Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease. The current standard treatment with glucocorticoids (GCs) leads to many adverse effects, and its effectiveness is questionable. Thus, it is critical and urgent to find new drug(s) for treatment of IPF. Baicalin (BAI) is an attractive candidate for this purpose. Herein, utilizing shotgun lipidomics, we revealed that IPF could lead to a lipid disorder of the liver in an animal model induced by bleomycin and confirmed through histopathological studies of the lung. Lipidomics further demonstrated that this disorder could virtually be corrected after treatment with BAI, but not with dexamethasone (DEX) (a commonly used GC for treatment of IPF). In contrast, the treatment with DEX did not improve IPF but led to tremendous alterations in hepatic lipidomes and accumulation of fat in the liver, which was very different from the lipid disorder induced by IPF. The underpinning mechanisms of the IPF-resultant lipid disorder and DEX-induced lipotoxicity as revealed by shotgun lipidomics were extensively discussed. Taken together, the current study showed that IPF could lead to hepatic lipid disorder, which can be treated with BAI, and demonstrated that lipidomics could be a powerful tool for drug screening.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-014-9714-4) contains supplementary material, which is available to authorized users.
KEY WORDS: baicalin, hepatic lipidome, idiopathic pulmonary fibrosis, multidimensional mass spectrometry, shotgun lipidomics
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, irreversible, and ultimately fatal lung disease of unknown etiology. It usually occurs between 50 and 70 years of age and is characterized by the histological pattern of usual interstitial pneumonia (1,2). The onset of its symptoms is gradual, beginning with nonproductive cough, minor signs of inflammation, and exertional dyspnea. However, it almost always causes rapid fibrotic destruction of the lung, and the survival time from the onset of symptoms ranges from 4 to 5 years (3,4).
Bleomycin (BLM) is a chemotherapeutic antibiotic used for treatment of some types of carcinomas but exhibits a dose-dependent pulmonary toxicity in most patients (5). Intratracheal instillation of BLM into the lungs of animal species results in symptoms and lung damage that essentially resemble human IPF, both histologically and physiologically. Thus, BLM has been widely utilized in animal models to cause injury and fibrotic lesions in the lung interstitium (6–10). In contrast, inhibition of lung inflammation and lipid peroxidation has been employed as therapeutic strategies for IPF (11–13).
So far, there is no cure or even effective treatment for IPF. In clinic, oral glucocorticoids (GCs) have been used as the standard treatment of IPF for a long time (4). Dexamethasone (DEX) is a synthetic member of GC and is commonly used in the treatment of IPF. DEX treatment may lead to symptomatic relief, but it appears neither to halt the progression of fibrosis nor to improve life expectancy (14). Moreover, high-dosage and/or long-term treatment with DEX leads to severe adverse effects, including hepatic injury (15,16). Adverse hepatic effects (e.g., hepatic steatosis (17), hepatic insulin resistance (15), and activation of GC receptors (16)) are very common, because the liver plays a key role in the control of glucose and lipid homeostasis and DEX is metabolized primarily in the liver (18). DEX treatment elevates fatty acid synthesis (19,20) and causes triacylglycerol (TAG) accumulation in the liver, which contributes to hepatic insulin resistance and pathophysiology of metabolic syndrome (21). Furthermore, liver disease is one of the major problems in IPF due to oxygen deficiency. Accordingly, it is critical and urgent to discover new drug(s) for treatment of IPF. Recently, the application of baicalin (BAI), a flavonoid compound, for treatment of IPF has been given great attention (22). It has been reported that BAI possesses the anti-inflammatory, anti-oxidative, and anti-tumor functions, while also scavenging free radicals (23–26). However, there is little information regarding the effects of BAI on IPF-induced hepatic lipid changes.
Herein, we reported a lipidomics study by using multidimensional mass spectrometry-based shotgun lipidomics (MDMS-SL) (27–29) on evaluating the effects of BAI on hepatic lipidomes of a mouse model of IPF that was induced by BLM and validated through the histopathological examination of lung tissues. To our great surprise, shotgun lipidomics revealed a significant lipid disorder in the liver of the IPF animal model. Treatment of IPF animals with BAI not only cured the lung complication, but also virtually corrected all the hepatic lipid disorder. In contrast to the BAI treatment, treatment of the model with DEX resulted in remarkable alterations in different lipid classes and molecular species and severe accumulation of TAG amount. Moreover, lipidomics studies also allowed us to uncover the biochemical mechanisms responsible for the altered hepatic lipidomes of the IPF model and following its treatments.
MATERIALS AND METHODS
Materials
Bleomycin A5 hydrochloride was obtained from Nippon Kayaku Co., Ltd, Japan. DEX acetate and BAI were purchased from Shanghai Sine Pharm Corp., Ltd and Shanxi Yongjian Pharmaceutical Co., Ltd, China, respectively. All synthetic phospholipids, N-lauroyl sphingomyelin (N12:0 SM), N-heptadecanoyl ceramide (N17:0 Cer), and triheptadecenoyl glycerol (T17:1 TAG), were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). The prefix “N” denotes the amide linkage in lipids. All of the solvents were obtained from Burdick and Jackson (Honeywell International Inc., Muskegon, MI). All other reagents were at least analytical grade and purchased from Fisher Scientific (Pittsburgh, PA), Sigma-Aldrich Chemical Company (St. Louis, MO), or as specified.
Animal Experiments and Tissue Collection
Adult male ICR mice (8 weeks old) weighing 22–24 g were used in the study. The mice were purchased from the Center Animal House of Zhejiang Chinese Medical University and maintained in cages at an ambient temperature of 22–25°C and relative humidity of 60–70% with free access to water and food. All of the procedures were conducted according to the Ethics Committee for the Use of Experimental Animals at Zhejiang Chinese Medical University. Thirty-two mice were randomly divided into four groups (eight mice per group): a saline-water group (the control group), a BLM-water group (the model group), a BLM-DEX treatment group (the DEX group), and a BLM-BAI treatment group (the BAI group). The control group was intratracheally injected with saline in a volume of 1 ml/kg and orally treated with distilled water. The other three groups were intratracheally injected with BLM (3.5 mg/kg) in a volume of 1 ml/kg and treated orally with water, DEX (3 mg/kg), and BAI (100 mg/kg) daily, respectively. The concentrations of BLM, DEX, and BAI employed were determined based on the literature and the previous experiment (22).
Mice were euthanized after treatment for 26 days. All tissue samples were collected. Then, the tissue was lavaged with phosphate-buffered saline (PBS) until no blood was present within them. Half of the left lung was fixed in 4% phosphate-buffered paraformaldehyde for histopathological preparation. After that, all samples were stored in liquid nitrogen for further research. The liver tissue was pulverized into fine powder with a stainless-steel mortar and pestle at the temperature of liquid nitrogen.
Histopathological Evaluation
Histopathological evaluation was performed as previously described (22). Mouse lung tissues were fixed in 4% phosphate-buffered paraformaldehyde (pH 7.4) and processed in routine paraffin embedding. Serial sections were cut and stained with hematoxylin and eosin (H&E) and a modified Masson’s trichrome kit (30). Quantitative image analysis of the area of fibrosis fraction after Masson’s trichrome staining was performed utilizing Image-Pro Plus software 5.0 (Media Cybernetics, Silver Springs, MD).
Preparation of Lipid Extracts from Liver Samples
A powder sample (∼20 mg) from each liver tissue was weighed and further homogenized in 0.5 ml of ice-cold, diluted (0.1×) PBS by using sonification (Branson Ultrasonic Bath). A protein assay was performed on individual homogenates by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) using bovine serum albumin as standard. Lipids were extracted from each sample by employing a modified procedure of Bligh and Dyer extraction (31) in the presence of internal standards that were in a premixed solution for global lipid analysis and added into each liver tissue sample based on its protein content. Therefore, the lipid levels of liver samples can be normalized to the protein concentration and quantified directly. The lipid extracts were dried with nitrogen, redissolved in 1:1 CHCl3/MeOH with a volume of 200 μl/mg protein (present in the original sample), capped, and stored at −20°C for mass spectrometric (MS) analysis as previously described (28).
MS Analysis of Lipids
A triple-quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an automated nanospray apparatus (TriVersa NanoMate, Advion Bioscience Ltd, Ithaca, NY) and Xcalibur system software was utilized in the study as previously described (32). Each lipid extract solution was diluted to <50 pmol of total lipids/μl with 1:2:4 CHCl3/MeOH/isopropanol prior to infusion to the mass spectrometer. The diluted solution was directly infused to the mass spectrometer through the NanoMate device.
As previously described, the majority of lipid species are composed of a small number of building blocks that include backbones, head groups, and aliphatic chains (28,33). These building blocks were exploited to identify individual lipid species in the MDMS-SL platform and determined through the use of two powerful tandem MS techniques (i.e., neutral loss scan (NLS) and precursor ion scan (PIS)) in a mass-ramp fashion (28). In brief, identification of individual species was achieved with the following three determinants. First, each lipid class or category was selectively ionized through intrasource separation as previously described (34). Second, a full MS scan detecting the precursor ions corresponding to the molecular species of a class of interest was specifically acquired in the appropriate mass region. Other approaches such as derivatization were utilized for those lipid classes that are in low abundance and/or unionizable (29,35,36). Finally, multiple PISs and/or NLSs related to the lipid class(es) of interest were acquired in the mass regions, among which one or more PISs or NLSs are sensitive and specific to the lipid class or the category of lipid classes of interest. The full MS scan and the set of PIS and/or NLS were referred to as the 2-D MS data since these scans are used to construct a 2-D mass spectrum as previously described (33). Individual lipid species, including fatty acyl chains, isomeric and isobaric species, and regiospecific positions, corresponding to a molecular ion displayed in the full MS scan were identified through examining the crossing peaks corresponding to the building blocks with the molecular ion as previously described (28).
In MS analysis, the first and third quadrupoles were used as independent mass analyzers with a mass resolution setting of 0.7 Th, and the second quadrupole served as a collision cell for tandem MS analysis. Usually, a 1-min period of signal averaging in the profile mode was employed for each full MS scan. For tandem MS analysis, a 2- to 5-min period of signal averaging in the profile mode was employed, and the collision gas pressure was set at 1.0 mT, while the collision energy varied according to the classes of lipids as described previously (28). All of the mass spectra were automatically obtained by a customized sequence subroutine operated under Xcalibur software (28).
Data Processing
MS data processing, including baseline correction, 13C de-isotoping, peak intensity comparison, and quantitation, was performed by custom-programmed Microsoft Excel macros (28). All the concentrations of lipid species were normalized to the protein content. Data were subjected to multivariate analysis in the form of unsupervised principal component analysis (PCA) using SIMCA-P+ (V11.0.0.0 Umetrics AB, Umea, Sweden) and Microsoft Office Excel 2003 (Microsoft, Redmond, WA). Data were scaled to Pareto variance by dividing each variable by the square root of the standard deviation of the variable. In PCA, the first principal component represents of the largest amount of correlated variation in the data set, and so on, until an adequate description of the data set is achieved. To elucidate changes of each lipid species, Student’s unpaired t tests were conducted to determine significant differences between the groups. A value of P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Histopathological Changes of the Lung and Alterations in Animal Body Weight After Treatment
To determine the histopathological changes of the lung with the treatments, lung tissues from different mouse groups of control, model, DEX, and BAI were collected after 26 days post-BLM or post-saline injection and stained with H&E or Masson’s trichrome for alveolitis and fibrosis (collagen fibers) assessment (Fig. 1). The sections from the control group displayed normal structure and no pathological changes as examined by a light microscope (Fig. 1a). H&E staining of the tissue sections from the model group (Fig. 1b) showed a lot of IPF-type pathological changes, such as inflammatory cell infiltration, large fibrous area, and collapsed alveolar spaces. This observation indicated that the BLM-induced model in the study was successful. Although fibrotic lesions were observed in both DEX (Fig. 1c) and BAI (Fig. 1d) groups, the extent of fibrosis and inflammation in the BAI-treated group was substantially less severe in comparison to that in the DEX-treated group. To quantitatively confirm histological observations, the area of fibrosis fraction, which appeared in blue in the sections stained with Masson’s trichrome, was assessed by quantitative image analysis. The results from different groups after normalization to the model group were shown (Fig. 1e). The results suggest that both drugs were attractive agents in the inhibition of the development of lung fibrosis induced by BLM, but BAI was a more attractive drug candidate for treatment of IPF.
Fig. 1.
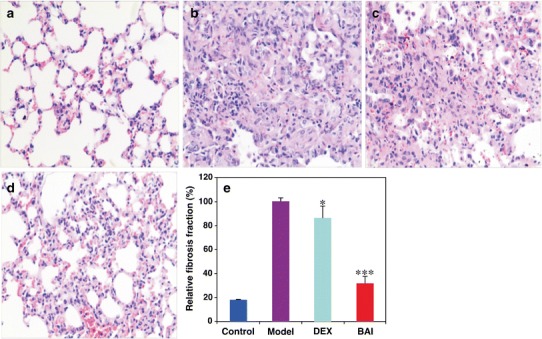
Collagen deposition in the lung of mice induced by BLM with or without treatment as assessed by staining. Lung tissue sections of mice in the control group (a), the BLM-induced model group (b), the BLM-induced model group treated with DEX (c), and the BLM-induced model group treated with BAI (d) were collected on the 26th day post-BLM injection with standard procedures as described in the “MATERIALS AND METHODS” section. H&E staining was visualized by using photomicroscope (Leica DM LB2; Wetzlar, Germany). Each image represents the staining experiments from at least five animals of individual group and was obtained with ×200 magnification from origin. The effects of different treatments on BLM-induced lung fibrosis fraction were assessed quantitatively by quantitative image analysis of Masson’s trichrome staining 26 days post-BLM injection (e). The data represent means ± SD from the individual animals (control, n = 5; model, n = 4; DEX, n = 5; and BAI, n = 4). *P < 0.05 and ***P < 0.001 compared with that of the model group
In the study, the changes of animal body weight after separate treatment were also documented. After 26 days post-BLM or post-saline injection, mice with or without treatment were weighed. It was found that body mass of the model (26.4 ± 5.3 g, P < 0.05), DEX (22.1 ± 3.0 g, P < 0.001), and BAI (26.4 ± 3.9 g, P < 0.01) groups was significantly reduced in comparison to that of the control group (30.1 ± 2.6 g).
Multivariate Analysis of Lipidomics Data Revealed That BAI Is an Attractive Drug Candidate for Treatment of BLM-Induced IPF
PCA describes the largest variation in data using a few orthogonal latent variables. Thus, an overview of the data, including trends and groupings, can be detected. PCA was carried out and the score plot was obtained. The first two principal components, the first principal component (PC1) and the second principal component (PC2), were plotted in a score plot and examined for any clustering trends (Fig. 2a). The PC1 separated the samples with respect to hepatic lipid levels and accounted for 33.2% of the total variance with the data, whereas the PC2 accounted for 20.7%. It clearly indicated that administration of DEX led to the worsening of hepatic lipid metabolism. The PCA score plot absolutely separated the mice administered with DEX from the controls (Fig. 2a). Moreover, the differences in the amounts of lipid species between BAI-treated and control mice were much less than those between model and control mice. These results indicated that treatment of BAI can correct IPF-induced hepatic lipid disorders to a great degree. The corresponding loading plot (Fig. 2b) indicated that many phospholipids and sphingolipids contributed to the separation of different groups. In order to understand the possible biochemical mechanisms, we compared the changes of hepatic lipids in classes between different groups (see below).
Fig. 2.
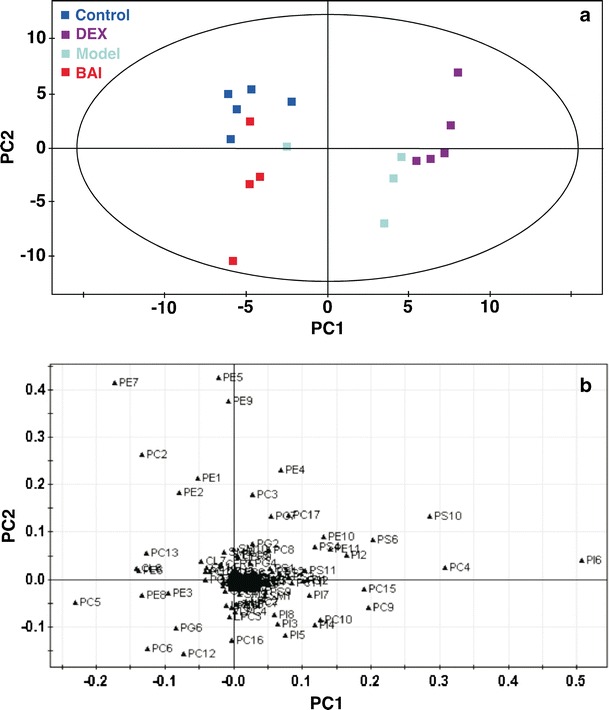
Multivariate analysis of lipidomics data. The PCA score (a) and loading (b) plots were obtained based on the mass levels of individual hepatic lipid species of mice of different groups: control (n = 5), model (n = 4), DEX (n = 5), and BAI (n = 4). The detailed information about the abbreviations of lipid species is given in Table S2
Altered Hepatic Mitochondrial Lipid Amount in the Treated Mice
Cardiolipin (CL) is a unique class of phospholipid species containing four fatty acyl chains and located almost exclusively in the inner mitochondrial membrane (37). The fatty acyl chain composition of CL is composed predominantly of linoleic acid and tetralinoleoyl CL (T18:2 CL), which accounts for more than 50 mol% of total CL in the majority of mammalian organs and is the most abundant species (38). The amount of T18:2 CL is a direct indicator of mammalian mitochondrial function (37,39,40). MDMS-SL analysis of lipid extracts from mouse livers demonstrated significant reduction of amounts of T18:2 CL in the model (3.42 ± 0.27 nmol/mg protein, P < 0.001), DEX (2.74 ± 0.53 nmol/mg protein, P < 0.001), and BAI (3.82 ± 0.20 nmol/mg protein, P < 0.001) groups relative to that in the control group (4.51 ± 0.10 nmol/mg protein) (Fig. 3a). Compared with those in the model group and after treatment with BAI, the amount of T18:2 CL in mouse liver treated with BAI significantly increased although this level was still a little lower than that of the control group. Among these groups of mice, the group treated with DEX showed the lowest amount of T18:2 CL. These results indicate that mitochondrial function was significantly impaired in mouse liver of the BLM-induced IPF model and it was made worse after treatment with DEX. The results suggest that the reduced CL abundance in BLM-induced IPF may associate with the liver injury in the pathology, which may be related to the oxygen deficiency caused by IPF. In contrast, the present data also indicate that treatment with BAI may largely recover the T18:2 CL amount and, thus, mitochondrial function as expected. Of course, further experiments are needed to support this conclusion.
Fig. 3.
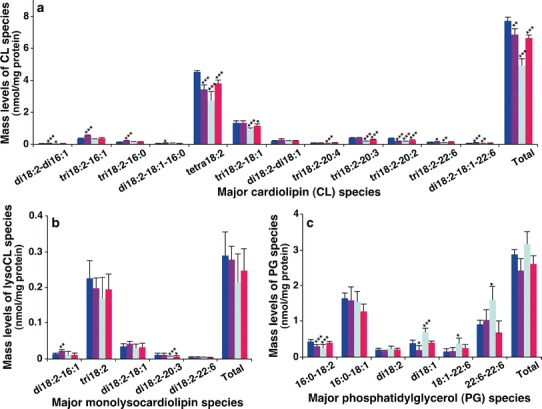
Comparison of the amounts of cardiolipin, monolysocardiolipin, and phosphatidylglycerol species present in hepatic lipid extracts from different mouse groups. Liver tissue samples of the control (n = 5, blue), model (n = 4, purple), DEX (n = 5, green), and BAI (n = 4, red) groups were collected and lipid extracts were prepared by using a modified Bligh and Dyer procedure as described in the “MATERIALS AND METHODS” section. The amounts of CL (a), MLCL (b), and phosphatidylglycerol (PG) (c) species were identified and quantified by MDMS-SL as previously described (28). The data represent means ± SD from the separate animals. Only the major species were displayed. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with those in the control group
Regarding the biochemical mechanism responsible for the reduced CL amount in the model group, the significantly increased amounts of a couple CL species containing relatively short fatty acyls provided some insights. Specifically, significant increases in the amounts of tri18:2–16:1 and tri18:2–16:0 CL species (Fig. 3a), as well as a few other minor CL species containing one or two shorter fatty acyl chains than 18 carbon atoms, were present in the model group. These data indicate that the reduced CL abundance may be due to insufficient remodeling of newly synthesized CL species as previously described (41). The amounts of these CL species containing relatively short fatty acyl chain(s) were not affected after treatment with either DEX or BAI (Fig. 3a), indicating that CL remodeling was corrected after treatment. Other alternative mechanisms leading to the reduced CL amount may exist.
In order to understand the mechanism(s) responsible for the reduced CL amount in the DEX group, MDMS-SL analysis was further performed to determine the levels of monolysocardiolipin (MLCL), which is a product of CL degradation. The significantly increased abundance of 18:2–18:2–16:1 MLCL further supports the impaired CL remodeling as an underlying mechanism for the reduced CL abundance in the model group (Fig. 3b). It was found that there were no significant differences in the levels of predominant MLCL species (i.e., tri18:2 MLCL) or total MLCL between different groups (Fig. 3b). In contrast, MDMS-SL even detected the significant reduction of a minor MLCL species present in both BAI and DEX groups (Fig. 3b). These results suggest that the increased degradation of CL species was unlikely a causal factor leading to the reduction of CL levels present in mouse liver after treatment with DEX.
Phosphatidylglycerol (PG) is a precursor of CL biosynthesis. CL can be synthesized de novo through the condensation of PG and cytidine diphosphate-diacylglycerol (CDP-DAG) in mitochondria, and the reaction is unique to CL and catalyzed by CL synthase (42). Moreover, PG species also play a vital role in the maintenance of mitochondrial structure and function (43). MDMS-SL analysis demonstrated that there existed significantly reduced amounts of 16:0–18:2 and di18:1 PG species in the model group compared with the control group (Fig. 3c). This observation further suggests that the reduced amount of CL in mouse liver of the model group did not result from the abnormal CL biosynthesis. In contrast, MDMS-SL revealed the increased amounts of a couple of PG species in the DEX group, which might count on the disturbed CL biosynthesis and lead to the reduced amount of CL species in this group. These abnormalities in PG species were largely recovered after treatment with BAI compared with the model group, but still existed, which is consistent with the partially recovered CL amount after treatment with BAI.
Altered Phosphatidylcholine and Lysophosphatidylcholine Amounts in Mouse Liver After Treatment
Phosphatidylcholine (PC) is a class of the major phospholipid components of eukaryotic cellular membranes. It plays an important role as a precursor of signaling molecules and as a key element of lipoproteins (44–46). Aberrant hepatic PC metabolism significantly reduces the levels of circulating lipoproteins (47). Compared with the control group, MDMS-SL analysis showed significant changes of the amount of many PC species in both model and DEX groups whereas the amount of PC species in the liver of mice after treatment with BAI was essentially identical to that of the control group with respect to those abundant PC species (Fig. 4a).
Fig. 4.
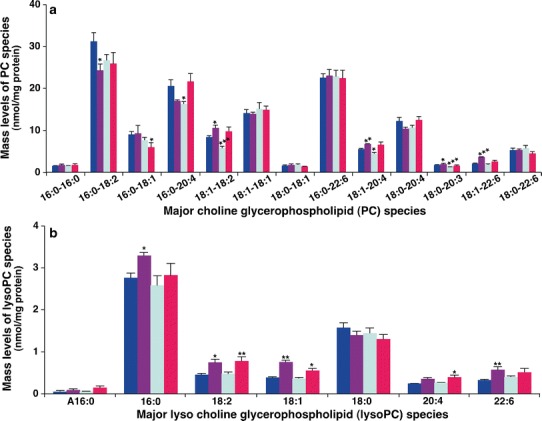
Comparison of the amount of phosphatidylcholine and lysophosphatidylcholine species present in hepatic lipid extracts from different mouse groups. Liver tissue samples of the control (n = 5, blue), model (n = 4, purple), DEX (n = 5, green), and BAI (n = 4, red) groups were collected and lipid extracts were prepared by using a modified Bligh and Dyer procedure as described in the “MATERIALS AND METHODS” section. The abundance of PC (a) or lysoPC (b) species was identified and quantified by MDMS-SL as previously described (28). The data represent means ± SD from the individual animals. Only the major species were displayed. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with those in the control group. The letter “A” stands for alkyl (ether-linked) species
Reduction of PC amount could result from the activation of phospholipase activity (e.g., phospholipase A2). MDMS-SL analysis demonstrated that the amounts of a large number of lysoPC species were significantly increased including 16:0, 18:1, 18:2, and 22:6 lysoPC species in the model group (Fig. 4b) with a total of 7.15 ± 0.55 nmol/mg protein (P < 0.01) in comparison to that of 5.78 ± 0.55 nmol/mg protein in the control group. Treatment with BAI essentially corrected the increased mass levels of lysoPC species induced by IPF, particularly those abundant lysoPC species (Fig. 4b). These data suggest that the reduction of PC levels induced by BLM may be largely due to the activation of phospholipase activity, leading to the accumulation of lipotoxic lysoPC species. The study further suggests that the recovery of the accumulated lysoPC amount after treatment with BAI is likely due to the inhibition of phospholipase activation, which is consistent with the results from previous studies (48,49).
In contrast to the accumulation of lysoPC levels in the model group, unchanged lysoPC levels after treatment with DEX (Fig. 4b) did not support the activation of phospholipase activity. The reduced PC amount after DEX treatment was likely associated with the increased synthesis of PS through the activity of PS synthase 1 (50), which is consistent with the reduced phosphatidylethanolamine (PE) amount to synthesize PS through PS synthase 2 (50) (see below).
Altered Hepatic Levels of Other Phospholipid Classes in the Treated Mice
In the study, in order to further evaluate the efficacy of BAI for treatment of IPF, MDMS-SL was also employed to determine the amounts of other phospholipid classes including phosphatidylinositol (PI), phosphatidic acid (PA), PE, and phosphatidylserine (PS). Similar to PC, these phospholipids play essential roles in membrane architecture, cell division, and function and serve as the depot of lipid second messengers.
PA is the critic substrate for biosynthesis of PI, PG, PE, and PC, as well as TAG species. MDMS-SL analysis of hepatic PA species demonstrated that the amounts of all PA species in the DEX group substantially reduced to 60.4 ± 10.7 pmol/mg protein from 126.0 ± 18.8 pmol/mg protein in the control group (a reduction of 52.06 ± 2.38%). In contrast, there were no significant differences of the amount of any single PA species of the other groups compared to the control one. These results indicate that the majority of PA amount in the DEX group was used for the enhanced TAG biosynthesis (see below), leading to the deficiency in PA amount. This observation also suggests that the altered levels of hepatic phospholipid species in the model group were likely caused by other factors (see above in the case of PC) and that unchanged PA levels in the BAI group were likely due to the protective effects of BAI on hepatic lipidome. The decreased PA levels could also be due to the increased CDP-DAG synthesis pathway, which should have resulted in the increased amount of PI in the DEX group (see below).
Measurement of the PI amount by MDMS-SL revealed very different levels of hepatic PI species from the groups. Specifically, MDMS-SL analysis demonstrated a predominant 18:0–20:4 PI species, which accounted for approximately 70 mol% of total PI amount in liver samples (Fig. 5a). The amount of this PI species in the control and BAI groups was essentially identical, whereas there were significant increases in this PI species and total PI species of both model (P < 0.05) and DEX groups (P < 0.01) compared to the control group (Fig. 5a). The increased amount of PI species after DEX treatment was consistent with the enhanced CDP-DAG synthesis pathway as discussed above. The increased amount of PI species in the model group was likely due to alterations in signaling since the kinetics of PI synthesis and accumulation has important implications for the levels and availability of these signaling metabolites (51).
Fig. 5.
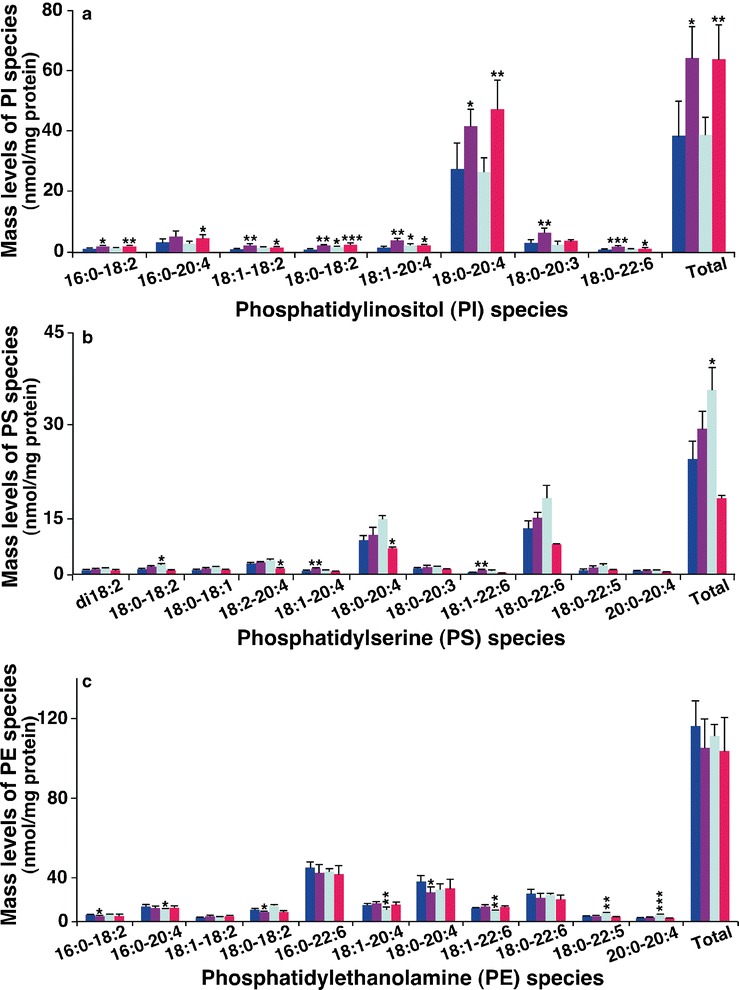
Comparison of the peak intensities of phosphatidylinositol species or the amount of phosphatidylserine and phosphatidylethanolamine species present in hepatic lipid extracts from different mouse groups. Liver tissue samples of the control (n = 5, blue), model (n = 4, purple), DEX (n = 5, green), and BAI (n = 4, red) groups were collected and lipid extracts were prepared by using a modified Bligh and Dyer procedure as described in the “MATERIALS AND METHODS” section. The amount of PI (a), PS (b), or PE (c) species was identified and quantified by MDMS-SL as previously described (28). The data represent means ± SD from the individual animals. Only the major species were displayed. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with those in the control group
The amount of one of the major species of PS (18:0–20:4) as well as the total amount of PS in the DEX group significantly increased (P < 0.05) compared to the control group (Fig. 5b). This result is consistent with the increased PS biosynthesis as evidenced by the significantly reduced both PC and PE levels through activation of PS synthase activity as aforementioned. The levels of a couple of minor PS species significantly increased (P < 0.01) in the model group relative to the controls (Fig. 5a). The increased PS species in the model group might involve signaling and/or cell apoptosis. The amount of hepatic PS species minimally changed in the BAI group relative to the control group, indicating the protective effects of BAI on cellular membrane structure and function.
PE, which is a class of the second most abundant phospholipids, plays an important role in cellular structure and function. MDMS-SL analysis demonstrated that BAI treatment could bring any altered hepatic PE levels in the model group to the normal levels as presented in the control group (Fig. 5c). DEX treatment led to alterations in some PE species, but not the total amount (Fig. 5c), which is likely due to the compartmental effects of PS biosynthesis, which occurs in mitochondria.
Changes of the Mass Levels of Hepatic Ceramide and Sphingomyelin After Treatment
Cer is an important class of sphingolipids, serving as the second messengers in a variety of cellular responses, such as regulation of cell growth, viability, differentiation, and senescence (52), and being a central component in sphingolipid metabolism and catabolism (53). SM is the major component of microdomains in cellular membranes (54). MDMS-SL revealed the changes of the mass levels of a few hepatic Cer species in both model and DEX groups relative to the control group (Fig. 6). However, these changed levels of both Cer and SM species did not occur in the BAI group (Fig. 6), indicating the protective effects on hepatic sphingolipid metabolism after treatment with BAI.
Fig. 6.
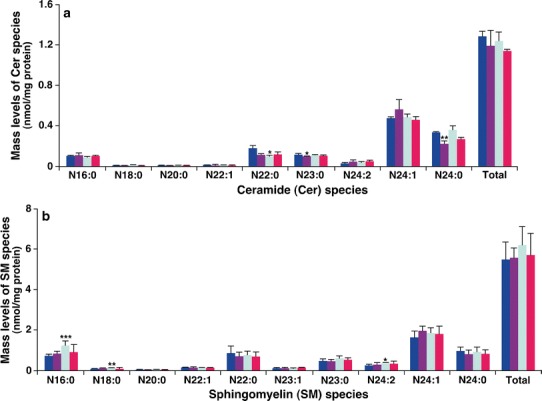
Comparison of the amount of ceramide and sphingomyelin species present in hepatic lipid extracts from different mouse groups. Liver tissue samples of the control (n = 5, blue), model (n = 4, purple), DEX (n = 5, green), and BAI (n = 4, red) groups were collected and lipid extracts were prepared by using a modified Bligh and Dyer procedure as described in the “MATERIALS AND METHODS” section. The amount of ceramide (a) or sphingomyelin (SM) (b) was identified and quantified by MDMS-SL as previously described (28). The data represent means ± SD from the individual animals. Only the major species were displayed. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with those in the control group. “N” stands for the amide linage of the acyl chain
Altered Amount and Composition of Hepatic Triglyceride Fatty Acyls in the Treated Mice
It is well known that treatment with GC is associated with hepatic steatosis (17). MDMS-SL analysis confirmed that DEX treatment increased the hepatic TAG level from 18.4 ± 1.1 nmol/mg protein in the control group to 49.0 ± 7.9 nmol/mg protein in the DEX group (an increase of >150 mol%; P < 0.01) (Fig. 7b, c). In contrast, the amount of hepatic TAG species in the BAI group reduced to 8.8 ± 1.3 nmol/mg protein (a reduction of 50 mol%; P < 0.001) after treatment. These results indicate that DEX treatment resulted in tremendous accumulation of TAG in the liver regardless of the lower body mass relative to the control group, suggesting that DEX treatment could lead to centripetal obesity. In contrast, BAI treatment did not lead to fat accumulation in the liver and further body mass reduction. Moreover, it is well known that the increased hepatic TAG levels were accompanied with insulin resistance and nonalcoholic fatty liver (steatosis), indicating hepatocellular injury (15,55). The increased hepatic amount of TAG in the DEX group was obviously due to the increased biosynthesis of TAG evidenced by the significantly decreased amount of PA species. Previous studies have demonstrated that GC could stimulate the activities of related enzymes that involve the biosynthesis, increased uptake, or reduced secretion of lipids, or a combination of these factors (18,20).
Fig. 7.
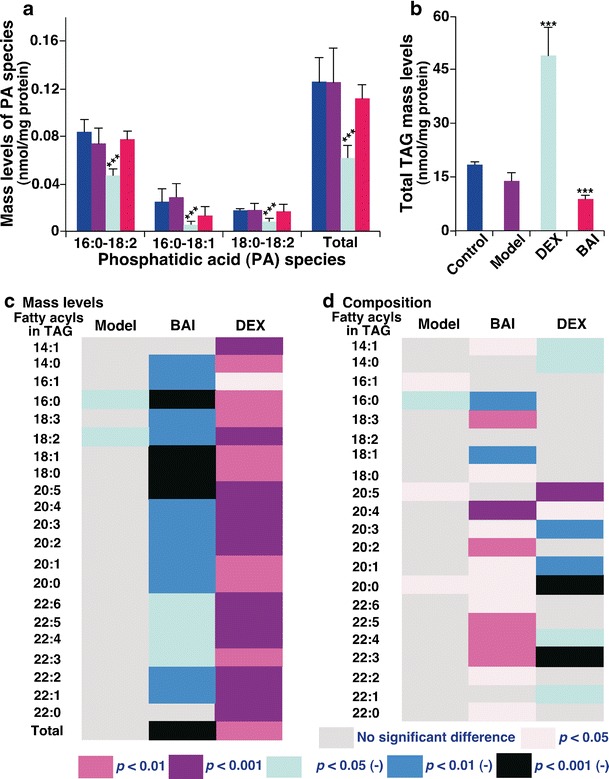
Comparison of the abundance and composition of PA, triacylglycerol, and fatty acyls present in triacylglycerol pools of hepatic lipid extracts from different mouse groups. Liver tissue samples of the control (n = 5, blue), model (n = 4, purple), DEX (n = 5, green), and BAI (n = 4, red) groups were collected and lipid extracts were prepared by using a modified Bligh and Dyer procedure as described in the “MATERIALS AND METHODS” section. The abundance of TAG species was identified and quantified by MDMS-SL as previously described (28,58). The data of PA (a) and TAG (b) species represent means ± SD from the individual animals. ***P < 0.001 compared with those in the control group. The amount of individual fatty acyl chain was derived from the amount of the identified and quantified TAG species. The heat maps showed the P values of statistical significance of the amount (c) and composition (d) of individual fatty acyls relative to the counterparts in the control group, respectively. The negative symbol after the P values in the heat maps indicates the reduction of the values in comparison to those in the control group
Moreover, the heat map of fatty acyl composition in TAG pools showed intriguing tendency (Fig. 7d). Specifically, very long and/or polyunsaturated fatty acyls were predominant in the fatty acyl profiles of TAG species after treatment with BAI whereas a very different profile from the BAI group was present in the DEX group where the compositions of these very long and/or polyunsaturated fatty acyls significantly decreased. The ratios of long-chain polyunsaturated fatty acyls in TAG were decreased in the DEX group, which is identical to what has been reported in severe fatty liver induced by obesity and ethanol (56,57). These data indicate that hepatic steatosis associated with GC treatment might be not only from the accumulation of TAG amount, but also affected from the different levels of fatty acyls present in the TAG pools. The protective effects of BAI on hepatic lipidomes obviously were evidenced by both the reduced accumulation of TAG and the increased levels of polyunsaturated fatty acyls in the TAG pools.
In summary, the current study showed that BLM-induced IPF resulted in alterations in hepatic cellular phospholipids, including CL, PC, PE, PG, PI, PS, lysoCL, and lysoPC species, some sphingolipids, and TAG was examined. Moreover, it was demonstrated that both DEX and BAI have beneficial effects on BLM-induced IPF, but BAI has been found to be more attractive and beneficial with fewer adverse effects on hepatic lipid metabolism compared to DEX. Specifically, after administration with BAI, the majority of these lipid classes at the molecular species levels recovered to normal as shown in the control group. In addition, BAI treatment not only entirely restored the normal hepatic lipid profile, but also greatly improved the fat abundance and composition. Taken together, the results from the current study indicate that BAI is a potential drug for treatment of IPF and that MDMS-SL is a powerful tool for drug screening.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(XLSX 58 kb)
Acknowledgments
We thank Dr. Miao Wang at Sanford-Burnham Medical Research Institute for his technical help. This work was supported by the Natural Science Foundation of Zhejiang Chinese Medical University (No. 711200F002) to C.H., the Natural Science Foundation of Zhejiang Province of China (No. LQ12H28004) to Y.W., a program sponsored by Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents to C.W., and the National Basic Research Program “973” of China (No. 2014CB5430) to Y.F.
Abbreviations
- BAI
Baicalin
- BLM
Bleomycin
- Cer
Ceramide
- CL
Cardiolipin
- CDP
Cytidine diphosphate
- DAG
Diacylglycerol
- DEX
Dexamethasone
- GCs
Glucocorticoids
- IPF
Idiopathic pulmonary fibrosis
- MDMS-SL
Multidimensional mass spectrometry-based shotgun lipidomics
- MLCL
Monolysocardiolipin
- MS
Mass spectrometry or mass spectrometric
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PG
Phosphatidylglycerol
- PI
Phosphatidylinositol
- PS
Phosphatidylserine
- SM
Sphingomyelin
- TAG
Triacylglycerol
Footnotes
Changfeng Hu and Yiqi Wang contributed equally to this work.
Contributor Information
Xianlin Han, Email: xhan@sanfordburnham.org.
Chengping Wen, Email: cpwen.zcmu@yahoo.com.
References
- 1.Society AT Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Ryu JH, Colby TV, Hartman TE. Idiopathic pulmonary fibrosis: current concepts. Mayo Clin Proc. 1998;73:1085–101. doi: 10.4065/73.11.1085. [DOI] [PubMed] [Google Scholar]
- 4.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341:1264–9. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am Rev Respir Dis. 1986;133:321–40. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- 6.Alder S, Shimaoka K, Tsukada Y. Bleomycin toxicity. J Am Med Assoc. 1976;235:2814. doi: 10.1001/jama.235.26.2814c. [DOI] [PubMed] [Google Scholar]
- 7.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- 8.MacNee W, Rahman I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50(Suppl 1):S53–8. doi: 10.1136/thx.50.Suppl_1.S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2002;162:225–31. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 10.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–87. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, et al. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17:1228–35. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- 12.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–9. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 13.Daba MH, Abdel-Aziz AA, Moustafa AM, Al-Majed AA, Al-Shabanah OA, El-Kashef HA. Effects of L-carnitine and ginkgo biloba extract (EG b 761) in experimental bleomycin-induced lung fibrosis. Pharmacol Res. 2002;45:461–7. doi: 10.1006/phrs.2002.0985. [DOI] [PubMed] [Google Scholar]
- 14.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–82. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–23. doi: 10.1042/CS19980388. [DOI] [PubMed] [Google Scholar]
- 16.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/S0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 17.Hill RB., Jr Fatal fat embolism from steroid-induced fatty liver. N Engl J Med. 1961;265:318–20. doi: 10.1056/NEJM196108172650704. [DOI] [Google Scholar]
- 18.Wang M. The role of glucocorticoid action in the pathophysiology of the metabolic syndrome. Nutr Metab (Lond) 2005;2:3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamant S, Shafrir E. Modulation of the activity of insulin-dependent enzymes of lipogenesis by glucocorticoids. Eur J Biochem. 1975;53:541–6. doi: 10.1111/j.1432-1033.1975.tb04097.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang CN, McLeod RS, Yao Z, Brindley DN. Effects of dexamethasone on the synthesis, degradation, and secretion of apolipoprotein B in cultured rat hepatocytes. Arterioscler Thromb Vasc Biol. 1995;15:1481–91. doi: 10.1161/01.ATV.15.9.1481. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 22.Gong LK, Li XH, Wang H, Zhang L, Chen FP, Cai Y, et al. Effect of Feitai on bleomycin-induced pulmonary fibrosis in rats. J Ethnopharmacol. 2005;96:537–44. doi: 10.1016/j.jep.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24:31–6. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- 24.Nagashima S, Hirotani M, Yoshikawa T. Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry. 2000;53:533–8. doi: 10.1016/S0031-9422(99)00593-2. [DOI] [PubMed] [Google Scholar]
- 25.Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–50. doi: 10.1016/S0304-4165(99)00152-X. [DOI] [PubMed] [Google Scholar]
- 26.Shen YC, Chiou WF, Chou YC, Chen CF. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171–81. doi: 10.1016/S0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of the cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multi-dimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81:4356–68. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev. 2012;31:134–78. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–9. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 31.Cheng H, Guan S, Han X. Abundance of triacylglycerols in ganglia and their depletion in diabetic mice: implications for the role of altered triacylglycerols in diabetic neuropathy. J Neurochem. 2006;97:1288–300. doi: 10.1111/j.1471-4159.2006.03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X, Yang K, Gross RW. Microfluidics-based electrospray ionization enhances intrasource separation of lipid classes and extends identification of individual molecular species through multi-dimensional mass spectrometry: development of an automated high throughput platform for shotgun lipidomics. Rapid Commun Mass Spectrom. 2008;22:2115–24. doi: 10.1002/rcm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X, Gross RW. Shotgun lipidomics: multi-dimensional mass spectrometric analysis of cellular lipidomes. Expert Rev Proteomics. 2005;2:253–64. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 34.Han X, Yang K, Yang J, Fikes KN, Cheng H, Gross RW. Factors influencing the electrospray intrasource separation and selective ionization of glycerophospholipids. J Am Soc Mass Spectrom. 2006;17:264–74. doi: 10.1016/j.jasms.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Han RH, Han X. Fatty acidomics: global analysis of lipid species containing a carboxyl group with a charge-remote fragmentation-assisted approach. Anal Chem. 2013;85:9312–20. doi: 10.1021/ac402078p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Hayakawa J, Yang K, Han X. Characterization and quantification of diacylglycerol species in biological extracts after one-step derivatization: a shotgun lipidomics approach. Anal Chem. 2014;86:2146–55. doi: 10.1021/ac403798q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 38.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 40.He Q, Han X. Cardiolipin remodeling in diabetic heart. Chem Phys Lipids. 2014;179:75–81. doi: 10.1016/j.chemphyslip.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Kiebish MA, Bell R, Yang K, Phan T, Zhao Z, Ames W, et al. Dynamic simulation of cardiolipin remodeling: greasing the wheels for an interpretative approach to lipidomics. J Lipid Res. 2010;51:2153–70. doi: 10.1194/jlr.M004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mejia EM, Nguyen H, Hatch GM. Mammalian cardiolipin biosynthesis. Chem Phys Lipids. 2014;179:11–6. doi: 10.1016/j.chemphyslip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–85. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 44.Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, et al. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B. 1986;83:551–4. doi: 10.1016/0305-0491(86)90295-6. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta. 2008;1778:1676–95. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 46.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta. 1821;2012:754–61. doi: 10.1016/j.bbalip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Kyo R, Nakahata N, Sakakibara I, Kubo M, Ohizumi Y. Baicalin and baicalein, constituents of an important medicinal plant, inhibit intracellular Ca2+ elevation by reducing phospholipase C activity in C6 rat glioma cells. J Pharm Pharmacol. 1998;50:1179–82. doi: 10.1111/j.2042-7158.1998.tb03331.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Feng G, Jiang J, Tian H, Chen L, Cai Y, et al. Effect of baicalin and octreotide on the expression levels of P-selectin protein in multiple organs of rats with severe acute pancreatitis. J Gastroenterol Hepatol. 2009;24:1753–62. doi: 10.1111/j.1440-1746.2009.05902.x. [DOI] [PubMed] [Google Scholar]
- 50.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–87. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Gaspar ML, Aregullin MA, Jesch SA, Nunez LR, Villa-Garcia M, Henry SA. The emergence of yeast lipidomics. Biochim Biophys Acta. 2007;1771:241–54. doi: 10.1016/j.bbalip.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/S0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 53.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkatesan S, Rideout JM, Simpson KJ. Microsomal delta 9, delta 6 and delta 5 desaturase activities and liver membrane fatty acid profiles in alcohol-fed rats. Biomed Chromatogr. 1990;4:234–8. doi: 10.1002/bmc.1130040605. [DOI] [PubMed] [Google Scholar]
- 57.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37:1499–507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 58.Han X, Gross RW. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2001;295:88–100. doi: 10.1006/abio.2001.5178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 58 kb)


