Abstract
Activation of liver X receptors (LXRs) can improve glucose tolerance in insulin-independent diabetes, however, whether similar effects can be achieved in insulin-dependent diabetes remains unclear. Here, we evaluated the anti-diabetic activity of T0901317, a potent agonist of LXRs, in diabetic mice induced by streptozotocin, and our data demonstrate that T0901317 is most effective when combined with cold treatment of animals. Treatment with T0901317 improved glucose tolerance of diabetic mice, which was associated with repressed expression of key genes involved in hepatic gluconeogenesis such as Pepck and G6p. Combined treatment by T0901317 and cold exposure reduced transcription of gluconeogenic genes to similar levels. Intriguingly, combined treatment greatly increased expression of Ucp1, Cidea, Dio2, and Elvol3 predominantly in the inguinal white adipose tissue, consequently leading to browning of this fat pad, and resulting in further improvement of glucose tolerance which was associated with increased protein levels of UCP1 and GLUT4. Collectively, these results suggest that browning of white adipose tissue via cold exposure in combination with activation of liver X receptors is an alternative and effective strategy to manage insulin-dependent diabetes.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-015-9746-4) contains supplementary material, which is available to authorized users.
KEY WORDS: browning of white adipose tissue, cold exposure, diabetes, liver X receptor, streptozotocin, T0901317
INTRODUCTION
Liver X receptors (LXRs), including LXRα and LXRβ, are nuclear receptors that play central roles in regulating lipid metabolism and glucose homeostasis through transcriptional regulation of their target genes (1,2). Upon ligand binding, the activated LXRs upregulate expression of Abca1, Abcg1 and Cyp7a1, leading to reversal of cholesterol transport and elevation of cholesterol metabolism (3,4). LXRs also play crucial roles in glucose homeostasis (5). Activation of LXRs by synthetic agonists such as T0901317 and GW3965 has been reported to improve glucose tolerance in diabetic mice and rats (6–8) via repressing gluconeogenesis in the liver and enhancing glucose absorption and utilization in peripheral tissues (6). In addition, we have recently reported that activation of LXRs by T0901317 protects mice from high fat diet-induced insulin resistance and glucose intolerance through modulating the transcription of a set of genes involved in energy metabolism (9). These previous studies support the notion that activation of LXRs results in beneficial effects in glucose metabolism in high-fat diet-induced type 2 diabetes. An obvious question is whether such an effect is also achievable in insulin-dependent type 1 diabetes.
A recent study by Gunawardana et al. clearly shows that subcutaneous transplants of brown adipose tissue (BAT) can correct hyperglycemia in STZ-induced diabetic mice, leading to a reversal of diabetes (10). Moreover, Stanford et al. reported that transplantation of BAT protected mice from diet-induced glucose intolerance, further highlighting the critical role of BAT in glucose metabolism (11). In addition to natural BAT, certain white adipose tissue (WAT) can be converted to a “brown-like” state with prolonged cold exposure (12). This leads us to raise the hypothesis that browning of WAT via cold exposure may help enhance the anti-diabetic effect of T0901317 in insulin-dependent diabetic mice.
To test this hypothesis, we established a streptozotocin (STZ)-induced diabetic mice model and treated the animals with T0901317 with or without daily cold exposure. Our results show that browning of WAT via daily cold exposure greatly improved the anti-diabetic effect of T0901317 in these diabetic mice. These results suggest that browning of WAT via cold exposure represents a sound strategy in enhancing the beneficial effect of LXR activation on managing type-1 diabetes.
MATERIALS AND METHODS
Animals
Eight-week old C57BL/6 mice purchased from Charles River (Wilmington, MA) were housed under a standard 12-h light-dark cycle. The use of mice (male, ∼25 g) in this study was compliant with relevant policies, and the animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Georgia (Protocol Number, A2014 07-008-Y1-A0).
Development and Treatment of Diabetic Mice
Four groups of mice (n = 5 for each group) were employed in this study. STZ was freshly prepared using a citrate buffer (pH 4.0) before injections. Group 1 mice were intraperitoneally (i.p.) injected with the citrate buffer and used as the control, and those in the last three groups were injected (i.p.) with STZ (50 mg/kg) daily for 5 days to establish diabetes. Three days after the last injection of STZ, animals were treated with saline or T0901317 (10 mg/kg, i.p.). One group of T0901317-treated mice was exposed to 4°C daily for 6 h and the other exposed to 25°C as the control. The treatment was performed daily and continued for 5 days.
Induction and Confirmation of Browning of WAT
Diabetic animals exposed daily for 6 h at 4 or 25°C for 5 days were euthanized using carbon dioxide. This intermittent cold exposure condition was selected based on several previous publications showing that this condition was necessary for inducing browning of the white adipose tissue in mice (12–14). BAT, epididymal white adipose tissue (EWAT), and inguinal white adipose tissue (IWAT) were dissected and stored for gene expression analysis and histochemical examination. In a separate experiment, we also verified the cold exposure-induced browning of white adipose tissue in non-drug-treated normal mice by following the same procedure.
Glucose Tolerance Test and Determinations of Blood Insulin and FGF21
Mice who received the final treatment of T0901317 with or without cold exposure were fasted for 6 h. The protocol for glucose tolerance test has been previously reported (15). In brief, glucose dissolved in phosphate buffered saline was injected (i.p.) into mice (2 g/kg) and the time considered as time zero. Blood glucose levels at 0, 30, 60, and 120 min were determined using glucose test strips and glucose meters. Blood samples at 0 and 30 min were collected from tail veins for determination of insulin concentration using a commercially available ELISA kit (#10-1113-01, Mercodia Developing Diagnostics). In a separate experiment, we assessed glucose tolerance of normal mice with or without cold exposure by performing the same procedure. We also measured circulating FGF21 levels of these mice using an ELISA kit purchased from Aviscera Bioscience (#SK00145-08, Santa Clara, CA) by following a protocol provided by the manufacturer.
Liver Triglyceride Determination
Liver triglyceride level was measured using a previously reported method (16,17). Briefly, liver samples (200–400 mg per sample) were homogenized using a solution consisting of chloroform and methanol (2:1) and incubated overnight at 4°C. These samples were centrifuged at 12,800g for 20 min at 4°C. Supernatants were collected, dried, and re-dissolved in 5% Triton-X100. Triglyceride concentration was determined following the instruction of the commercial kit (#TR22203, Thermo-Scientific).
Determination of Blood Concentration of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)
Blood samples were collected from heart cavities immediately after euthanizing mice. These samples were centrifuged at 1500g for 5 min to isolate serum for blood aspartate aminotransferase (AST) and alanine aminotransferase (ALT) determinations. The measurements were performed following the instruction of the kits (#TR70121 and #TR71121, Thermo-Scientific).
Gene Expression Analysis
Tissue samples were freshly collected and immediately frozen at −80°C until use. Total RNA was isolated using an RNeasy kit (#74804) purchased from QIAGEN (Valencia, CA). Purified RNA samples were dissolved in RNase-free water and reverse-phase polymerase chain reaction (RT-PCR) was conducted using a Superscript RT III enzyme kit (Invitrogen, #11752-050). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green as detection reagent. The primers for qRT-PCR analysis were synthesized at Sigma (St. Louis, MO), and their sequences are listed in Supplementary Table 1. Melting curve analyses of all real-time PCR product were conducted and showed a single DNA duplex.
Hematoxylin-Eosin Staining
Tissues were freshly collected from animals, fixed overnight using buffered formalin at neutral pH, and dehydrated against gradients of ethanol before embedded in paraffin. Tissue sections were cut at 6 μm in thickness and dried at 37°C for 1 h. Hematoxylin-eosin (H&E) staining was conducted following the instructions of a commercial kit (#3500, BBC Biochemical). The tissue sections were examined using an optical microscope (ECLIPSE Ti, Nikon), and size measurements of adipocytes were conducted using NIS-Elements imaging platform from Nikon Instruments Inc. (Melville, NY).
Oil-Red O Staining for Assessment of Lipid Content in the Liver
Liver samples were freshly collected and immediately frozen in liquid nitrogen. The tissue samples were cut at 8 μm in thickness using a Cryostat. The procedure for Oil-red O staining has been previously described (18). Briefly, liver sections were immediately placed on slides and fixed in buffered formalin for 30 min before being completely washed with phosphate buffered saline. These sections were washed with 60% isopropanol for 5 min and then stained with freshly prepared Oil-red O working solution (Electron Microscopy Sciences) for 30 min and counterstained with hematoxylin for 1 min.
Immunofluorescence Histochemical Study
Tissue samples embedded in paraffin were cut to 6 μm in thickness and dried at 37°C for 1 h. The sections were immersed in citrate buffer (pH 6.0) and processed in a thermostatic water bath (95°C, 30 min) for antigen retrieval. These sections were blocked using 10% normal serum with 1% bovine serum albumin in Tris-buffered saline for 2 h at 25°C before incubation with primary antibodies overnight at 4°C. The following primary antibodies were used: antibody against UCP1 (#ab10983, Abcam), antibody against insulin (#4590, Cell Signaling), and antibody against GLUT4 (#ab654, Abcam). A secondary antibody conjugated with fluorescent dye (#4412, Cell Signaling) was utilized for imaging and visualization.
Statistics
The results were expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance. A p value below 0.05 (p < 0.05) was considered significantly different.
RESULTS
Anti-diabetic Effects of T0901317 and Cold Exposure on Diabetic Mice
The T0901317 treated diabetic mice with or without cold exposure had comparable body weight, food intake and water intake (data not shown). Treatment with T0901317 of animals received five daily injections of STZ reduced STZ-induced hyperglycemia by an average decrease of 33.2 mg/dL in non-fasting glucose. Daily cold exposure at 4°C greatly improved the potency of T0901317 with an additional reduction of 94.8 mg/dL (Fig. 1b). To evaluate the overall effect of the treatment on glucose tolerance, we performed IPGTT. Treatment with T0901317, with or without daily cold exposure, significantly suppressed the elevation of blood glucose level in IPGTT (Fig. 1c). The area under curve (AUC) calculation confirms the conclusion (Fig. 1d). In a separate experiment, we found that cold exposure without T0901317 treatment was capable of increasing glucose tolerance in normal mice (Supplementary Figure 1).
Fig. 1.
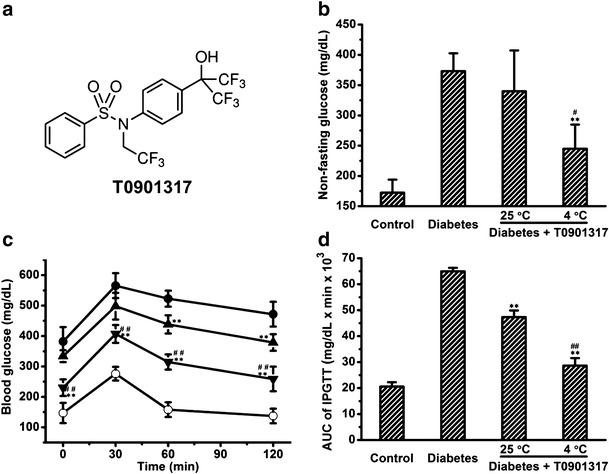
Anti-diabetic effect of T0901317 with or without cold exposure on diabetic mice. a Molecular structure of T0901317. b Determination of non-fasting glucose levels. (c) Results of IPGTT (○, control mice; ●, diabetic mice treated with saline; ▲, diabetic mice treated with T0901317; ▼, diabetic mice treated with a combination of T0901317 and cold exposure). d AUC analysis of IPGTT. Values in b–d represent average ± SD (n = 5). **p < 0.01 compared with diabetic mice, # p < 0.05 compared with diabetic mice treated with T0901317 at 25°C
Cold Exposure-Induced Browning of WAT
To confirm 5 days of daily cold exposure-induced browning of WAT, in a separate experiment, we performed histochemical examinations in BAT, epididymal white adipose tissue (EWAT) and inguinal white adipose tissue (IWAT) using H&E staining in normal mice. Cold exposure-induced apparent morphological changes in IWAT, making its morphology more like that of BAT (Fig. 2a). Meanwhile, cold exposure also changed the morphology of BAT and EWAT, but not as much as that of IWAT (Fig. 2a), suggesting that the primary adipose tissue affected by cold exposure is IWAT. To further confirm that cold exposure-induced browning of WAT in these mice, we selected a set of BAT signature genes and measured their expression levels. Cold exposure modulated the expression of these genes with different manners in BAT, EWAT, and IWAT (Fig. 2b–d). In BAT, cold exposure increased the expression of Dio2, Elovl3, Pgc1α, and Ucp1, but not Cidea and Prdm16 (Fig. 2b). In EWAT, cold exposure increased expression of Elovl3, Pgc1α, and Prdm16, but not the others (Fig. 2c). Interestingly, cold exposure elevated expression of other genes in IWAT (Fig. 2d), especially Prdm16 (∼11.9-fold) and Ucp1 (∼6.1-fold). We also detected an increase in the amount of the UCP1 protein in IWAT using IHC staining (Fig. 2e). These results show that UCP1 levels in IWAT of mice with cold exposure were significantly higher than that of control, demonstrating that cold exposure efficiently induced browning of this tissue.
Fig. 2.
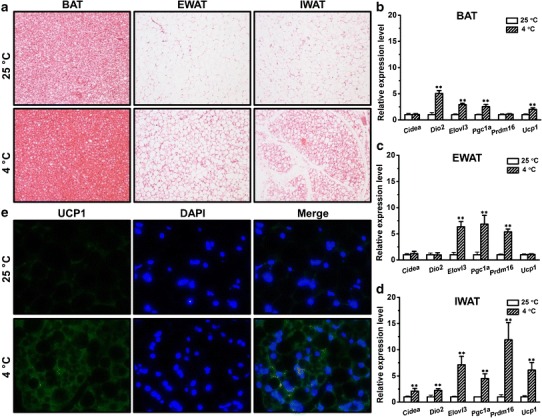
Cold exposure-induced browning of WAT in metabolically normal mice. a Representative images of H&E staining of sections of BAT (left), epididymal white adipose tissue (EWAT) (middle) and inguinal white adipose tissue sections (IWAT) (right). b The mRNA levels of the selected genes in BAT. c The mRNA levels of the selected genes in the EWAT. d The mRNA levels of the selected genes in IWAT. e Representative images of immunohistochemical staining of IWAT sections (the green and blue colors represent positive signals for UCP1 and the nuclei, respectively). Values in b–d represent average ± SD (n = 5). **p < 0.01 compared with mice kept at 25°C
The T0901317 Treatments With or Without Cold Exposure Neither Increased the Glucose-Stimulated Insulin Secretion nor Corrected the STZ-induced Tissue Damage in Pancreases
One explanation for the beneficial effect of T0901317 and cold exposure is that the treatment reversed the insulin deficiency established by STZ. To investigate the impact of treatment on insulin levels, we measured blood insulin level using ELISA. Mice injected with STZ show lower levels of fasting insulin compared to control (Fig. 3a). Neither T0901317 treatment alone nor combined treatment increased the glucose-stimulated insulin secretion (Fig. 3b). To investigate the impact of treatments on the morphology of pancreatic islets, we conducted H&E staining. Consistent with blood insulin levels, mice injected with STZ showed tissue damages in their pancreases, and these damages were not repaired by the treatment of T0901317 with or without cold exposure (Fig. 3c). We also examined insulin levels in pancreatic islets using IHC staining. In line with the above results, all diabetic mice showed lower levels of insulin in islets compared to control (Fig. 3d). These results suggest that the effects exerted by T0901317 with or without cold exposure are not mediated through the repair of pancreatic insulin secretion.
Fig. 3.
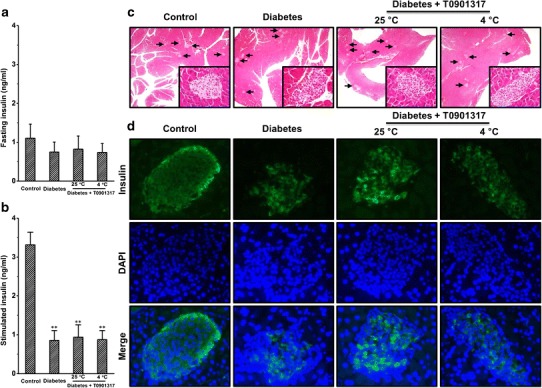
Treatment by T0901317 with or without cold exposure neither increased the glucose-stimulated insulin secretion nor corrected the dysfunction of pancreatic islets. a Determination of fasting insulin. b Determination of glucose-stimulated insulin secretion. c Representative images of H&E staining of pancreas tissue sections. d Representative images of immunohistochemical staining of pancreatic tissue sections (the green and blue colors represent positive signals for insulin and the nuclei, respectively). Values in a–b represent average ± SD (n = 5). **p < 0.01 compared with control mice
T0901317 Treatments With or Without Cold Exposure did not Cause Liver Dysfunction in Diabetic Mice
To examine the impact of treatments on the morphology and lipid levels in the liver, we performed H&E and Oil-red O staining. No apparent changes were observed among treated and control animals (Fig. 4a). We also conducted quantitative measurement of triacylglycerol in the liver and obtained similar results (Fig. 4b). Next, we measured blood concentrations of AST and ALT. Both enzymes were within the normal range after T0901317 treatment with or without cold exposure (Fig. 4c, d), demonstrating that the treatments did not cause liver dysfunction in the diabetic mice.
Fig. 4.
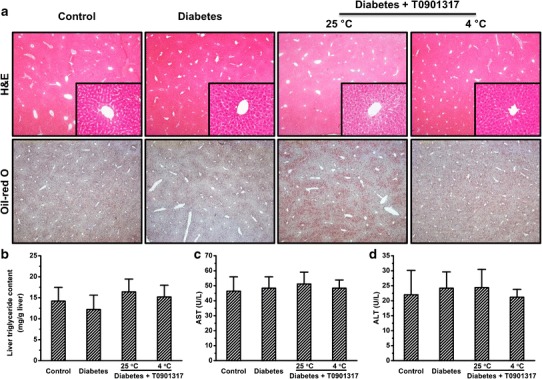
T0901713 treatments did not lead to fatty liver or liver dysfunction. a Representative images of H&E (upper panel) and Oil-red O staining (lower panel) of liver tissue sections. b Quantitative determination of liver triglyceride. c Blood aspartate aminotransferase levels. d Blood alanine aminotransferase levels. Values in a–b represent average ± SD (n = 5)
T0901317 Treatment With or Without Cold Exposure Modulated Gene Expression in Livers
To confirm LXRs were activated by T0901317 treatment, we examined the mRNA levels of Cyp7a1, Abca1, and Abcg1 in livers, the LXRs target genes. Treatment with T0901317 increased the expression of Cyp7a1, Abca1, and Abcg1 by ∼4.0-, ∼2.2-, and ∼4.1-fold, respectively (Fig. 5a–c). Cold exposure did not significantly affect the elevated expression of these three target genes (Fig. 5a–c). We also determined the expression of Srebp1c which is strongly involved in lipogenesis in the liver, and found that T0901317 treatment alone or in combination with cold exposure increased the expression of this gene by ∼7.7- and ∼6.8-fold, respectively (Fig. 5d). Next, we determined expression of Pepck and G6p which are key genes for hepatic gluconeogenesis. Treatment by T0901317 with or without cold exposure decreased expression of both genes to a similar level (Fig. 5e, f). In a separate experiment, we found that cold exposure without T0901317 treatment greatly elevated expression of Fgf21 in the liver of normal mice (Supplementary Figure 2).
Fig. 5.
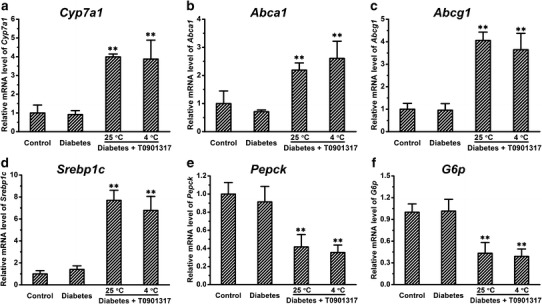
Treatment by T0901317 with or without cold exposure had similar effect in modulating the expression of genes in the liver. a–c Expression of LXR target genes. d Expression of Srebp1c. e–f The mRNA levels of genes involved in hepatic gluconeogenesis. Values in a–f represent average ± SD (n = 5). **p < 0.01 compared with control mice
Cold Exposure Changed the Morphology of IWAT in Diabetic Mice Treated with T0901317
To examine the impact of treatments on morphology of adipose tissues, we made tissue sections and performed H&E staining. Diabetic mice showed slight morphological changes in EWAT compared to control (Fig. 6a). Similar results were observed in IWAT (Fig. 6a). Consistent with previous results obtained in normal mice (Fig. 2a), cold exposure markedly changed the morphology of IWAT in diabetic mice treated with T0901317 (Fig. 6a). No apparent morphological change was seen in BAT after treatments (Fig. 6a). We also performed quantitative determinations. Although injections of STZ tended to decrease the size of EWAT cells, no statistically significant differences were seen among the four groups of animals (Fig. 6b). In IWAT, T0901317 treatment with cold exposure greatly decreased the size of adipocytes, leading to an average reduction of ∼53% compared to the size in control animals (Fig. 6c).
Fig. 6.
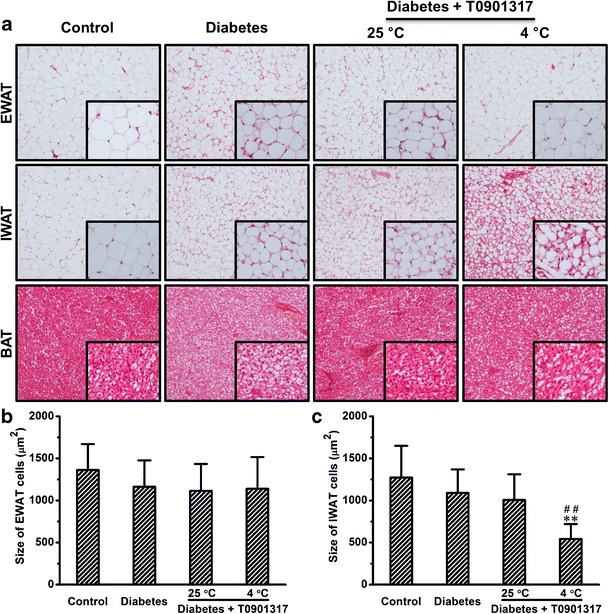
Cold exposure changed the morphology of IWAT in mice treated with T0901317. a Representative images of H&E staining. b Quantitative analysis of epididymal white adipose tissue (EWAT). c Quantitative analysis of inguinal white adipose tissue (IWAT). Values in b–c represent average ± SD of total number of adipocytes seen in five tissue slices (50 adipocytes per slice). **p < 0.01 compared with control mice, ## p < 0.01 compared with diabetic mice treated with T0901317 at 25°C
Cold Exposure Increased the Expression of BAT Signature Genes in IWAT of Diabetic Mice Treated with T0901317
To confirm cold exposure-induced browning of IWAT in these diabetic mice treated with T0901317, we determined the expression levels of several BAT signature genes using quantitative PCR. Cold exposure greatly increased Ucp1 expression by ∼115.8-fold, which was confirmed by regular PCR and agarose gel electrophoresis (Fig. 7a, b). In addition, cold exposure markedly increased expression of Cidea, Dio2, and Elovl3 by ∼39.0-, ∼12.1-, and ∼22.9-fold, respectively (Fig. 7c–e).
Fig. 7.
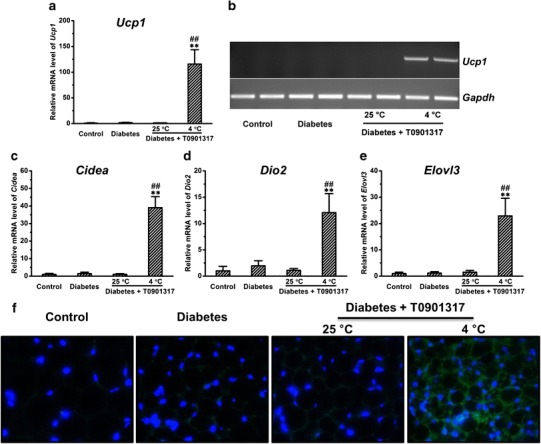
Cold exposure greatly increased the expression of BAT signature genes in the inguinal white adipose tissue of mice treated with T0901317. a Expression of Ucp1. b Results of agarose gel examination of Ucp1 expression. c Expression of Cidea. d Expression of Dio2. e Expression of Elovl3. f Representative images of immunohistochemical staining of sections of inguinal white adipose tissue. The green and blue colors represent positive signals for UCP1 and the nuclei, respectively. Values in (a), (c)–(e) represent average ± SD (n = 5). **p < 0.01 compared with control mice, ## p < 0.01 compared with diabetic mice treated with T0901317 at 25°C
Cold Exposure Increased UCP1 and GLUT4 Protein Levels in IWAT of Diabetic Mice Treated with T0901317
To further confirm that cold exposure-induced browning of IWAT in these diabetic mice, we performed IHC staining using antibody against UCP1 which is a specific marker for BAT. Consistent with the results of gene expression (Fig. 7a, b), mice treated with a combination of T0901317 and cold exposure showed significantly higher levels of UCP1 in IWAT (Fig. 7f). We also examined GLUT4 protein levels in IWAT using IHC and found that browning of IWAT was associated with higher levels of GLUT4 in this tissue (Fig. 8).
Fig. 8.
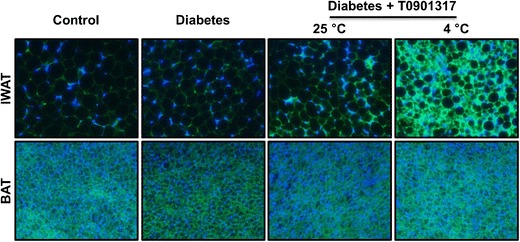
Browning of inguinal white adipose tissue was associated with higher levels of GLUT4 in diabetic mice treated with T0901317. Representative images of immunohistochemical staining of sections of inguinal white adipose tissue (upper panel) and brown adipose tissue (lower panel). The green and blue colors represent positive signals for GLUT4 and the nuclei, respectively
DISCUSSION
In this study, we demonstrate that browning of WAT via cold exposure greatly improved the anti-diabetic effect of T0901317 in STZ-induced insulin-dependent diabetes (Fig. 1). Improvement in glucose tolerance by cold exposure is not mediated by the repair of pancreas, since browning of WAT was not associated with an increase in glucose-stimulated insulin secretion (Fig. 3). This improvement did not relate to the hepatic gluconeogenesis, because both treatments repressed the transcription of gluconeogenic genes to similar levels (Fig. 5). The cold exposure-induced browning of WAT was associated with higher levels of GLUT4 in this tissue (Fig. 8), suggesting that the improvement is mediated, at least partly, by enhanced glucose absorption in this fat pad.
The limited anti-diabetic effect of T0901317 injected (i.p.) at 10 mg/kg in insulin-dependent mice (Fig. 1) may be due to its weak activity in stimulating insulin secretion from pancreatic β-cells in vivo (Fig. 3a, b). The effects of activation of LXRs in pancreatic β-cells have been extensively studied in vitro. Studies by several groups showed that T0901317 increases insulin secretion from MIN6 cells, INS-1 cells, and human islets, respectively (19–21). However, reports from Scholz et al. show that activation of LXRs only suppressed inflammation in human islets without a significant effect on insulin secretion (22), and a study by Meng et al. even indicates that T0901317 impaired insulin secretion from MIN6 cells (23). These differences may be caused by varied conditions employed in these studies. In the current study, we also examined the impact of T0901317 on insulin secretion and the data clearly show that treatment with T0901317 did not increase basal fasting insulin or glucose-stimulated insulin secretion, and it did not repair the STZ-induced pancreatic tissue damage (Fig. 3).
Hepatic gluconeogenesis plays a crucial role in regulating glucose homeostasis (24). A previous study by Cao et al. shows that activation of LXRs by T0901317 inhibited expression of several genes involved in hepatic gluconeogenesis, leading to reduced hepatic glucose outputs and decreased levels of plasma glucose in diabetic rats (7). Laffitte et al. also reported that activation of LXRs suppressed the gluconeogenic program including Pepck and G6p, leading to an improved glucose tolerance in diet-induced obese mice (6). In agreement with these earlier studies, our data reveal that treatment with T0901317 greatly suppressed the expression of Pepck and G6p in livers of diabetic mice (Fig. 5). However, browning of WAT did not further decrease the transcription of these genes (Fig. 5), suggesting that the improvement in glucose tolerance induced by cold exposure is not mediated via suppressing hepatic gluconeogenesis.
Glucose absorption in peripheral tissue is important in maintaining glucose homeostasis, and GLUT4 is a pivotal transporter for this process (25–27). Previous studies by Dalen et al. and Laffitte et al. demonstrated that LXRs play a crucial role in controlling transcription of Glut4 (6,28). In line with these reports, our previous study also shows that activation of LXRs by T0901317 significantly increased mRNA levels of Glut4 in multiple peripheral tissues (9). In the present study, our data show that browning of IWAT was associated with higher levels of GLUT4 in this tissue, indicating that the improved glucose tolerance might be achieved, at least in part, through the increased GLUT4 expression in this converted fat pad. However, considering that the GLUT4-mediated glucose uptake is insulin-dependent and both T0901317-treated animals had impaired capability of insulin secretion, the increased GLUT4 expression may not be the only mechanism.
BAT is actively involved in lipid and glucose metabolism (29). Recent studies clearly show that transplantation of BAT can increase glucose tolerance in diabetic rodents (10, 11), highlighting the crucial role of BAT in glucose metabolism. The molecular mechanism underlying this metabolic benefit remains poorly understood, and it is likely that a panel of secreted proteins from BAT such as FGF21 and neuregulin 4 (Nrg4) may play pivotal roles in this process (11,30,31). In addition to the endogenous natural BAT, some WAT depots can be converted to a “brown-like” state (32). For example, several studies provide convincing evidence proving that multiple proteins including BMP7, BDNF, irisin, and FGF21 can efficiently initiate browning of WAT in mice (12,33–37). In addition to the molecules mentioned above, cold exposure, as a simple physical approach, can also induce browning of WAT in mice (12). Consistent with these studies, our data show that cold exposure efficiently induced the browning of IWAT, as evidenced by the appearance of UCP1-positive adipocytes in this tissue (Figs. 2 and 7), and browning of IWAT markedly increased the potency of T0901317, improving glucose tolerance of diabetic mice (Fig. 1).
The beneficial effects of cold exposure in improving glucose and lipid metabolism have been increasingly appreciated in a variety of animal models as well as in humans, although the underlying mechanism remains largely elusive (12,38,39). Recent investigations unveiled that cold exposure strongly induces browning of white fat and activates brown fat, which subsequently releases a distinctive group of proteins (40). Among these released proteins, FGF21 is the major component having an insulin-independent glucose-lowering activity (41). Remarkably, FGF21 is capable of restoring euglycemia in diabetic mice primarily through inducing browning of white fat and increasing adaptive thermogenesis in brown fat (42). At the cellular level, FGF21 is able to stimulate glucose uptake into adipocytes in an insulin-independent manner (43). Moreover, FGF21 has a direct effect in stimulating glucose uptake by skeletal muscle (44). Mechanistically, the FGF21-mediated restoration of euglycemia is mediated by complex pathways and may be contributed collectively by induced browning of white fat, increased thermogenesis in brown fat, elevated expression of glucose transporters in metabolically active tissues, stimulated glucose uptake in muscle and fat, and suppressed gluconeogenesis in the liver (42–44). Given that FGF21 has an insulin-independent hypoglycemic activity and its expression was greatly increased in response to cold exposure (Supplementary Figure 2), it is highly likely that the increased FGF21 expression contributes substantially to the metabolic benefits of cold exposure observed in the present study. However, some other molecules may also be involved and the detailed underlying mechanism needs to be elucidated in future work.
In conclusion, we demonstrate in this study that browning of WAT via cold exposure can greatly improve the anti-diabetic effect of T0901317 in diabetic mice. Our results suggest that browning of WAT via cold exposure or molecules such as FGF21, Irisin, and Nrg4 that mimic this effect would be an excellent strategy in managing insulin-dependent diabetes.
Electronic supplementary material
Cold exposure increased glucose tolerance in metabolically normal mice. a. Glucose profiles of IPGTT. (b) AUC analysis of IPGTT. Values in (a) and (b) represent average ± SD (n = 5). ** p < 0.01 compared with control mice kept at 25°C. (GIF 127 kb)
Cold exposure increased mRNA and protein levels of FGF21 in normal mice. (a) Expression of FGF21 in the liver and brown adipose tissue. (b) Protein level of FGF21 in blood. Values in (a) and (b) represent average ± SD (n = 5). ** p < 0.01 compared with control mice kept at 25°C. (GIF 67 kb)
(DOCX 16 kb)
Acknowledgments
The study was supported in part by grants from NIH (RO1EB007357 and RO1HL098295). We thank Ms. Ryan Fugett for English editing.
Conflict of Interest
The authors claim no conflicts of interest.
References
- 1.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–14. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffensen KR, Gustafsson JA. Putative metabolic effects of the liver X receptor (LXR) Diabetes. 2004;53(Suppl 1):S36–42. doi: 10.2337/diabetes.53.2007.S36. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204(3):233–40. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 4.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/S0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 5.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445(7124):219–23. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 6.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100(9):5419–24. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J Biol Chem. 2003;278(2):1131–6. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 8.Gao M, Bu L, Ma Y, Liu D. Concurrent activation of liver X receptor and peroxisome proliferator-activated receptor alpha exacerbates hepatic steatosis in high fat diet-induced obese mice. PLoS One. 2013;8(6):e65641. doi: 10.1371/journal.pone.0065641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M, Liu D. The liver X receptor agonist T0901317 protects mice from high fat diet-induced obesity and insulin resistance. AAPS J. 2013;15(1):258–66. doi: 10.1208/s12248-012-9429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61(3):674–82. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HS, Qiao L, Bosco C, Leong LH, Lytle N, Feng GS, et al. Intermittent cold exposure enhances fat accumulation in mice. PLoS One. 2014;9(5):e96432. doi: 10.1371/journal.pone.0096432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS One. 2014;9(1):e85876. doi: 10.1371/journal.pone.0085876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M, Ma Y, Liu D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm Res. 2013;30(11):2940–50. doi: 10.1007/s11095-013-1125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90(1):420–6. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 17.Gao M, Liu D. Resveratrol suppresses T0901317-induced hepatic fat accumulation in mice. AAPS J. 2013;15(3):744–52. doi: 10.1208/s12248-013-9473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu L, Gao M, Qu S, Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. AAPS J. 2013;15(4):1001–11. doi: 10.1208/s12248-013-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efanov AM, Sewing S, Bokvist K, Gromada J. Liver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cells. Diabetes. 2004;53(Suppl 3):S75–8. doi: 10.2337/diabetes.53.suppl_3.S75. [DOI] [PubMed] [Google Scholar]
- 20.Green CD, Jump DB, Olson LK. Elevated insulin secretion from liver X receptor-activated pancreatic beta-cells involves increased de novo lipid synthesis and triacylglyceride turnover. Endocrinology. 2009;150(6):2637–45. doi: 10.1210/en.2008-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogihara T, Chuang JC, Vestermark GL, Garmey JC, Ketchum RJ, Huang X, et al. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J Biol Chem. 2010;285(8):5392–404. doi: 10.1074/jbc.M109.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz H, Lund T, Dahle MK, Collins JL, Korsgren O, Wang JE, et al. The synthetic liver X receptor agonist GW3965 reduces tissue factor production and inflammatory responses in human islets in vitro. Diabetologia. 2009;52(7):1352–62. doi: 10.1007/s00125-009-1366-z. [DOI] [PubMed] [Google Scholar]
- 23.Meng ZX, Yin Y, Lv JH, Sha M, Lin Y, Gao L, et al. Aberrant activation of liver X receptors impairs pancreatic beta cell function through upregulation of sterol regulatory element-binding protein 1c in mouse islets and rodent cell lines. Diabetologia. 2012;55(6):1733–44. doi: 10.1007/s00125-012-2516-2. [DOI] [PubMed] [Google Scholar]
- 24.Konig M, Bulik S, Holzhutter HG. Quantifying the contribution of the liver to glucose homeostasis: a detailed kinetic model of human hepatic glucose metabolism. PLoS Comput Biol. 2012;8(6):e1002577. doi: 10.1371/journal.pcbi.1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5(4):237–52. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Gao M, Zhang C, Ma Y, Bu L, Yan L, Liu D. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol Ther. 2013;21(10):1852–61. doi: 10.1038/mt.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159(2):318–32. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J Biol Chem. 2003;278(48):48283–91. doi: 10.1074/jbc.M302287200. [DOI] [PubMed] [Google Scholar]
- 29.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 30.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306(8):E945–64. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–43. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–36. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–38. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Ma Y, Cui R, Liu D. Hydrodynamic delivery of FGF21 gene alleviates obesity and fatty liver in mice fed a high-fat diet. J Control Release. 2014;185:1–11. doi: 10.1016/j.jconrel.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M, Liu D. Gene therapy for obesity: progress and prospects. Discov Med. 2014;17(96):319–28. [PubMed] [Google Scholar]
- 38.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 39.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109(25):10001–5. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab. 2013;305(5):E567–72. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 41.Adams AC, Kharitonenkov A. FGF21: the center of a transcriptional nexus in metabolic regulation. Curr Diabetes Rev. 2012;8(4):285–93. doi: 10.2174/157339912800840505. [DOI] [PubMed] [Google Scholar]
- 42.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124(2):515–27. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27(3):286–97. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cold exposure increased glucose tolerance in metabolically normal mice. a. Glucose profiles of IPGTT. (b) AUC analysis of IPGTT. Values in (a) and (b) represent average ± SD (n = 5). ** p < 0.01 compared with control mice kept at 25°C. (GIF 127 kb)
Cold exposure increased mRNA and protein levels of FGF21 in normal mice. (a) Expression of FGF21 in the liver and brown adipose tissue. (b) Protein level of FGF21 in blood. Values in (a) and (b) represent average ± SD (n = 5). ** p < 0.01 compared with control mice kept at 25°C. (GIF 67 kb)
(DOCX 16 kb)


