Abstract
Objective
Elevated plasma D-dimer (DD) is associated with decreased survival among patients with breast, lung, and colon cancers. The present study clarifies the prognostic significance of pretreatment plasma DD levels in patients with epithelial ovarian cancer (EOC).
Methods
We investigated pretreatment DD levels and other variables for overall survival using univariate and multivariate analyses in 134 consecutive patients with EOC stages II to IV who were initially treated between November 2004 and December 2010.
Results
The median follow-up period was 53 (7–106) months. Univariate analysis significantly associated elevated pretreatment DD (≥2.0 μg/mL) levels to poor 5-year overall survival rates irrespective of previously treated venous thromboembolism (72.2% vs 52.6%, P = 0.039). Cancer antigen 125 levels of 200 U/mL or higher (P = 0.011), distant metastases (P = 0.0004), residual tumors (P < 0.0001), and International Federation of Gynecology and Obstetrics stage III/IV (P = 0.0033) were also poor prognostic factors. Multivariate analysis independently associated DD levels of 2.0 μg/mL or higher (P = 0.041), distant metastases (P = 0.013), and residual tumors (P < 0.0001) with poor overall survival.
Conclusions
High pretreatment DD levels are associated with poor overall survival in patients with EOC independently of venous thromboembolism and tumor extension and might comprise a promising prognostic biomarker for patients with EOC.
Key Words: Plasma D-dimer, Epithelial ovarian cancer, Prognosis
Trousseau reported an association between cancer and venous thromboembolism (VTE) in 1865, and activated coagulation and fibrinolysis in patients with malignant disease have recently been discovered.1,2 High D-dimer (DD) levels are a poor prognostic factor in patients with lung, prostate, cervical, breast, and colorectal cancer.3 Plasma DD levels (mean, 2.65 μg/mL) correlated with tumor volume, progression rate, and survival in 84 patients with metastatic breast cancer.4 Elevated plasma levels of DD (>0.65 μg/mL) were associated with decreased survival and a poor response to treatment in 78 patients with lung cancer.5 Survival was significantly shorter in a group with high DD levels (>0.85 μg/mL) among 96 patients with colon cancer, and the DD level was an independent prognostic factor in a multivariate analysis.6 However, the relationship between pretreatment DD levels and the survival of patients with ovarian cancer has not been elucidated.
Plasma DD is generated via the degradation of fibrin by plasmin, and the level increases with enhanced fibrinolysis secondary to enhanced coagulation. Based on this mechanism, the plasma DD levels are used as an index to screen for deep vein thrombosis (DVT), with reported positive and negative predictive values of 36% to 44% and 89% to 100%, respectively.7–10 We showed that DD levels are useful in screening for VTE before treating ovarian, endometrial, and cervical cancers, and found that clear cell adenocarcinoma is a risk factor for VTE.11–13
The present study assesses relationships between pretreatment plasma DD levels and overall survival in patients with ovarian cancer.
MATERIALS AND METODS
Patients
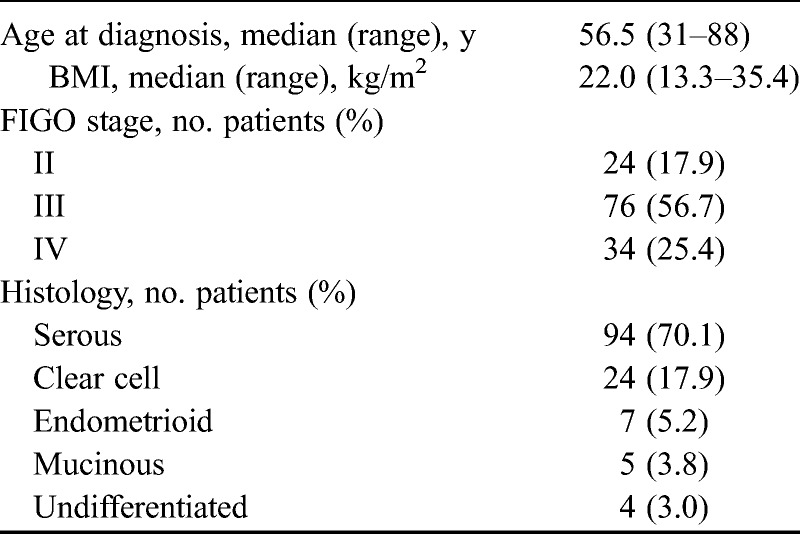
We enrolled 134 patients with stages II to IV who had undergone primary treatment of pathologically diagnosed epithelial ovarian cancer (EOC) between November 2004 and December 2010 at the University of Tsukuba Hospital. The stages classified according to the International Federation of Gynecology and Obstetrics (FIGO) system (1988) were II, III, and IV in 24, 75, and 35 patients, respectively. The histologic subtypes were serous, clear cell, endometrioid, and mucinous types of adenocarcinoma and undifferentiated carcinoma in 94, 24, 7, 5, and 4 patients, respectively. The median age of the patients was 56.5 (31–88) years, and their median body mass index (BMI) was 22.0 kg/m2 (range, 13.3–35.4 kg/m2; Table 1). The ethics committee of the University of Tsukuba Hospital approved the study protocol.
TABLE 1.
Characteristics of 134 patients with EOC
Measurement of Plasma DD Levels
Peripheral blood samples were collected from all patients before treatment, and DD levels were measured. Blood samples were collected from an antecubital vein into plastic tubes using a 2-tube technique, and the first 4 to 5 mL was discarded. Whole blood was anticoagulated by adding a 9:1 volume of 0.11 M sodium citrate, and then citrated plasma was separated by centrifugation at 3000 rpm for 10 minutes and frozen at −20°C for up to 3 days. Plasma DD levels were measured using STA-Liatest D-Di latex (Diagnostica Stago, Asnières, France) sensitized with anti-DD mouse monoclonal antibody to induce a latex coagulation reaction, then turbidity was quantified by spectrophotometry. The cutoff value for plasma DD was 0.5 μg/mL.
Management of EOC
Among the 134 patients, 74 underwent primary debulking surgery (PDS) with postoperative chemotherapy and 60 received neoadjuvant chemotherapy (NAC), followed by interval debulking surgery (IDS) and postoperative chemotherapy. The basic surgical procedure in both groups comprised total abdominal hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and pelvic as well as para-aortic lymphadenectomy. Tumors were dissected from patients with peritoneal dissemination to achieve optimal debulking, and 95.5% of the patients received paclitaxel plus carboplatin as a first-line chemotherapeutic regimen (Table 2).
TABLE 2.
Primary treatments and chemotherapy regimens (n = 134)
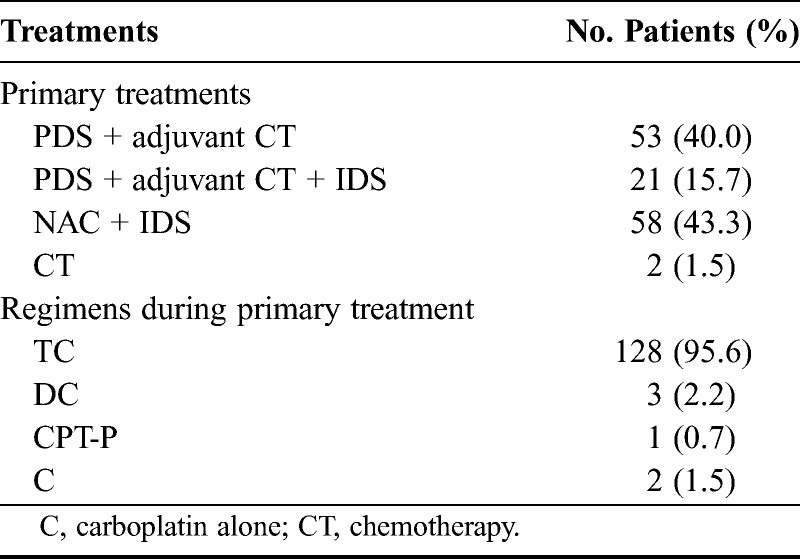
A 1-stage procedure was selected for 65 of the 74 patients who underwent PDS or upfront surgery. Stage II, IIIa, or IIIb tumors in 19 patients were completely removed without residual tumors. Stage IIIc or IV tumors in 20 of 46 patients were completely removed without residual tumors; those in 17 patients were optimally debulked by surgery with residual tumors less than 1 cm in diameter, and 8 were treated by suboptimal surgery with residual tumors greater than 1 cm. The remaining 21 patients were treated by salpingo-oophorectomy with or without hysterectomy to pathologically diagnose ovarian cancer at PDS. All 21 patients underwent second-stage optimal debulking surgery as complete surgery or restaging laparotomy including pelvic and para-aortic lymphadenectomy or biopsies. Seventy-four patients received postoperative chemotherapy with paclitaxel + carboplatin (TC; n = 68), docetaxel + carboplatin (DC; n = 3), irinotecan + cisplatin (CPT-P; n = 1), and carboplatin alone (n = 2).
Sixty-one patients were treated with NAC because optimal debulking at primary surgery was not likely to be feasible (n = 31), because surgical duration was reduced due to complications including VTE before treatment (n = 11), and because of assignment to the NAC group in a phase III trial of upfront debulking surgery vs NAC for stage III/IV ovarian, tubal, and peritoneal cancers (n = 18).14 Of these 60 patients, 2, in whom EOC was diagnosed based on biopsy of a disseminated lesion before treatment, died of disease progression before IDS. Thus, the remaining 58 patients underwent IDS after 3 or 4 cycles of the TC regimen as follows: complete surgery (n = 35), optimal surgery with residual tumors less than 1 cm in diameter (n = 16), and suboptimal surgery (n = 7). Of the 58 patients treated by IDS, 51 received 4 or 5 cycles of the same chemotherapy regimen as before the surgery, whereas the chemotherapy regimen was changed after IDS in the remaining 7 (CPT-P, n = 2; paclitaxel + cisplatin, n = 2; DC, n = 1; docetaxel + nedaplatin, n = 1; paclitaxel + nedaplatin, n = 1; Table 2).
Management of VTE Before Primary Treatment
After blood screening for DD, VTE before the start of treatment was detected in 33 (24.6%) of 134 patients by ultrasound and computed tomography (CT); 23, 8, and 1 patients had DVT alone, both DVT and pulmonary embolism (PE), and PE alone, respectively. Venous thromboembolism was silent in 32 patients and symptomatic only in 1 with DVT and PE.
All 33 patients with VTE received anticoagulant therapy before the initial treatment and after PDS or IDS. In addition, an inferior vena cava filter was placed or NAC was administered to 17 of these patients according to severity of VTE, stage of ovarian cancer, likely histology of the tumor, cytological evidence of malignancy, and the performance status of patients. Thus, we managed patients with VTE using only anticoagulant therapy (n = 18), PDS with placement of an inferior vena cava filter (n = 5), and NAC followed by IDS (n = 10).
None of these patients developed clinical manifestations of VTE during treatment of ovarian cancer including surgery and chemotherapy.
Statistical Analysis
Data were statistically analyzed using JMP 9.0 software (SAS Institute, Cary, NC). Survival rates were determined using the Kaplan-Meier method and analyzed using the log-rank test. Multivariate analyses proceeded using the Cox hazard model. Whether or not pretreatment DD levels, pretreatment VTE, age, BMI, distant metastases, histology, FIGO stage, residual tumors after primary surgery, lymph node swelling greater than10 mm in short axis determined by CT, massive ascites, cancer antigen 125 (CA125) level, and tumor size on magnetic resonance imaging could serve as prognostic factors was analyzed using univariate analysis. Factors with P values less than 0.05 in the univariate analysis were further assessed by multivariate analysis that included factors with the lowest P values as confounding factors.
RESULTS
Survival
The median follow-up period excluding patients who died was 47 months (range, 23–100 months). The 5-year overall survival rate for all patients was 61.6% (stages II, III, and IV: 90.7%, 62.8%, and 35.2%, respectively).
Relationship Between the Plasma DD Level and Overall Survival in Univariate Analysis
The median DD level for all patients was 5.4 μg/mL (range, 0.1-36.6 μg/mL). Univariate analysis showed that the overall survival of patients with DD levels at least 1.5 μg/mL (P = 0.098), at least 2.0 μg/mL (P = 0.039), and at least 2.5 μg/mL (P = 0.091) was poorer than that in those with DD levels below these respective cutoff values. Figure 1 shows survival curves of patients classified according to DD levels less than 2.0 μg/mL or levels at least 2.0 μg/mL, which was the cutoff level with the lowest P value (P = 0.039). The hazard ratio for the risk of death in DD level at least 2.0 compared with less than 2.0 μg/mL was 2.01 (95% confidence interval [CI], 1.07–4.64). The 5-year overall survival rates were 52.6% and 72.2% in patients with DD levels at least 2.0 μg/mL and less than 2.0 μg/mL, respectively. According to the supportive analysis of receiver operating characteristic curve based on a univariate logistic model, the cutoff value of DD level was 2.2 μg/mL and the area under the curve was 0.55 (Fig. 2).
FIGURE 1.
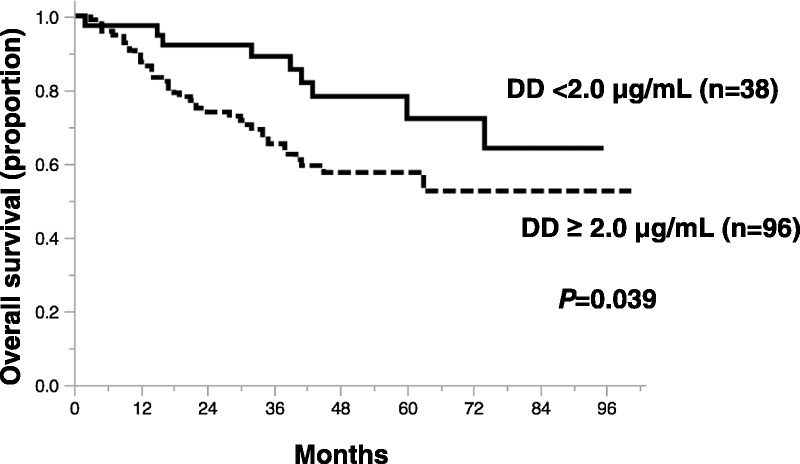
Kaplan-Meier survival curves of patients with ovarian cancer according to pretreatment plasma DD levels. Prognosis is significantly poorer for patients with DD ≥ 2.0 μg/mL (n = 96) than patients with DD < 2.0 μg/mL (n = 38; P = 0.039).
FIGURE 2.
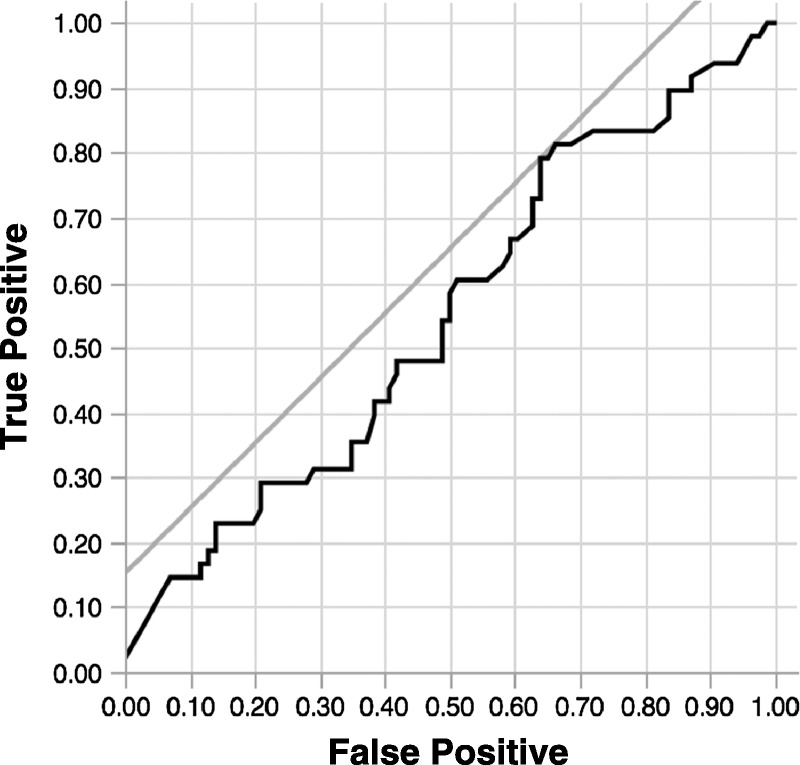
The cutoff values of DD level was 2.2 μg/mL, and the area under the curve was 0.55.
High DD Level Is a Poor Prognostic Factor Independently of VTE
D-dimer levels were significantly lower in patients without than with VTE (6.6 ± 6.7 vs 9.8 ± 6.3 μg/mL, P < 0.0007). Univariate analysis did not uncover a significant difference in overall survival between patients with and without VTE (P = 0.592). Figure 3 shows survival curves of patients with DD levels at least 2.0 μg/mL classified according to the presence of pretreatment VTE. The 5-year overall survival rate for 29 patients with pretreatment VTE and DD ≥ 2.0 μg/mL was 60.0%, and that for 67 patients without pretreatment VTE and DD ≥ 2.0 μg/mL was 49.0%.
FIGURE 3.
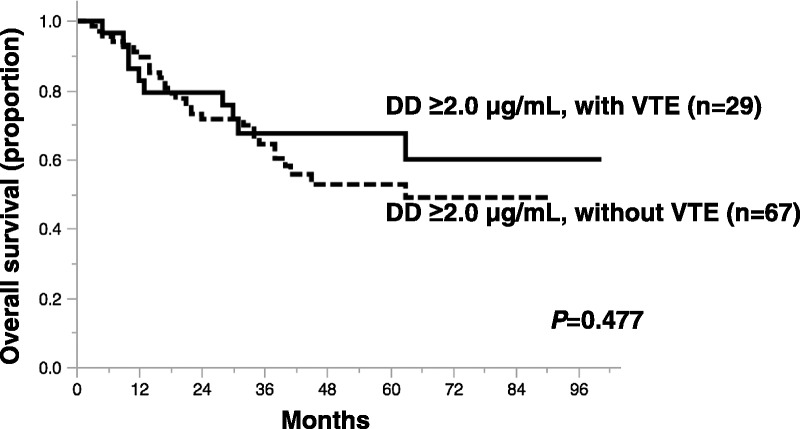
Kaplan-Meier survival curves of patients with ovarian cancer with DD ≥ 2.0 μg/mL, with or without VTE. High DD levels comprise poor prognostic factor regardless of pretreatment VTE (P = 0.477).
Univariate Analysis of Other Prognostic Factors
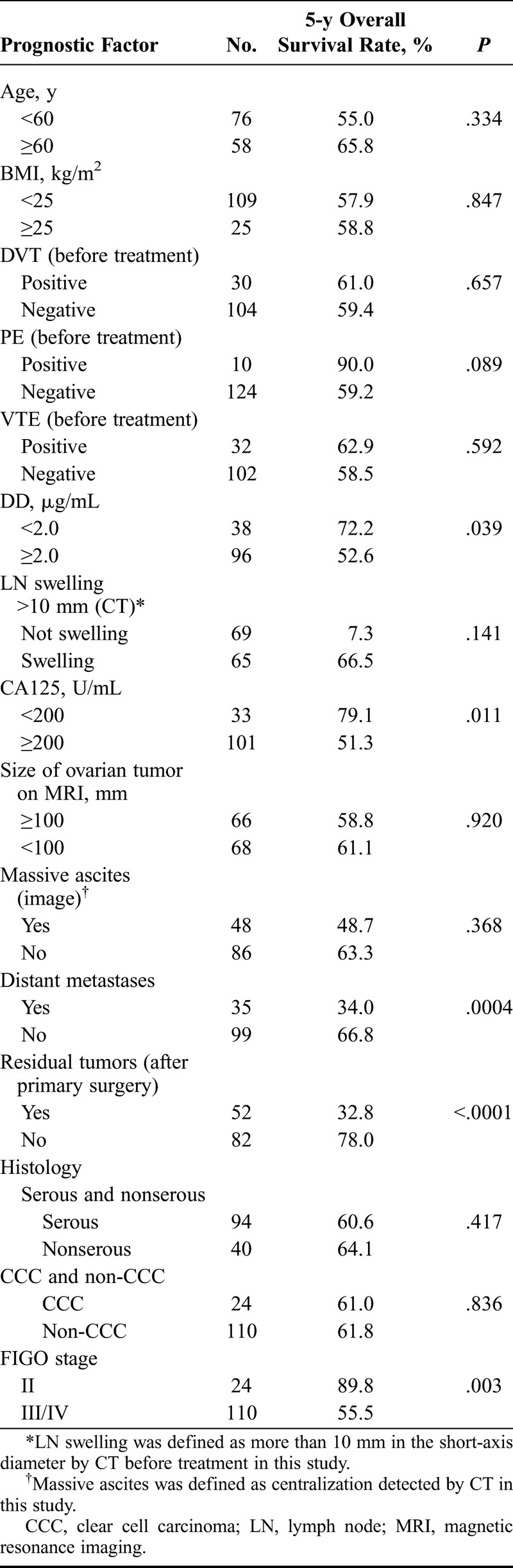
Univariate analysis of prognostic factors other than DD level showed that CA125 ≥ 200 U/mL (P = 0.011), distant metastases (P = 0.0004), residual tumors after primary surgery (P < 0.0001), and FIGO stage III/IV (P=0.003) were significant prognostic factors (Table 3). Age, BMI, tumor size, lymph node swelling, massive ascites, histology, and VTE did not significantly influence overall survival.
TABLE 3.
Univariate analysis of prognostic factors respectively with EOC
Multivariate Analysis
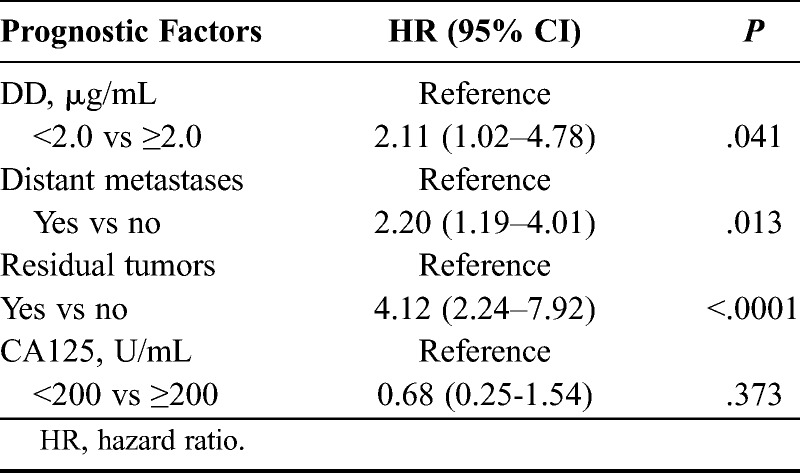
We included DD ≥ 2.0 μg/mL, CA125 ≥ 200 U/mL, distant metastases, and residual tumors as variables in a multivariate analysis based on the outcome of the univariate analysis. Only DD ≥ 2.0 μg/mL (P = 0.041), distant metastases (P = 0.013), and residual tumors (P < 0.0001) were selected as independent factors associated with a poor prognosis (Table 4).
TABLE 4.
Multivariate analysis for the 4 representative risk factors of ovarian cancer
DISCUSSION
A high pretreatment DD level (≥2.0 μg/mL) was a significant and poor prognostic factor. Five-year overall survival rates significantly decreased with increasing DD levels. D-dimer can reflect the extent and course of advanced ovarian cancer more effectively than CA125.15 Elevated DD levels were specific but not sensitive for macroscopically positive second-look findings of ovarian cancer.16 These studies indicated that measuring plasma DD levels during treatment might be useful for evaluating persistent ovarian cancer. However, they could not determine whether pretreatment DD levels are useful for predicting the survival of patients with ovarian cancer. Our findings suggested that pretreatment DD levels could serve as a promising prognostic biomarker associated with poor overall survival in patients with ovarian cancer.
We found here that the association between high DD levels and a poor prognosis was independent of VTE. High DD levels reportedly predict VTE in patients with ovarian cancer,10 and epidemiological studies have associated VTE with a poor prognosis and 3-fold increased risk of mortality in the general cancer population.17–19 However, the present study did not identify pretreatment VTE as a poor prognostic factor, and poor survival was not mediated by VTE in patients with elevated levels of DD. These findings indicated the possibility that high DD levels are a poor prognostic factor regardless of pretreatment VTE.
Univariate analysis identified CA125 levels 200 U/mL or higher (P = 0.011), distant metastases (P = 0.0004), residual tumors after primary surgery (P < 0.0001), and FIGO stage III/IV (P = 0.003) as other factors that were significantly associated with a poor prognosis. Multivariate analysis that included these variables selected only DD ≥ 2.0 μg/mL (P = 0.041), distant metastases (P = 0.013), and residual tumors (P < 0.0001) as mutually independent factors associated with a poor prognosis. In multivariate analysis, we adjusted for distant metastases, which is generally related to prognosis, instead of FIGO stage III/IV. When we analyzed it using FIGO stage III/IV, only residual tumors were an independent factor. Elevated plasma levels of DD in patients with ovarian cancer are associated with deceased survival independently of tumor extension.
D-dimer is a global indicator of coagulation activation and fibrinolysis. Several studies have associated high DD levels with both risk of VTE and tumor progression in patients with cancer. Procoagulant activity with elevated DD might be mediated by tissue factor (TF) expression in cancer.20 Increased TF expression induces excess fibrin formation and results in VTE and increased DD levels in patients with cancer. In addition to TF, products of the coagulation cascade triggered by TF activation, such as factor Xa, thrombin, and fibrin, also promote tumor growth and metastasis.21
Anticoagulation provided by low-molecular-weight heparin (LMWH) might favorably affect the survival of patients with cancer.22 A randomized clinical trial of 302 patients with metastasized or locally advanced solid tumors found an overall hazard ratio of 0.75 (95% CI, 0.59–0.96) for mortality with a median survival of 8 months in patients treated with nadroparin for 6 weeks compared with 6.6 months in a placebo group.23 A meta-analysis also showed that heparin significantly increases survival in patients with malignant tumors.24 Experimental studies have shown that LMWH can interfere with angiogenesis, adhesion of cancer cells to vascular endothelium, and invasion.25 Our observation that overall survival was similar between patients with and without VTE might be due to a higher frequency of treating VTE with LMWH.
In conclusion, high pretreatment DD levels are associated with poor overall survival in patients with EOC independently of VTE and tumor extension and might comprise a promising prognostic biomarker for patients with EOC.
We are presently planning a prospective study to determine whether anticoagulant therapy could help to improve the prognosis of patients with ovarian cancer and high pretreatment DD levels.
ACKNOWLEDGMENTS
This research was supported, in part, by a Grant-in-Aid for Scientific Research (No. 26861310 and No. 26462513) from the Ministry of Education, Science, and Culture, Japan.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Trousseau A. Phlegmasia alba dolens. Clinique medicale de l’Hotel-Dieu de Paris, 3rd edition. Paris: J-B Balliere et Fils, 1865: 654– 712. [Google Scholar]
- 2. Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009; 29: 332– 336. [DOI] [PubMed] [Google Scholar]
- 3. Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012; 97: 1158– 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dirix LY, Salgado R, Weytjens R, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002; 86: 389– 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol). 2007; 19: 494– 498. [DOI] [PubMed] [Google Scholar]
- 6. Oya M, Akiyama Y, Okuyama T, et al. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001; 31: 388– 394. [DOI] [PubMed] [Google Scholar]
- 7. Bounameaux H, Cirafici P, de Moerloose P, et al. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991; 337: 196– 200. [DOI] [PubMed] [Google Scholar]
- 8. Harrison KA, Haire WD, Pappas AA, et al. Plasma D-dimer a useful tool for evaluating suspected pulmonary embolus. J Nucl Med. 1993; 34: 896– 898. [PubMed] [Google Scholar]
- 9. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003; 349: 1227– 1235. [DOI] [PubMed] [Google Scholar]
- 10. Righini M, Le Gal G, De Lucia S, et al. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb Haemost. 2006; 95: 715– 719. [PubMed] [Google Scholar]
- 11. Satoh T, Oki A, Uno K, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007; 97: 1053– 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Satoh T, Matsumoto K, Uno K, et al. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br J Cancer. 2008; 99: 1034– 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satoh T, Matsumoto K, Tanaka YO, et al. Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb Res. 2013; 131: 127– 132. [DOI] [PubMed] [Google Scholar]
- 14. Onda T, Matsumoto K, Shibata T, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers. Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008; 38: 74– 77. [DOI] [PubMed] [Google Scholar]
- 15. Rella C, Coviello M, De Frenza N, et al. Plasma D-dimer measurement as a marker of gynecologic tumors: comparison with Ca 125. Tumori. 1993; 79: 347– 351. [DOI] [PubMed] [Google Scholar]
- 16. Rose PG, Terrien JM, Baker S. Plasma D-dimer and peritoneal CA-125 levels as predictors of disease status in ovarian carcinoma. J Surg Oncol. 1994; 56: 168– 171. [DOI] [PubMed] [Google Scholar]
- 17. Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006; 166: 458– 464. [DOI] [PubMed] [Google Scholar]
- 18. Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007; 25: 70– 76. [DOI] [PubMed] [Google Scholar]
- 19. Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006; 24: 1112– 1118. [DOI] [PubMed] [Google Scholar]
- 20. Uno K, Homma S, Satoh T, et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007; 96: 290– 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009; 22: 49– 60. [DOI] [PubMed] [Google Scholar]
- 22. Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009; 27: 4902– 4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005; 23: 2130– 2135. [DOI] [PubMed] [Google Scholar]
- 24. Kakkar AK. Low-molecular-weight heparin and survival in patients with malignant disease. Cancer Control. 2005; 12: 22– 30. [DOI] [PubMed] [Google Scholar]
- 25. Smorenburg SM, Van Noorden CJ. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001; 53: 93– 105. [PubMed] [Google Scholar]


