 2–3 |
t
1/2 = 0.3–24 days (photolysis half-lives) |
Yes | |
Considering the highly conservative nature of PEC3 estimates, and hence, estimates of MOEp3, a prioritization evaluation was performed for all 16 PhACs for which an MOEp3 of less than 10 was estimated. The goal of this evaluation was to develop, from the list of 16 PhACs, a sub-list of priority PhACs for which the development of comprehensive ADI values, extensive monitoring in Canadian drinking waters, and geo-spatially explicit contaminant fate modelling would be recommended. The results of the prioritization evaluation are summarized in Table IV. Overall, each of the 16 PhACs were prioritized unless there was extensive MEC data (n > 100) in finished drinking waters suggesting negligible risk or, in the absence of extensive MECs, there was compelling evidence to suggest that the PhAC being considered was highly unlikely to reach drinking water intakes due to a high sorption and/or degradation potential.
Of the 16 PhACs, MECs in finished drinking water were only available for three: ibuprofen, estrone, and estriol. MEC data for ibuprofen suggested that it poses negligible risk to human health and therefore can be considered to not be a priority compound. However, given the fact that ibuprofen is the one PhAC that is detected with the highest frequency in finished Canadian drinking waters (see Table II), it carries the potential to be used as an indicator compound for the contamination of Canadian drinking waters with PhACs. Extensive monitoring data (n = 236) for estriol and estrone in Canada has only yielded zero and one positive detections, respectively. Therefore, these PhACs are essentially undetected in finished Canadian drinking water samples. This is to be expected as both estriol and estrone are known to degrade in the environment (16). In conjunction with the lack of detection of these PhACs, one must also recognize that the p-ADI developed in the current assessment for estrone and estriol of 0.00014 μg/kg × day is highly conservative (see Appendix O). Consider that the ADI for estradiol, due to its veterinary uses, has been estimated by the Joint FAO/WHO Expert Committee on Food Additives to be 0.050 μg/kg × day (see Appendix O), a level that is 360 times higher than the conservative p-ADI developed for estriol and estrone in the current assessment. Yet estrone and estriol have been suggested to be 2 and 17 times less potent than estradiol, respectively, when administered orally in the rat uterotrophic bioassay (74). Overall, it is concluded that estrone and estriol should not be considered as compounds of concern for priority follow-up. However, a photolysis product of estrone, lumiestrone, has been shown to be estrogenic and persistent (75) and therefore warrants further consideration.
Prioritized PhACs
For the remaining 13 PhACs, no monitoring data was available for finished Canadian drinking waters and, further, there was no compelling evidence to suggest that their respective sorption and/or degradation potentials would prevent their loads from being transmitted to drinking water intakes (see Table IV). Therefore, it is concluded that they should be prioritized for further studies. Each of the 13 prioritized PhACs are briefly discussed below.
An MOEp3 of 0.08 was estimated for triiodothyronine, which is a thyroid hormone that is almost entirely released to the Canadian aquatic environment due to endogenous excretions (see Appendix K). Upon its release to the environment, triiodothyronine is expected to be mobile and persistent (see Table IV) but it has yet to be monitored in drinking waters from Canada or elsewhere. In fact, to date, there is only the report of a single influent and a single effluent sample from a Finnish sewage treatment plant that have been analyzed for the environmental presence of this PhAC (76). In both samples analyzed, triiodothyronine was found to be below the detection limit of 2–4 ng/L (76).
The MOEp3 for the other thyroid hormone, thyroxine, was estimated to be 1.7. Almost 94% of the load of thyroxine to the Canadian environment results from endogenous excretions (see Appendix K). Thyroxine is also predicted to be persistent and sufficiently mobile upon its release to the environment (see Table IV and (77)). To date, environmental monitoring of thyroxine within the Canadian environment has not been conducted. In fact, the measurement of thyroxine has only been reported in a single influent and a single effluent sample from a Finnish sewage treatment plant (76). Thyroxine was detected in both the influent and the effluent at levels of 64 and 22 ng/L, respectively. If this effluent level is divided by a commonly assumed dilution factor of 10 (54), a surface water concentration of 2.2 ng/L can conservatively be estimated. If a further surface water concentration of 2.2 ng/L is used as an exposure concentration, an MOE of 1.8 (3.9 ng/L (PNECdw for thyroxine) divided by 2.2 ng/L) can be predicted.
An MOEp3 of 2.4 was estimated for ramipril, which is used for the treatment of hypertension and congestive heart failure. Ramipril is expected to be released to the Canadian aquatic environment primarily through its direct use in the general population, as only a small fraction of the PhAC is sourced through hospitals (see Fig. 7). Upon its release to the environment, ramipril is expected to be mobile and subject to some degradation (see Table IV). Ramipril remains to be monitored in Canadian drinking or surface waters. However, an extensive monitoring campaign in France (78), failed to yield a single positive detection for Ramipril in 285 finished drinking water samples for a limit of detection of 5 ng/L. The PEC3 estimated here for ramipril of 20 ng/L may have been overestimated due to an assumption made about the metabolism of the PhAC; that is, based on metabolic disposition data available for ramipril; it was unclear what fraction of the PhAC is excreted unchanged via the fecal route (see Appendix D). Therefore, it was conservatively assumed that the net fecal elimination of the PhAC of approximately 40% was in the form of unchanged ramipril. If this assumption had not been made, the PEC3 estimate for ramipril would have only been 0.5 ng/L and, consequently, its MOEp3 would have been estimated at the negligible risk level of 98.
Fig. 7.
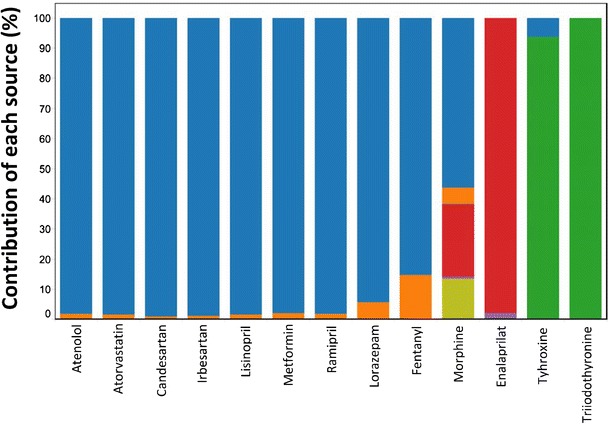
Sources of prioritized PhACs: M
CG ( ); M
CH (
); M
CH ( ); M
endo(
); M
endo( ); M
OC(
); M
OC( ); M
OH(
); M
OH( ); M
OID (
); M
OID ( )
)
An MOEp3 of 2.6 was estimated for candesartan, which is typically used for the treatment of hypertension. Candesartan is primarily released to the Canadian aquatic environment through its use in the general population, with hospital use accounting for less than 1% of its environmental load (see Fig. 7). Upon its release to the environment, candesartan is expected to be highly mobile and persistent (see Table IV and (79)). Candesartan is yet to be monitored in Canadian environmental matrices. Outside of Canada, only limited environmental occurrence data for this PhAC have been reported (80) but concentrations as high as 1100 ng/L for candesartan have been reported in German surface waters (80). If this surface water maximum is conservatively taken as a reasonable estimate of exposure concentration, an MOE of 0.04 is calculated, which is well below the risk trigger level of 1.0 and, thus, could be of concern. Note that, on a per capita basis, the use of candesartan in Germany is nearly twice the level with which the drug is used in Canada (27,28,80).
Lisinopril is an angiotensin-converting enzyme-inhibiting PhAC that is typically used for the treatment of hypertension. An MOEp3 of 3.9 was estimated for lisinopril. Lisinopril is primarily released to the Canadian aquatic environment through its use in the general population, with only 2% of the environmental load coming from hospitals (see Fig. 7). Lisinopril is expected to be highly mobile and persistent upon its release to the environment (see Table IV). Canadian environmental matrices have yet to be monitored for the presence of lisinopril. Furthermore, no drinking water monitoring data for this PhAC even from locations outside Canada could be located in the literature. However, levels as high as 3300 ng/L have been reported for this PhAC in effluents from sewage treatment plants in the USA (81). If this measured maximum is divided by a conservative dilution factor of 10, a highly conservative surface water concentration of 330 ng/L can be predicted. Further, if this surface water concentration is used as an estimate of the exposure concentration, an MOE of 0.3 [98 ng/L (PNECdw for lisinopril) divided by 330 ng/L] can be predicted, which is below the trigger threshold of 1.0 and, therefore, could be of concern. That being said, the above evaluation is highly conservative.
Atorvastatin is a PhAC that is used to lower blood cholesterol. An MOEp3 of 4.8 was estimated for this compound. Atorvastatin is almost entirely released to the Canadian aquatic environment due to its use in the general population, with less than 1% of the PhAC being sourced through Canadian hospitals (see Fig. 7). Upon its release to the environment, the environmental load of atorvastatin load would be expected to be somewhat attenuated due to sorption to sediments and degradation (see Table IV). Atorvastatin has yet to be monitored in Canadian drinking waters, but reports exist of it being monitored in Canadian surface waters. To date, 27 Canadian surface water samples have been analyzed for the presence of atorvastatin (see Appendix R) in which atorvastatin was detected with a frequency of 33% and at a maximum concentration of 59 ng/L (see Appendix R). If this maximum concentration in surface waters is conservatively used as an estimate of exposure concentration, an MOE of 6.7 is calculated [390 ng/L (PNECdw for atorvastatin) divided by 59 ng/L]. Analyses of 18 American finished drinking water samples did not yield a single positive detection for atorvastatin (29). Furthermore, when the associated source waters were analyzed, atorvastatin was only detected in 2 of the 19 samples analyzed with a maximum of 2 ng/L (29). However, atorvastatin was reported to be present at a level of 1 ng/L in a finished drinking water sample from Spain (83). With a MEC of 1 ng/L, an MOE of 390 is calculated, thereby indicating negligible risk to human health.
Lorazepam is a benzodiazepine that is used for the treatment of anxiety disorders. Lorazepam is predominantly expected to be released to the Canadian aquatic environment due to its use in the general population; however, as much as 6% of the PhAC is sourced through hospitals (see Fig. 7). Upon its release to the environment, Lorazepam is expected to be mobile and subject to degradation (see Table IV). Canadian environmental matrices have yet to be monitored for the presence of lorazepam. However, lorazepam was not detected in finished French and Spanish drinking waters (83,84). The per capita consumption of lorazepam in France is similar to the level with which the PhAC is used in Canada (14,27,28).
Fentanyl is an opioid that is used as an anesthetic and to relieve pain. As much as 15% of the PhAC is sourced through Canadian hospitals, while the remainder is made available to the general population through pharmacies (see Fig. 7). An environmental load of fentanyl would be expected to be attenuated due to sorption onto sediments and degradation (see Table IV). Fentanyl has yet to be monitored in Canadian surface or drinking waters. However, fentanyl was found to be below limits of quantification/detection in the influent and the effluent of a Canadian sewage treatment plant (85). Fentanyl was also not detected in finished Dutch and Spanish drinking waters (86,87). However, an extensive survey of European effluents was able to detect fentanyl with a detection frequency of less than 1% and with a maximum concentration of 1.6 ng/L (88). It is also worth noting that, in recent years, there has been a steady increase in the use of fentanyl in Canada, with an increase of 50% recorded over the period of 2005 to 2012 (89).
Atenolol is a beta blocker that is used for the treatment of cardiovascular diseases. It is primarily released to the Canadian environment through its use in the general population, with only 2% of the PhAC being sourced through hospitals (see Fig. 7). Upon its release to the environment, atenolol is expected to be mobile and subject to degradation. Canadian drinking waters have yet to be monitored for the presence of atenolol. However, nineteen (19) Canadian surface water samples have been analyzed for the presence of this PhAC (see Appendix R). In these samples, atenolol was detected with a frequency of 47% and at a measured maximum concentration of 53 ng/L (see Appendix R). If the measured maximum in surface water of 53 ng/L is conservatively taken as an exposure concentration, an MOE of 18 is calculated, indicating negligible risk. Atenolol has been detected in finished drinking waters in the USA with a maximum concentration of 26 ng/L (1). In contrast, atenolol was not detected in English and French finished drinking waters when analyzed with detection limits of 2 and 25 ng/L, respectively (78,90).
Metformin is an anti-diabetic drug for which an MOEp3 of 9.0 was estimated (see Table IV). Metformin is almost entirely released to the Canadian aquatic environment due to its use in the general population (see Fig. 7). Upon its release to the environment, metformin is expected to be mobile and subject to some degradation (see Table IV). Metformin has yet to be monitored in Canadian drinking and surface waters, but it has been monitored in the influent and effluent of a Canadian sewage treatment plant equipped with a membrane bioreactor (91) and the discharges of untreated wastewater by the city of Victoria in the Province of British Columbia (92). Metformin was measured in concentrations as high as 110,000 ng/L in one of the discharges of untreated wastewater from the city of Victoria (92). Outside Canada, metformin was not detected in finished Dutch drinking waters when sought using an analytical method with a detection limit of 50 ng/L (93).
Enalaprilat is an angiotensin-converting enzyme (ACE) inhibitor that is typically used for the treatment of hypertension and certain types of chronic heart failures. Even though enalaprilat is clinically used in Canada (31), almost the entire load of the PhAC in the Canadian environment is expected to be due to the use of the precursor pro-drug enalapril (see Appendix I). Enalapril, and therefore enalaprilat, is primarily used in the general population with a relatively minor fraction of the PhAC being sourced through hospitals (see Fig. 7). Upon its release to the environment, enalaprilat is expected to be mobile and subject to degradation (see Table IV). Canadian environmental matrices have yet to be monitored for the presence of enalaprilat. Further, no drinking water monitoring data for the PhAC from locations outside of Canada could be identified. However, levels as high as 150 ng/L have been reported for this compound in effluents from sewage treatment plants in the USA (81). If this measured maximum is divided by a dilution factor of 10, a highly conservative surface water concentration of 15 ng/L is predicted and an MOE of 6.5. Note that enalaprilat is poorly bioavailable upon oral ingestion (60) and this is one of the reasons for why enalaprilat is clinically administered via intravenous injections.
Morphine is an opioid that is typically used as a painkiller. As discussed earlier, morphine in the Canadian environment arises from a number of sources. Of these, the single most important source is the use of morphine itself in the general population (Fig. 7). However, other sources still account for an estimated 42% of the net environmental load (see Fig. 7). Upon its release to the environment, morphine is expected to be mobile and liable to significant degradation (see Table IV). To date, morphine has not been monitored in Canadian surface or drinking waters, however it has been measured in effluents from Canadian sewage treatment plants at levels as high as 69 ng/L (94). If this maximum concentration measurement is divided by a dilution factor of 10, a highly conservative surface water concentration of 6.9 ng/L is predicted along with an MOE of 28, indicating negligible risk. Outside Canada, morphine was not detected in finished Dutch and German drinking waters (87,95).
Irbesartan is a drug that is used for the treatment of high blood pressure and certain kidney diseases. Irbesartan is primarily released to the Canadian aquatic environment due to its use in the general population, with hospitals being a minor source for the PhAC’s environmental load. Upon its release to the environment, irbesartan would be expected to be mobile and possibly liable to degradation (see Table IV). Canadian environmental matrices have yet to be monitored for the presence of irbesartan. Outside Canada, irbesartan could not be detected in finished Spanish drinking waters (83,96). However, an extensive survey of European effluents was able to detect irbesartan with a 100% detection frequency and a maximum of 17,900 ng/L (88).
It is worth noting that of the 13 prioritized PhACs, ADIs for 8 PhACs were ultimately estimated using respective LOTD values and a highly conservative safety factor, SF, of 10,000. Specifically, these PhACs were: triiodothyronine, thyroxine, ramipril, lisinopril, atorvastatin, lorazepam, atenolol, and enalaprilat (see Appendix O). A SF of 10,000 was used for triiodothyronine and thyroxine because they are endogenous thyroid hormones. For the other six PhACs, a SF of 10,000 was used because they were either pregnancy class D or X drugs. However, had the default SF of a 1000 been used instead, MOEp3 for 7 PhACs listed above would have been estimated at negligible risk levels (i.e., MOEp3 > 10), while the MOEp3 for triiodothyronine would have still remained below the trigger limit of 1.0 for possible risk. Nevertheless, since the goal here was to prioritize the human health risk of PhACs in a manner that false negative classifications were minimized the use of highly conservative SF remains. That being said, it is important to appreciate the conservative nature of our analysis, both in estimating exposure and accounting for effects on human health.
Overall, our results are consistent with those reported by others (1,4,15–25) in that most PhACs, even when evaluated highly conservatively, are deemed unlikely to pose a human health risk when considered individually. Interestingly, lisinopril, atenolol, and enalaprilat, three PhACs prioritized here, were also among the six shown to pose the highest relative, yet low, risk to the health of the population in the USA (81).
A limitation of evaluations performed with MOEp3 estimates, as has been the focus thus far, is that the risk outcome for a given PhAC could be highly sensitive to the assumptions made about its metabolic conversion and removal in sewage treatment plants. Therefore, it is also of value to perform risk evaluations with PEC1 estimates to see for which PhACs a risk outcome of “possible risk” resides in assumptions made about their metabolic conversion and removal in sewage treatment plants. Such an evaluation, which is sometimes referred to as the “total residue approach” (97), conservatively assumes that a given PhAC’s metabolites and transformation products are as potent as the PhAC itself and further assumes that the total residue is not removed in sewage treatment plants.
The results of a risk evaluation based on PEC1 estimates (MOEp1) are summarized in Appendix Q. The results show that if the total residue approach is assumed, only seven PhACs of the evaluation set would yield an MOEp1 value less than the trigger limit of 1.0 for “possible risk”. Specifically, these seven PhACs were triiodothyronine, estriol, cocaine, morphine, estrone, thyroxine, and ramipril (see Appendix Q). As discussed earlier, triiodothyronine and estriol also trigger “possible risk” based on PEC3 estimates (see Appendix Q). An evaluation with PEC2 estimates (i.e., considering metabolic conversion but discounting removal in sewage treatment plants) triggers possible risk for thyroxine as well as triiodothyronine and estriol (see Appendix Q). Therefore, the possible risk outcomes of cocaine, morphine, estrone, and ramipril reside in assumptions made about their metabolism, while that for thyroxine resides in assumptions made about its metabolism and subsequent removal in sewage treatment plants. Of the latter five PhACs, estrone likely poses a negligible risk to human health, as discussed earlier.
Cocaine is extensively metabolized by humans with between less than 1 and 8% of the drug being excreted unchanged (12). Of the various metabolites of cocaine, only norcocaine and cocaethylene are active (98). Norcocaine is twice as potent as cocaine, while cocaethylene is equipotent (87,98). However, only less than 0.1 and 0.7% of an administered dose of cocaine are excreted as norcocaine and cocaethylene, respectively (12). In a number of investigations, it has been shown that cocaine undergoes significant depletion in sewage treatment plants (85), which includes partitioning onto sewage sludge (99). Canadian surface or drinking waters have yet to be monitored for the presence of cocaine or its active metabolites. However, cocaine could not be detected in finished Dutch and English drinking waters (87,90). In contrast, cocaine has been detected in tap waters at unidentified locations by Boleda et al. (100) to a maximum of 2.9 ng/L, which translates to an estimated MOE of 64 representing a negligible risk. Norcocaine could not be detected in finished Dutch and Spanish drinking waters (87,100). Cocaethylene was detected to a level of 0.9 ng/L in Spanish tap waters and not detected in finished Dutch drinking waters (87,100). An exposure concentration of 0.9 ng/L for cocaethylene translates to a negligible risk MOE of 192 (187 ng/L (i.e., the PNECdw for cocaine—since cocaethylene is equipotent)—divided by 0.9 ng/L). Overall, it appears that cocaine and its active metabolites are unlikely to be present in Canadian drinking waters at levels that result in risks to human health.
The remaining three PhACs, morphine, thyroxine, and ramipril were already among the list of PhACs prioritized through a risk evaluation with MOEp3 values. Nevertheless, their metabolism and removal in sewage treatment plants warrants further discussion.
Morphine largely results from its direct clinical use and the clinical and illicit use of its precursors. For the PEC1 estimate for morphine, it was conservatively assumed that the entire mass of morphine itself and its clinical and illicit precursors end up in the environment as morphine. Therefore, the PEC1 estimate for morphine not only accounts for the total residue of morphine, but also of its precursors. This results in a highly artificial, yet conservative, estimate of PEC1 for morphine. Morphine is primarily excreted by humans as its glucuronide conjugates (12). Since such conjugates are known to revert to the parent compound upon their release to the environment (12), they were considered as the parent compound when PEC2 and PEC3 estimates were calculated for morphine. The only other active metabolite of morphine is normorphine, which has been suggested to be one-fourth as potent as morphine (101). Additionally, less than 5% of an administered dose of morphine is excreted as normorphine (12). Normorphine is yet to be monitored in Canadian environmental matrices. However, it was not detected when looked for in tap waters from a number of countries (100).
Ramipril is in fact a pro-drug for the active metabolite ramiprilat (31). Approximately 9% of an administered dose of ramipril is eliminated via urine and bile as ramiprilat and its glucuronide conjugate (102). Much like ramipril, ramiprilat has not been monitored to date in Canadian environmental matrices. However, an extensive monitoring campaign of finished drinking waters in France found ramiprilat in a single sample of the 285 samples analyzed. Specifically, the single positive detection was between the study’s limit of detection (not reported) and the limit of quantification (5 ng/L). Ramiprilat was found in 2 of the 285 samples source water samples analyzed, again at levels between the limit of detection and the limit of quantification for the study. Other than this study, little monitoring data has been reported for the environmental occurrence of ramiprilat. Recognizing this, ramiprilat is included in the list of priority compounds that warrant further study.
Thyroxine is primarily released to the Canadian environment through endogenous excretions (see Appendix K). Nevertheless, important uncertainties are present in arriving at the net environmental load for this PhAC (see Appendix F). In addition, the treatment plant removal rate used for this PhAC results from the analysis of a single sample (76). Therefore, these uncertainties with respect to the environmental fate and loading of thyroxine indicate that further study of this PhAC is warranted.
Agricultural Contributions
In Canada, the aquatic releases of 31 of the 335 PhACs in the evaluation set are also expected to result from agricultural sources since these compounds are either endogenously excreted by animals (e.g., estrone) and/or are exogenously administered or fed to animals (e.g., erythromycin) (see Table V). Extensive MEC data for a PhAC, if available, can be taken to include agricultural sources, but such sources were not considered in the PEC estimates calculated here. Of the 31 PhACs for which agricultural contributions are expected, extensive MEC data was only available for six of them (see Table V). For these six, an evaluation using MEC values has already suggested that they pose a negligible risk to human health (see Tables IV and VI). Therefore, the release of these PhACs from human and agricultural sources likely represents a negligible risk to human health due to their potential presence in finished drinking waters.
Table V.
PhAC of the Evaluation Set that are Also Expected to be Released to the Canadian Aquatic Environment Through Agricultural Sources in Addition to Their Human Sources
| PhAC | ATC class | Veterinary sources | Measured | Predicted | |||
|---|---|---|---|---|---|---|---|
| Exogenous use | Endogenous excretion | n/pc | MOEm-max d | MOEp3 e | |||
| Extensive MECs | Estrone | G | ✓ | 237/1 | 4 | 2.3 | |
| Estradiol | G | ✓ | ✓ | 237/0 | Not detected | 1500 | |
| Estriol | G | ✓ | 236/0 | Not detected | 0.6 | ||
| Trimethoprim | J | ✓ | 221/1 | 7333 | 3700 | ||
| Tetracycline | J | ✓ | 214/5 | 5500 | 1600 | ||
| Erythromycin | J | ✓✓ | 214/13 | 890 | 8400 | ||
| Limited MECs | Testosterone | G | ✓ | ✓ | 68/0 | Not detected | 13,000 |
| Pentoxifylline | C | ✓ | 63/0 | Not detected | 101,000 | ||
| Penicillin G | J | ✓✓(b) | 32/0 | Not detected | 84 | ||
| Scant MECs | Sulfapyridine | J | ✓ | 4/0 | Not detected | 113 | |
| Enalapril | C | ✓ | 3/0 | Not detected | 59 | ||
| No MECs | Triiodothyronine | H | ✓ | – | 0.1 | ||
| Thyroxine | H | ✓ | – | 1.7 | |||
| Docusate | A | ✓(a) | – | 15 | |||
| Amoxicillin | J | ✓ | – | 28 | |||
| Levodopa | N | ✓ | – | 38 | |||
| Dihydrotestosterone | No code | ✓ | – | 120 | |||
| Hydrocortisone | D | ✓ | ✓ | – | 260 | ||
| Ampicillin | J | ✓ | – | 640 | |||
| Androstenedione | No code | ✓ | – | 1400 | |||
| Atropine | S | ✓ | – | 1600 | |||
| Dexamethasone | H | ✓ | – | 2200 | |||
| Lidocaine | C | ✓ | – | 3000 | |||
| Prednisolone | S | ✓ | – | 3500 | |||
| Acetylsalicylic acid | N | ✓ | – | 4500 | |||
| Ketoprofen | M | ✓ | – | 9100 | |||
| Methylprednisolone | H | ✓ | – | 9900 | |||
| Selegiline | N | ✓ | – | 36,000 | |||
| Gentamicin | J | ✓ | – | 60,300 | |||
| Omeprazole | A | ✓ | – | 74,000 | |||
| Chlorhexidine | D | ✓ | – | 148,000 | |||
| Neomycin | J | ✓✓ | – | 9,540,000 | |||
a(✓) is a source (31)
b(✓✓) expected to be a significant sources since the PhAC is authorized for use in animal feed (82)
cBased on data compiled from the following sources: (39–45), n number of samples analyzed, p number of positive detections
dSee Table III for further details
eEstimated using Eq. 5
Of the remaining 25 PhACs, limited (n > 10 and <100) and scant (n > 1 and <10) MEC data was available for 3 and 2 PhACs, respectively, while for 20 PhACs no MEC data was available at all (see Table V). In the absence of sufficient MEC data, the potential for the unaccounted agricultural contributions to push the MOE values for these PhACs to “possible risk” levels can be evaluated by considering the magnitude of their respective MOEp3 values. With the exception of thyroxine and triiodothyronine, the respective MOEp3 values are such that it is highly unlikely that the unaccounted for agricultural contributions for a listed PhAC are such that they will increase the MOE estimate to risk levels (see Table V). As an example of the logic used here, consider the PhAC amoxicillin, it is highly unlikely that the mass of amoxicillin administered for veterinary purposes (note: amoxicillin is not used in feeds) in Canada is such that, upon its inclusion in calculations, it would decrease its MOE from the value of 28 to a level less than the trigger limit of 1.0. For the two thyroid hormones, thyroxine and triiodothyronine, it seems likely that agricultural contributions could significantly add to their risk levels, given that their respective MOE values based on human sources alone already are just above or and an order of magnitude below the risk trigger value of 1.0, respectively.
Even though, with the exception of the thyroid hormones, it is unlikely that the 31 PhACs listed in Table V will be used at such levels for agricultural purposes that they will end up posing human health risks due to their potential presence in finished drinking waters, it is still worth highlighting that a significant fraction of several of the PhACs listed in Table V could result from agricultural sources. For instance, Anderson et al. (103) estimated that, depending on the assumptions made for what fractions of agricultural excretions are routed to surface waters, the mass of estrogens (i.e., estrone, estradiol, and estriol) contributed by agricultural sources to surface waters in the USA could vary from as little as 29% to as much as 91% of the net load of such PhACs. An equivalent analysis for Canada remains to be performed. In addition to estrogens, a significant fraction of the surface water loads of a number of antibiotics may result from agricultural sources. In Canada, nine antibiotics share agricultural and human uses (see Table V); however, it is only the antibiotics used in animal feeds that are expected to have a significant agricultural contribution. Of the nine, only neomycin, penicillin G, and erythromycin are approved for use in animal feeds on Canadian farms (82). Due to the lack of input data, it is currently not possible to estimate for each of these three PhACs what fraction of their respective surface water loads arises from agricultural as opposed to human sources. However, the net mass of antibiotics used in Canada for agricultural purposes is available (104) and this can be used with the net mass of antibiotics used by humans (27,28) to estimate, that during 2006, the staggering amount of nearly 2000 tonnes of antibiotics were used in Canada (see Appendix T). Alarmingly, 88% of this mass was used for agricultural purposes, another 10% was used for clinical purposes in the general population, and the remaining 2% was sourced through hospitals. Due to an importation loophole in regulations in Canada (105), some estimate that the use of antibiotics for agricultural purposes may be 33% higher than 1766 tonnes shown in Appendix T. If this is true, the total mass of antibiotics used in Canada could be 30% higher and the net agricultural contribution to the total load could be as high as 90%. This estimate clearly highlights the need to account for agricultural sources for certain compounds.
Sufficiency of Existing Monitoring Data
As indicated earlier, nearly 6000 analyses covering 47 PhACs, have been performed in Canada to evaluate the presence of PhACs in Canadian drinking waters. This raises the question as to whether the existing monitoring data are sufficient. Specifically, it is worth asking the question: Are we monitoring what needs to be monitored?
Ultimately, the need to monitor a PhAC should reside in the risk posed. Table IV suggests that of the 16 PhACs found to have MOEP3 values less than 10, extensive monitoring data was only available for three of them (i.e., estriol, estrone, and ibuprofen), whereas no monitoring data in Canadian drinking waters was available for the remaining 13. Further, of those 13, only atenolol and atorvastatin have been sought in a limited number of Canadian surface waters (see Appendix R). Therefore, most PhACs which pose a relatively high human health risk have yet to be monitored in Canada drinking water supplies.
A case can also be made to monitor those PhACs that are expected to be found at the highest concentrations in the environment and, therefore, carry a higher potential to end up in drinking waters (106). Again, the existing monitoring data are found to be wanting (see Table I) in that most PhACs predicted to occur in the Canadian environment at high concentration are yet to be monitored in Canadian surface and drinking waters while for others only limited data has been reported thus far. Consider that, of the top 50 PhACs in terms of estimated PEC3 values, only 8 have been extensively monitored in Canadian surface and drinking waters (see Table I). In fact, among this list of top 50 PhACs, no monitoring data are available for 34 and 40 PhACs in Canadian surface and drinking waters, respectively (see Table I). Daughton of the US Environmental Protection Agency has also highlighted the limitations of the existing monitoring data for PhACs (106) in that most PhACs expected to be found in the environment at high levels have yet to be monitored or have only been analyzed in a limited number of samples.
One could also make the case that the most potent PhACs (i.e., those with the lowest PNECdw values) should be monitored. Of the 50 most potent PhACs of the evaluation set, only 6 and 5 of them have been monitored in Canadian surface and drinking waters, respectively (see Appendix U).
Overall, it is clear that whether the evaluation is based on risk posed, predicted exposure concentrations, or potency, the existing monitoring data are found wanting.
General Considerations
Although it appears that most PhACs considered in this work pose a negligible risk to human health, several important caveats should be mentioned. Firstly, only 335 PhACs were considered in this study and, as such, a broadened evaluation encompassing the more than one thousand other PhACs in current use is warranted. Secondly, our evaluation focused on the risks posed by individual PhACs and, therefore, the results presented here cannot be taken to be representative of the risks posed by mixtures. Therefore, it remains an open question as to whether the presence of PhACs as mixtures in surface waters constitutes a significant risk to human health. By extension, it also remains unanswered whether the presence of a given PhAC or a mixture of PhACs interacts with other environmental pollutants to yield significant human health risks. Thirdly, it is possible that a greater number of PhACs pose eco-toxicological risks than they do human health risk, due to long-term exposure and greater sensitivity of biota to PhACs in the aquatic environment. Therefore, a complimentary investigation is warranted in which the eco-toxicological risks posed by the environmental release of PhACs are evaluated.
CONCLUSIONS
This research has focused on developing an understanding of the sources of PhACs to the environment and on evaluating their human health relevance with respect to their potential presence in drinking water. The approaches and models developed in this study were applied to an evaluation set of 335 PhACs in the Canadian context to establish the following:
Hospital contributions to the net load of individual PhACs were found to vary significantly from one case to another. For most PhACs, hospitals appeared to be a minor source. However, for 41 PhACs, greater than 70% of their respective environmental loads was estimated to be sourced through hospitals. Class L (i.e., antineoplastic and immunomodulating agents) of the evaluation set was found to be sourced primarily through hospitals.
Of the 14 PhACs for which endogenous contributions were found to be relevant, 9 were established to predominately almost entirely result from endogenous sources.
Of the six PhACs for which illicit contributions were found to be relevant, five were established to predominately almost entirely result from illicit sources.
Class J (i.e., antiinfectives for systemic use) and Class P (i.e., antiparasitic products) drugs of the evaluation set were found to be the two drug classes with highest median predicted exposure concentrations when accounting for removal through human metabolism and wastewater treatment. Therefore, on average, PhACs belonging to these drug classes are expected to be present in the environment in the highest concentrations. In contrast, Class L drugs were estimated to be the least abundant in the environment.
Class L and Class G (i.e., genito-urinary system and sex hormones) drugs of the evaluation set were established to be the most potent in their potential to cause human health effects. Conversely, Class J and Class M (i.e., musculo-skeletal system) drugs were established to be the least potent in their potential to yield human health effects.
A compilation of measurement data indicates that 20 PhACs have thus far been detected in Canadian finished drinking waters with an overall detection frequency of only 3%.
ADIs or TDIs were only available for 32 PhACs and, thus, for the remaining 303 PhACs provisional ADIs were developed. Of these, ADI values for 133, 164, 3, and 3 of the PhACs were based on LOTD, OEL, NSRL, and LTD10 values, respectively. For 220 PhACs, both LOTD and OEL values were available and in nearly 70% of these cases an OEL-based approach yielded the more conservative p-ADI.
A risk evaluation based on measurements could only be performed for 17 PhACs and all of them were found to pose a negligible risk to human health when considered individually. The same risk evaluation based on predictions, rather than measurements, suggested that 322 PhACs of the evaluation set when considered individually are expected to pose a negligible risk to human health due to their potential presence in drinking waters. However, the following 14 PhACs warrant prioritization for further study: triiodothyronine, thyroxine, ramipril and its metabolite ramiprilat, candesartan, lisinopril, atorvastatin, lorazepam, fentanyl, atenolol, metformin, enalaprilat, morphine, and irbesartan.
The currently available monitoring data for PhACs in Canadian surface and drinking waters was found to be wanting irrespective of whether their suitability was assessed based on risk posed, predicted exposure concentrations, or potency resulting in human health effects.
Electronic supplementary material
(DOC 14337 kb)
ABBREVIATIONS
- ADI
Acceptable daily intake
- ATC
Anatomical therapeutic chemical
- CCSA
Canadian Centre on Substance Abuse
- DPD
Drug Product Database
- LOTD
Lowest oral therapeutic dose
- MEC
Measured exposure concentration
- MOE
Margin of exposure
- MSDS
Material safety data sheets
- NSRL
No significant risk levels
- OECD
Organization for Economic Co-operation and Development
- OEL
Occupational exposure limits
- p-ADI
Provisional acceptable daily intake values
- PEC
Predicted exposure concentration
- PhAC
Pharmaceutically active compounds
- PNEC
Predicted no effect concentrations
- RCMP
Royal Canadian Mounted Police
- STP
Sewage treatment plants
- TDI
Tolerable daily intake
REFERENCES
- 1.Daughton CG. Pharmaceutical ingredients in drinking water: overview of occurrence and significance of human exposure. In: Halden RU, editor. Contaminants of emerging concern in the environment: ecological and human health considerations. Washington, DC: American Chemical Society; 2010. [Google Scholar]
- 2.MDH . Human health-based water guidance table. St. Paul: Minnesota Department of Health; 2013. [Google Scholar]
- 3.WHO . Pharmaceuticals in drinking-water: public health and environment water, sanitation, hygiene and health. Geneva: World Health Organization; 2011. [Google Scholar]
- 4.Cunningham VL, Binks SP, Olson MJ. Human health risk assessment from the presence of human pharmaceuticals in the aquatic environment. Regul Toxicol Pharmacol. 2009;53(1):39–45. doi: 10.1016/j.yrtph.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang QQ, Zhao JL, Ying GG, Liu YS, Pan CG. Emission estimation and multimedia fate modelling of seven steroids at the river basin scale in China. Environ Sci Technol. In press doi:10.1021/es501226h. [DOI] [PubMed]
- 6.Khan U, Nicell J. Assessment of the aquatic release and relevance of selected endogenous chemicals: androgens, thyroids and their in vivo metabolites. In: Halden RU, editor. Contaminants of emerging concern in the environment: ecological and human health considerations. Washington, DC: American Chemical Society; 2010. [Google Scholar]
- 7.Cardoso O, Porcher J-M, Sanchez W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: review of evidence and need for knowledge. Chemosphere. 2014;115:20–30. doi: 10.1016/j.chemosphere.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Khan U, Nicell J. Assessing the risk of exogenously consumed pharmaceuticals in land-applied human urine. Water Sci Technol. 2010;62(6):1335–45. doi: 10.2166/wst.2010.427. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan A, Halden RU. Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Sci Rep. 2014;4:3731. doi: 10.1038/srep03731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford KH, Thomas KV. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ Int. 2009;35(5):766–70. doi: 10.1016/j.envint.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Cook SM, VanDuinen B, Love NG, Skerlos SJ. Life cycle comparison of environmental emissions from three disposal options for unused pharmaceuticals. Environ Sci Technol. 2012;46(10):5535–41. doi: 10.1021/es203987b. [DOI] [PubMed] [Google Scholar]
- 12.Khan U, Nicell J. Refined sewer epidemiology mass balances and their application to heroin, cocaine and ecstasy. Environ Int. 2011;37(7):1236–52. doi: 10.1016/j.envint.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Khan U, Nicell J. Sewer epidemiology mass balances for assessing the illicit use of methamphetamine, amphetamine and tetrahydrocannabinol. Sci Total Environ. 2012;421–422:144–62. doi: 10.1016/j.scitotenv.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Besse J-P, Kausch-Barreto C, Garric J. Exposure assessment of pharmaceuticals and their metabolites in the aquatic environment: application to the French situation and preliminary prioritization. Hum Ecol Risk Assess. 2008;14(4):665–95. doi: 10.1080/10807030802235078. [DOI] [Google Scholar]
- 15.Bull RJ, Crook J, Whittaker M, Cotruvo JA. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul Toxicol Pharmacol. 2011;60(1):1–19. doi: 10.1016/j.yrtph.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell DJ, Mastrocco F, Nowak E, Johnston J, Yekel H, Pfeiffer D, et al. An assessment of potential exposure and risk from estrogens in drinking water. Environ Health Perspect. 2010;118(3):338–44. doi: 10.1289/ehp.0900654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham VL, Perino C, D’Aco VJ, Hartmann A, Bechter R. Human health risk assessment of carbamazepine in surface waters of North America and Europe. Regul Toxicol Pharmacol. 2010;56(3):343–51. doi: 10.1016/j.yrtph.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Schwab BW, Hayes EP, Fiori JM, Mastrocco FJ, Roden NM, Cragin D, et al. Human pharmaceuticals in US surface waters: a human health risk assessment. Regul Toxicol Pharmacol. 2005;42(3):296–312. doi: 10.1016/j.yrtph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Bruce GM, Pleus RC, Snyder SA. Toxicological relevance of pharmaceuticals in drinking water. Environ Sci Technol. 2010;44(14):5619–26. doi: 10.1021/es1004895. [DOI] [PubMed] [Google Scholar]
- 20.Kostich MS, Lazorchak JM. Risks to aquatic organisms posed by human pharmaceutical use. Sci Total Environ. 2008;389(2–3):329–39. doi: 10.1016/j.scitotenv.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Sedlak DL, Pinkston K, Huang C-H. Occurrence survey of pharmaceutically active compounds. Alexandria: AWWA Research Foundation; 2005. [Google Scholar]
- 22.Kumar A, Xagoraraki I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in US surface and finished drinking waters: a proposed ranking system. Sci Total Environ. 2010;408(23):5972–89. doi: 10.1016/j.scitotenv.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Watts C, Maycock D, Crane M, Fawell J, Goslan E. Desk based review of current knowledge on pharmaceuticals in drinking water and estimation of potential levels. London: Watts and Crane Associates; 2007. [Google Scholar]
- 24.de Jongh CM, Kooij PJF, de Voogt P, ter Laak TL. Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci Total Environ. 2012;427–428:70–7. doi: 10.1016/j.scitotenv.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 25.OECD . OECD health data. Paris: Organisation for Economic Co-operation and Development; 2013. [Google Scholar]
- 26.Roos V, Gunnarsson L, Fick J, Larsson DGJ, Rudén C. Prioritising pharmaceuticals for environmental risk assessment: towards adequate and feasible first-tier selection. Sci Total Environ. 2012;421:102–10. doi: 10.1016/j.scitotenv.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 27.Brogan IMS. Canadian compuscript audit database. Montreal: IMS Brogan; 2007. [Google Scholar]
- 28.Brogan IMS. Canadian drug store and hospital purchases audit database. Montreal: IMS Brogan; 2007. [Google Scholar]
- 29.Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol. 2009;43(3):597–603. doi: 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- 30.INCB . Psychotropic substances—2013 report. Vienna: International Narcotics Control Board; 2013. [Google Scholar]
- 31.Health Canada . Drug product database (DPD) Ottawa: Health Canada; 2013. [Google Scholar]
- 32.Lamshöft M, Grobe N, Spiteller M. Picomolar concentrations of morphine in human urine determined by dansyl derivatization and liquid chromatography–mass spectrometry. J Chromatogr B. 2011;879(13):933–7. doi: 10.1016/j.jchromb.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 33.Helwig K, Hunter C, MacLachlan J, McNaughtan M, Roberts J, et al. Micropollutant point sources in the built environment: identification and monitoring of priority pharmaceutical substances in hospital effluents. J Environ Anal Toxicol. 2014;3:177. [Google Scholar]
- 34.Riaz ul Haq M, Metcalfe C, Li H, Parker W. Discharge of pharmaceuticals into municipal sewers from hospitals and long-term care facilities. Water Qual Res J Can. 2012;47(2):140–52. doi: 10.2166/wqrjc.2012.015. [DOI] [Google Scholar]
- 35.STOWA. Inventarisatie van emissie van geneesmiddelen uit zorginstellingen. ZORG, Deel C. Amersfoor: Stichting Toegepast Onderzoek Waterbeheer (STOWA); 2011.
- 36.Miljøstyrelsen. Begrænsning af humane lægemiddelrester og antibiotikaresistens i spildevand med fokus på reduktion ved kilden. DHI og B. Halling-Sørensen; 2007.
- 37.Le Corre KS, Ort C, Kateley D, Allen B, Escher BI, Keller J. Consumption-based approach for assessing the contribution of hospitals towards the load of pharmaceutical residues in municipal wastewater. Environ Int. 2012;45:99–111. doi: 10.1016/j.envint.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Straub JO, Flückiger A. Proposal for an environmental quality standard according to the EU Water Framework Directive for the Anti-Hyperlipidaemic Pharmaceutical Bezafibrate. Poster presented at 20th SETAC Europe Annual Meeting: Seville; 2010.
- 39.Boyd GR, Reemtsma H, Grimm DA, Mitra S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ. 2003;311(1–3):135–49. doi: 10.1016/S0048-9697(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Ohman K, Metcalfe C, Ikonomou MG, Amatya PL, Wilson J. Pharmaceuticals and endocrine disruptors in wastewater treatment effluents and in the water supply system of Calgary, Alberta, Canada. Water Qual Res J Can. 2006;41(4):351–64. [Google Scholar]
- 41.Garcia-Ac A, Segura PA, Gagnon C, Sauvé S. Determination of bezafibrate, methotrexate, cyclophosphamide, orlistat and enalapril in waste and surface waters using on-line solid-phase extraction liquid chromatography coupled to polarity-switching electrospray tandem mass spectrometry. J Environ Monit. 2009;11(4):830–8. doi: 10.1039/b817570e. [DOI] [PubMed] [Google Scholar]
- 42.Kleywegt S, Pileggi V, Yang P, Hao C, Zhao X, Rocks C, et al. Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Sci Total Environ. 2011;409(8):1481–8. doi: 10.1016/j.scitotenv.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 43.MDDEP . Résultats du suivi des produits pharmaceutiques et de soins personnels ainsi que des hormones dans des eaux usées, de l’eau de surface et de l’eau potable au Québec (in French) Quebec City: MDDEP; 2011. [Google Scholar]
- 44.Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, et al. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ Toxicol Chem. 2010;29(1):79–89. doi: 10.1002/etc.27. [DOI] [PubMed] [Google Scholar]
- 45.Tabe S, Yang P, Zhao X, Hao C, Seth R, Schweitzer L, et al. Occurrence and removal of PPCPs and EDCs in the Detroit river watershed. Water Pract Technol. 2010 [Google Scholar]
- 46.Bouchard M, Morselli C, Gallupe O, Easton S, Descormiers K, Turcotte M, et al. Estimating the size of the Canadian illicit METH and MDMA markets: a multi-method approach. Ottawa: Public Safety Canada; 2012. [Google Scholar]
- 47.WHO . ATC classification system. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2013. [Google Scholar]
- 48.Fu W, Franco A, Trapp S. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Environ Toxicol Chem. 2009;28(7):1372–9. doi: 10.1897/08-233.1. [DOI] [PubMed] [Google Scholar]
- 49.Travis CC, Arms AD. Bioconcentration of organics in beef, milk, and vegetation. Environ Sci Technol. 1988;22:271–4. doi: 10.1021/es00168a005. [DOI] [PubMed] [Google Scholar]
- 50.Statistics Canada . CANSIM database. Ottawa: Statistic Canada; 2013. [Google Scholar]
- 51.CCSA . Misuse of opioids in Canadian communities—April Bulletin. Ottawa: Canadian Centre on Substance Abuse; 2013. [Google Scholar]
- 52.RCMP . Report on the illicit drug situation in Canada—2009. Ottawa: Royal Canadian Mounted Police; 2010. [Google Scholar]
- 53.Environment Canada . The municipal water and wastewater survey. Ottawa: Environment Canada; 2011. [Google Scholar]
- 54.Lehner B, Nicell J, Grill G, Khan U, Ariwi J. Down-the-drain geospatial fate model for substances in consumer products. Report Submitted by McGill University to Health Canada; 2013.
- 55.Environment Canada . Data sources and methods: municipal wastewater treatment indicator. Ottawa: Environment Canada; 2012. [Google Scholar]
- 56.RIVM . Evaluation of the model simple treat. Bilthoven: National Institute for Public Health and the Environment (RIVM); 2013. [Google Scholar]
- 57.Franco A, Fu W, Trapp S. The effect of pH on the sorption of ionizable chemicals: effects and modeling advances. Environ Toxicol Chem. 2008;28:458–64. doi: 10.1897/08-178.1. [DOI] [PubMed] [Google Scholar]
- 58.ECETOC . Environmental risk assessment of ionisable compounds. Brussels: European Centre for Ecotoxicology and Toxicology of Chemicals; 2013. [Google Scholar]
- 59.Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug information handbook. 20. Hudson: Lexi-Comp, Inc.; 2011. [Google Scholar]
- 60.www.UptoDate.com. UpToDate, Waltham, MA; 2013.
- 61.NIOSH . List of antineoplastic and other hazardous drugs in healthcare settings. Cincinnati: National Institute for Occupational Safety and Health; 2012. [Google Scholar]
- 62.Binks SP. Occupational toxicology and the control of exposure to pharmaceutical agents at work. Occup Med. 2003;53:363–70. doi: 10.1093/occmed/kqg116. [DOI] [PubMed] [Google Scholar]
- 63.Christensen FM. Pharmaceuticals in the environment—a human risk. Regul Toxicol Pharmacol. 1998;28(3):212–21. doi: 10.1006/rtph.1998.1253. [DOI] [PubMed] [Google Scholar]
- 64.California EPA . Proposition 65 safe harbor levels: no significant risk levels for carcinogens and maximum allowable dose levels for chemicals causing reproductive toxicity. Sacramento: California EPA; 2012. [Google Scholar]
- 65.Gold L. The Carcinogenic Potency Project (CPDB). Berkley: United States; 2011.
- 66.Health Canada . Federal contaminated site risk assessment in Canada. Part 1: guidance on human health preliminary quantitative risk assessment. Ottawa: Health Canada; 2013. [Google Scholar]
- 67.Weissbrodt D, Kovalova L, Ort C, Pazhepurackel V, Moser R, Hollender J, et al. Mass flows of X-ray contrast media and cytostatics in hospital wastewater. Environ Sci Technol. 2009;43(13):4810–7. doi: 10.1021/es8036725. [DOI] [PubMed] [Google Scholar]
- 68.FDA. Drugs@FDA. Silve Spring, Maryland: 2013.
- 69.Laurenson JP, Bloom RA, Page S, Sadrieh N. Ethinyl estradiol and other human pharmaceutical estrogens in the aquatic environment: a review of recent risk assessment data. AAPS J. 2014;16(2):299–310. doi: 10.1208/s12248-014-9561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan U, Nicell JA. Contraceptive options and their associated estrogenic environmental loads: relationships and trade-offs. PLoS ONE. 2014;9(3):e92630. doi: 10.1371/journal.pone.0092630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan U, van Nuijs ALN, Li J, Maho W, Du P, Li K, et al. Application of a sewage-based approach to assess the use of ten illicit drugs in four Chinese megacities. Sci Total Environ. 2014;487:710–21. doi: 10.1016/j.scitotenv.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 72.Oosterhuis M, Sacher F, ter Laak TL. Prediction of concentration levels of metformin and other high consumption pharmaceuticals in wastewater and regional surface water based on sales data. Sci Total Environ. 2013;442:380–8. doi: 10.1016/j.scitotenv.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 73.Straub J. An environmental risk assessment for human-use trimethoprim in European surface waters. Antibiotics. 2013;2(1):115–62. doi: 10.3390/antibiotics2010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shutt DA, Cox RI. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol. 1972;52:299–310. doi: 10.1677/joe.0.0520299. [DOI] [PubMed] [Google Scholar]
- 75.Trudeau VL, Heyne B, Blais JM, Temussi F, Atkinson SK, Pakdel F, et al. Lumiestrone is photochemically derived from estrone and may be released to the environment without detection. FMICB. 2011;2(Article 83):1–13. doi: 10.3389/fendo.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Svanfelt J, Eriksson J, Kronberg L. Analysis of thyroid hormones in raw and treated waste water. J Chromatogr A. 2010;1217(43):6469–74. doi: 10.1016/j.chroma.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 77.Howard PH, Muir DCG. Identifying new persistent and bioaccumulative organics among chemicals in commerce II: pharmaceuticals. Environ Sci Techol. 2011;45(16):6938–46. doi: 10.1021/es201196x. [DOI] [PubMed] [Google Scholar]
- 78.ANSES . Campagne nationale d’occurrence des résidus de médicaments dans les eaux destinées à la consommation humaine. Maisons-Alfort: Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail; 2011. [Google Scholar]
- 79.Ericson JF, Smith RM, Roberts G, Hannah B, Hoeger B, Ryan J. Experiences with the OECD 308 transformation test: a human pharmaceutical perspective. Integra Environ Assess Manag. 2014;10(1):114–24. doi: 10.1002/ieam.1457. [DOI] [PubMed] [Google Scholar]
- 80.Bayer A, Asner R, Schüssler W, Kopf W, Weiß K, Sengl M, et al. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ Sci Pollut Res. In press. doi:10.1007/s11356-014-3060-z. [DOI] [PubMed]
- 81.Kostich MS, Batt AL, Lazorchak JM. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the USA and implications for risk estimation. Environ Pollut. 2014;184:354–9. doi: 10.1016/j.envpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 82.Health Canada . Uses of antimicrobials in food animals in Canada: impact on resistance and human health. Ottawa: Health Canada; 2002. [Google Scholar]
- 83.Gros M, Rodriguez-Mozaz S, Barceló D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A. 2013;1248:104–21. doi: 10.1016/j.chroma.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 84.Vulliet E, Cren-Olive C, Grenier-Loustalot M-F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ Chem Lett. 2011;9:103–14. doi: 10.1007/s10311-009-0253-7. [DOI] [Google Scholar]
- 85.Rodayan A, Majewsky M, Yargeau V. Impact of approach used to determine removal levels of drugs of abuse during wastewater treatment. Sci Total Environ. 2014;487:731–9. doi: 10.1016/j.scitotenv.2014.03.080. [DOI] [PubMed] [Google Scholar]
- 86.Huerta-Fontela M, Galceran MT, Ventura F. Stimulatory drugs of abuse in surface waters and their removal in a conventional drinking water treatment plant. Environ Sci Technol. 2008;42(18):6809–16. doi: 10.1021/es800768h. [DOI] [PubMed] [Google Scholar]
- 87.van der Aa M, Bijlsma L, Emke E, Dijkman E, van Nuijs ALN, van de Ven B, et al. Risk assessment for drugs of abuse in the Dutch watercycle. Water Res. 2013;47(5):1848–57. doi: 10.1016/j.watres.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Loos R, Carvalho R, António DC, Comero S, Locoro G, Tavazzi S, et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013;47(17):6475–87. doi: 10.1016/j.watres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 89.Fischer B, Jones W, Rehm J. Trends and changes in prescription opioid analgesic dispensing in Canada 2005–2012: an update with a focus on recent interventions. BMC Health Serv Res. 2014;14(1):90. doi: 10.1186/1472-6963-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boxall ABA, Monteiro SC, Fussell R, Williams RJ, Bruemer J, Greenwood R, et al. Targeted monitoring for human pharmaceuticals in vulnerable source and final waters. London: Drinking Water Inspectorate; 2012. [Google Scholar]
- 91.Kim M, Guerra P, Shah A, Parsa M, Alaee M, Smyth SA. Removal of pharmaceuticals and personal care products in a membrane bioreactor wastewater treatment plant. Water Sci Technol. 2014;69(11):2221–9. doi: 10.2166/wst.2014.145. [DOI] [PubMed] [Google Scholar]
- 92.Lowe CJ. Pharmaceuticals, personal care products, illicit drugs and their metabolites in screened municipal wastewaters. Master’s Thesis. Victoria: University of Victoria; 2011.
- 93.Derksen A, ter Laak T. Human pharmaceuticals in the water cycle. Netherlands: Joint STOWA and KWR Report; 2013. [Google Scholar]
- 94.Yargeau V, Taylor B, Li H, Rodayan A, Metcalfe CD. Analysis of drugs of abuse in wastewater from two Canadian cities. Sci Total Environ. 2014;487:722–30. doi: 10.1016/j.scitotenv.2013.11.094. [DOI] [PubMed] [Google Scholar]
- 95.Hummel D, Löffler D, Fink G, Ternes TA. Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environ Sci Technol. 2006;40(23):7321–8. doi: 10.1021/es061740w. [DOI] [PubMed] [Google Scholar]
- 96.Huerta-Fontela M, Galceran MT, Ventura F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011;45:1432–42. doi: 10.1016/j.watres.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 97.EMA . Questions and answers on 'Guideline on the environmental risk assessment of medicinal products for human use'. London: European Medicine Agency; 2011. [Google Scholar]
- 98.NHTSA . Cocaine: drug profile. Washington, DC: National Highway Traffic Safety Administration; 2013. [Google Scholar]
- 99.Chari BP, Halden RU. Predicting the concentration range of unmonitored chemicals in wastewater-dominated streams and in run-off from biosolids-amended soils. Sci Total Environ. 2012;440:314–20. doi: 10.1016/j.scitotenv.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boleda MR, Huerta-Fontela M, Ventura F, Galceran MT. Evaluation of the presence of drugs of abuse in tap waters. Chemosphere. 2011;84(11):1601–7. doi: 10.1016/j.chemosphere.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 101.Block JH, BealeWilson JM. Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 102.Verho M, Luck C, Shelter WJ, Rangoonwala B, Bender N. Pharmacokinetics, metabolism and biliary and urinary excretion of oral ramipril in man. Curr Med Res Opin. 1995;13:264–73. doi: 10.1185/03007999509111551. [DOI] [PubMed] [Google Scholar]
- 103.Anderson P. Perspectives on estrogen sources and effects in us surface waters. Presentation given at CropLife America & RISE 2013 Spring Conference. Crystal City: Virginia; 2013.
- 104.Government of Canada . Canadian integrated program for antimicrobial resistance surveillance (CIPARS) 2009 Annual Report. Guelph: Public Health Agency of Canada; 2013. [Google Scholar]
- 105.Handa A, Webster P. Industry-led committee urges delay in closing loophole allowing import of unapproved antibiotics for animals. CMAJ. 2009;180:914–6. doi: 10.1503/cmaj.090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daughton CG. The Matthew effect and widely prescribed pharmaceuticals lacking environmental monitoring: case study of exposure-assessment vulnerability. Sci Total Environ. 2014;466:315–25. doi: 10.1016/j.scitotenv.2013.06.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 14337 kb)


