Abstract
Background
Wide metastasis is one of characteristics of ovarian cancer. Cancer stem cells, as a source in cancer invasion and metastasis, possess powerful potential of differentiation. Scaffolding IQ domain GTPase-activating protein 1 (IQGAP1) plays a key role in the invasion and metastasis of cancer cells, but IQGAP1’s role in cancer stem cells including ovarian cancer was unclear.
Methods
Spheroid culture with serum-free medium was used for enriching ovarian cancer stem cell-like cells (CSC-LCs) from 3AO cell line, and a medium with 10% fetal bovine serum was used to induce the differentiation of CSC-LCs. Immunofluorescence was for detecting the stem markers OCT4 and SOX2. The quantitative real-time-polymerase chain reaction and Western blotting were performed to determine the messenger RNA and protein expression of IQGAP1, respectively. The capacity of cell invasion was evaluated by transwell chamber assay.
Results
Ovarian CSC-LCs obtained through spheroid culture showed irregularly elongated appearance, CD24 negative, and OCT4 and SOX2 positive. IQGAP1 expression was decreased in ovarian CSC-LCs compared with parental 3AO cells, but increased de novo during the differentiation of CSC-LCs. Knockdown of IQGAP1 by specific small interfering RNA remarkably weakened invasion capacity of 2-day differentiated ovarian CSC-LCs.
Conclusions
Increased IQGAP1 expression during the differentiation of CSC-LCs is involved in an aggressive cell behavior, which may contribute to metastasis of ovarian cancer.
Key Words: IQGAP1, Ovarian cancer, Cancer stem cell-like cells, Differentiation, Invasion
Ovarian cancer remains the leading cause of cancer mortality in women despite advances in surgery and adjuvant chemotherapy in several decades. This high mortality is attributed in part to silencing aggressive behavior of ovarian cancer cells, which results in prompt and wide metastasis in the peritoneal cavity and the majority of patients being diagnosed with advanced-stage disease at the very start.1
With further research, genetic and phenotypic heterogeneity of malignancies need more complex models to describe the tumour-initiating properties. The cancer stem cell concept actually derives from prominence of normal tissue hierarchies to tumor and was reported as a population of undifferentiated cells from a primary tumor involved in tumor progression and metastasis. First identified in acute myloid leukemia and breast cancer,2,3 cancer stem cells have soon been demonstrated in other solid malignancies, such as brain tumor,4 colorectal cancer,5,6 ovarian cancer,7,8 and others. Markers of certain cancer stem cells were gradually identified for isolating the minor subpopulations. Our previous study has identified CD24(−) cells from serous ovarian cancer cell line 3AO during serum-free starvation culture, which have higher capacity of tumorigenesis, drug resistance, self-renewal, and differentiation abilities, which means that CD24(−) cells exhibit the characters of ovarian cancer stem cell-like cells (CSC-LCs).8,9
Unlike normal counterparts, cancer stem cells endow tumor-initiating metastasis capacity that facilitates the final step of the distant organ metastasis. In this process, dynamic rearrangement of cell-cell adhesion and polarized cell migration are 2 of the major events in tumor metastasis in which Rho family small GTPases and their effectors, IQ domain GTPase-activating protein 1 (IQGAP1), are involved. IQGAP1, from the IQGAP family including 3 members, is ubiquitously expressed in humans.10 IQGAP1 acts in the regulation of cytoskeletal architecture and cell adhesion through binding to various regulators/effectors.11 In the physiological processes, regulated by activated Rac1, E-cadherin–β-catenin–IQGAP1 complex exists in a dynamic equilibrium of strong and weak adhesion.12 The evidences have shown an altered IQGAP1 expression in various cancer tissues. Compared with normal tissues, IQGAP1 overexpression is found in colorectal carcinoma,13 breast cancer,14 astrocytoma,15 and squamous cell carcinoma of the head and neck.16 For instance, Clark et al17 identified by a screen of genes that IQGAP1 and its binding proteins calmodulin and ERK showed a greater than 2.5-fold increase in metastatic cells in a mouse model with metastatic melanoma. However, the role of IQGAP1 in cancer stem cell including ovarian cancer is unclear up to date.
In this study, we used spheres as an ovarian CSC-LC model to observe the change of IQGAP1 expression in ovarian CSC-LCs during their differentiation and the influence of IQGAP1 in invasion behavior of ovarian CSC-LCs in vitro. The aim of the study was to identify the role of IQGAP1 in ovarian CSC-LCs invasion capacity and possible contribution to aggressive behavior of ovarian cancer.
MATERIALS AND METHODS
Cell Line and Culture
Ovarian adenocarcinoma cell line 3AO was obtained from the Women’s Hospital School of Medicine, Zhejiang University. Cells were maintained in RPMI 1640 medium (Cellgro, Virginia) supplemented with 10% fetal bovine serum (FBS, Gibco). Ovarian CSC-LCs, the tumor spheroids, were cultured at a density of 50,000 cells/mL in serum-free DMEM/F12 medium (Cellgro, Virginia) composed of 10 ng/mL basic fibroblast growth factor and 20 ng/mL epidermal growth factor (PeproTech Inc, Rocky Hill, NJ), 1 mg/mL insulin (Sigma-Aldrich St Louis, MO), and 10 μL/mL B27 additive (Life Technologies, Carlsbad, CA) on ultralow attachment culture dishes (Corning, NY). All cells were maintained in a humidified atmosphere 5% CO2 at 37°C.
Differentiation Assay of Tumor Spheres
Six days after serum-free medium (SFM) culture, cells were returned into PRIM 1640 medium with 10% FBS in individual well of a 6-well culture plate. The readhered cancer stem cells were fed with FBS-supplemented medium every 2 days, and gene or protein expression was detected after plating using real-time polymerase chain reaction (PCR) and Western blot.
Flow Cytometry
For CD24 expression quantization, 3AO cells and tumor spheroids were trypsinized to single cell and washed twice with phosphate-buffered saline (PBS). Then, 1 × 106 cells were labeled with 1 unit of phycoerythrin mouse anti-human CD24 (BD Biosciences, New Jersey) and phycoerythrin Mouse IgG2a, κ Isotype Control (BD Biosciences, New Jersey).
Immunofluorescence Analysis
Briefly, for immunostaining of undifferentiated tumor spheres, cells were trypsinized to single cell, then plated onto poly-l-ornithine–coated glass cover slips in SFM for 4 hours. The 3AO cells, the adherent cells, usually grew on cover slips for 24 hours. Cells were treated with 4% paraformaldehyde, cold 0.25% Triton-X-100 (Sigma-Aldrich, St Louis, MO) following the protocol, and stained with primary antibodies against SOX2 (1:500, Santa Cruz, CA) and OCT4 (1:500, Santa Cruz, CA). Appropriate secondary antibodies (Beyotime, Jiangsu, China) were used. Cell nuclear DNA was stained with propidium iodide (Sigma-Aldrich, St Louis, MO).
Western Blot
Cells were washed with PBS twice, dissolved, and vortexed in lysis buffer. The cell extracts were incubated on ice for 30 minutes, centrifuged at 14,000 rpm for 30 minutes, and the supernatants were collected. Protein were loaded and separated on 8% SDS-PAGE gels and transferred onto 0.45-μm transfer membranes (Corning, NY). The membranes were blocked with PBS containing 5% dried milk and 0.05% Tween 20 for 1 hour, and then probed with antibodies against GAPDH (1:2000, Santa Cruz, CA), IQGAP1 (1:5000, BD biosciences, New Jersey). Subsequently, visualization used enhanced chemiluminescence detection kit for HRP (BioInd, Israel) with the appropriate secondary antibodies (goat anti-mouse IgG, 1:1000; goat anti-rabbit IgG, 1:1000; Beyotime, Shanghai, China), which contained horseradish peroxidase.
RNA Extraction and Quantitative Real-Time-PCR (qRT-PCR) Assay
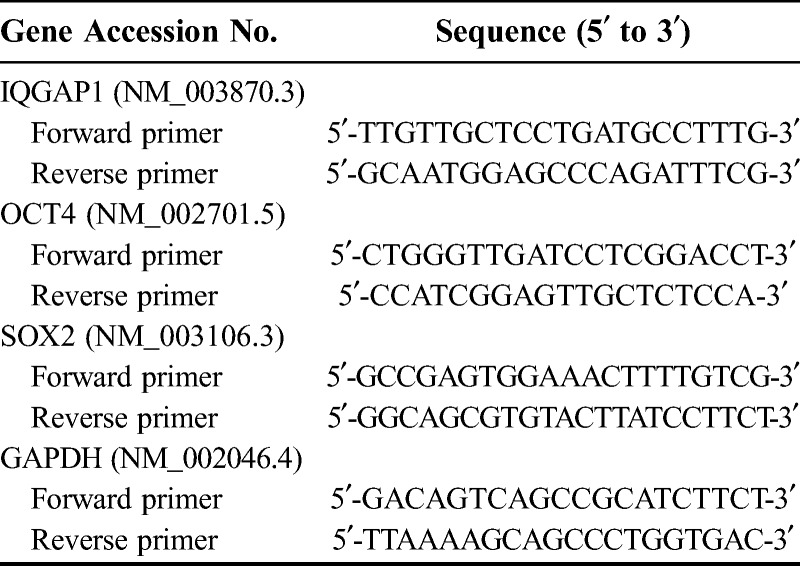
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s guidelines. Complementary DNA was reverse-transcribed with ReverTra Ace qPCR RT Kit (Toyobo, Japan). qRT-PCR was performed by Thunderbird Syber qPCR Mix (Toyobo, Japan) and Applied Biosystems 7900HT fast real-time PCR System (Life Technologies, Carlsbad, CA). The relative messenger RNA (mRNA) expression levels of all genes were calculated according to the 2-Δct method. The method to present relative gene expression was the comparative CT method. For normalization, GAPDH was used as an endogenous control. Primers and sequences were shown in Table 1.
TABLE 1.
Primer used for quantitative RT-PCR
Small Interfering RNA Transfection
Small interfering RNA (siRNA) targeting IQGAP1 and scramble siRNA-negative control were both synthesized by GenePharma (Shanghai, China). The oligonucleotide sequence was as follows: 5′-GCGACAAAGUCCUGAACAUTT-3′ (oligo-1), 5′-AAGUUCUACGGGAAGUAAUUGTT-3′ (oligo-2), and 5′-UUCUCCGAACGUGUCACGUTT-3′ (negative control). Before transfection, the cells were propagated to ∼60% confluency. Small interfering RNA and Lipofectamine RNAiMAX Transfection Reagent (Life Technologies, Carlsbad, CA) were diluted by Opti-Mem (Gibco, Life Technologies, Carlsbad, CA), respectively, according the guidelines. Cell grew for 48 to 72 hours before harvesting RNA and protein.
Transwell Invasion Assay
Cell invasion was routinely carried out in 8-μm pore polycarbonate membrane transwell chambers (Corning, NY). Inserts’ membranes were coated with Matrigel (BD Biosciences) in 37°C for 30 minutes. A total of 100,000 cells were resuspended in 200 μL SFM. A 500-μL medium supplemented with 10% FBS was placed in the wells beneath the inserts to act as a chemoattractant. Cells remaining in the chamber were removed with a cotton swab, and the migrated cells situated on the lower side of membranes were fixed and stained for 20 minutes in 10% ethanol containing 0.05% crystal violet. Assays were performed in triplicate, and all photographs were taken in microscope (×200) for graphical and statistical purposes.
Statistical Analysis
Statistical calculations were performed using SPSS 20.0. Comparisons between treatment groups were made with the 2-tailed Student t test. Results were presented as mean (SD). All P values were 2-sided, and P values less than 0.05 were considered statistically significant.
RESULTS
IQGAP1 Expression Is Decreased in Ovarian Cancer Stem Cells
To understand IQGAP1 expression in ovarian CSC-LCs cells, we initially obtained ovarian CSC-LCs through spheroid culture of 3AO cells with SFM. Over a 6-day culture period, the CSC-LCs were aggregated into almost 100-μm diameter spheroids (Fig. 1A). We further observed that CD24 expression was significantly decreased in CSC-LCs compared with parental 3AO cells by flow cytometric quantification (positive percentage 4.50 ± 1.88% vs 95.68 ± 0.68%, P < 0.001) (Fig. 1B). Compared with 3AO cells, the CSC-LCs showed remarkable immunoreactivity for stem cell markers OCT4 and SOX2 that were almost expressed in the nucleus (Fig. 1C). Simultaneously, mRNA expressions of OCT4 and SOX2 in CSC-LCs were increased to 2.24-fold (0.54-fold) and 1.51-fold (0.46-fold), respectively (P < 0.05) (Fig. 1D). In consistence with the expressions of CD24 and stem cell markers, the expressions of IQGAP1 mRNA and protein were decreased 77.7% (3.29%) and 45.1% (19%) (P < 0.05), respectively, in ovarian CSC-LCs compared with 3AO cells, as shown in Figures 1E and F.
FIGURE 1.

IQGAP1 expression is decreased in ovarian cancer stem cells from SFM culture. A, Morphology of aggregated ovarian CSC-LCs was identified to almost 100 μm tumor spheroids (×100). B, Flow cytometry analysis on CD24 expression in 3AO cells and ovarian CSC-LCs. C, Ovarian CSC-LCs, but not 3AO cells, retained the expression of stem cell markers OCT4 and SOX2 in nucleus (with DyLight 488, in green.). D, Stem cell markers OCT4 and SOX2 expression were compared between 3AO and CSC-LCs in mRNA level. E, The expression of IQGAP1 mRNA was compared between 3AO and CSC-LCs. F, The expression of IQGAP1 protein was compared between 3AO and CSC-LCs.
IQGAP1 Expression Is Increased During the Differentiation of Ovarian Cancer Stem Cells
To observe the differentiation process of ovarian CSC-LCs, we choose the FBS as the inducing agent. After culture in 10% FBS medium for 4 days, the morphology of CSC-LCs changed from irregularly elongated to densely cobblestone-like shape (Fig. 2A). Then we observed the dynamic decreasing of OCT4 and SOX2 expression from the first day to the fourth day by real-time PCR. Synchronously, the CSC-LCs in undifferentiated status exhibited highest level of stem cell markers SOX2 and OCT4. As time went on, OCT4 and SOX2 expressions were rapidly declined and reduced nearly to 18.37% (12.30%) and 40.7% (14.14%) at the fourth day (P < 0.05) of differentiation culture for CSC-LCs (Fig. 2B).
FIGURE 2.
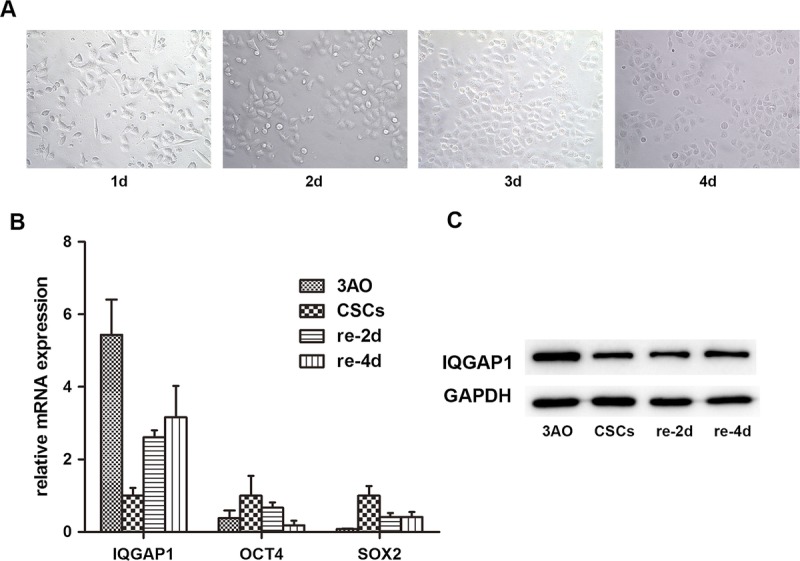
Ten percent FBS medium induces the differentiation of cancer stem cells. A, CSC-LCs morphological change of irregularly elongated to densely cobblestone-like shape from the first day to the fourth day incubated in 10% FBS medium. B, Comparison of the mRNA expression of IQGAP1, OCT4, and SOX2 among 3AO cells, CSC-LCs not adhered and readhered for 2 days and 4 days. C, Western blot showed increased expression of IQGAP1 protein during CSC-LCs differentiation.
Afterwards, we observed the change of IQGAP1 expression along with the differentiation of CSC-LCs by Western blot and real-time PCR and found that IQGAP1 expression was gradually increased after cell differentiation; IQGAP1 mRNA expression achieved 2.61-fold (0.19-fold) and 3.16-fold (0.87-fold), respectively, in cells readhered for 2 days and 4 days, and IQGAP1 protein expression achieved 1.51-fold and 1.67-fold, respectively, for 2 days and 4 days, respectively (Figs. 2B, C).
IQGAP1 Modulates Aggressive Behaviors of Ovarian Cancer Stem Cells
To understand the influence of increased IQGAP1 expression in the invasion capacity of ovarian CSC-LCs, we initially assessed the change of invasion capacity during their differentiation with transwell assays. In addition, we interestingly found enhanced invasion capability of ovarian CSC-LCs readhered for 2 days and 4 days, and the total numbers of migrated cells were 59.56 (6.28) per field in parental 3AO cells, 25.40 (4.04) in CSC-LCs readhered for 2 days, and 54.06 (3.93) for 4 days, respectively (P = 0.00071), as shown in Figure 3A. Together, our results suggest that the invasion capacity of ovarian CSC-LCs is enhanced along with increased IQGAP1 expression and cell differentiation.
FIGURE 3.

Invasion capacity of cells is enhanced after cell differentiation. A, The comparison of invasion capacity among 3AO cells, ovarian CSC-LCs, readhered for 2 days and 4 days by transwell assay. Invasion capacity of cells was enhanced following cell differentiation. B, IQGAP1 protein was knocked down 96.7% and 95.1% after siRNA oligo-1 and oligo-2 transfection, respectively, in 3AO cells. C, IQGAP1 mRNA was knocked 70.7% and 20.8% down after siRNA oligo-1 and oligo-2 transfection, respectively, in 3AO cells.
We further transfected 2 IQGAP1-specific siRNAs with different sequences and negative control into parental 3AO cells. IQGAP1 expression was significantly decreased by oligo-1 and oligo-2 in mRNA level compared with negative control, but the inhibitory rate of IQGAP1 expression achieved 96.7% (3.0%) in mRNA and 70.7% in protein by oligo-1 in 3AO cells whereas only 95.1% (3.2%) in mRNA and 20.8% in protein by oligo-2. Hence, siRNA oligo-1 was preferentially chosen in the next functional experiments (Figs. 3B, C). The siRNA oligo-1 specific to IQGAP1 and negative control were transfected into ovarian CSC-LCs readhered for 2 days, and the same numbers of cells were plated into transwell chambers and observed after 24 hours. The average number of cells transfected with siRNA oligo-1 was 19.00 (4.3) per field, whereas 40.00 (10.93) per field with negative control (P = 0.007858), as shown in Figure 4. Our findings suggest that IQGAP1 expression is positively correlated with the invasive capacity of cells, and inhibited IQGAP1 expression in ovarian CSC-LCs may facilitate to maintain cellular undifferentiation status.
FIGURE 4.

The cell invasion capacity is decreased after knocking down IQGAP1. Invasion capacity was decreased in ovarian CSC-LCs readhered for 2 days after siRNA oligo-1 was transfected.
DISCUSSION
The concept of the cancer stem cell arises from the observation of striking similarities between the self-renewal mechanisms of stem cells and cancer cells. Because normal somatic stem cells must self-renew and maintain a relative balance between self-renewal and differentiation, cancer can be contextualized as a disease of unregulated self-renewal.4
The consensus definition of a cancer stem cell is a cell within a tumor that possesses the capacity to self-renew and to generate the heterogeneous lineages of cancer cells that comprise the tumor, according to the American Association for Cancer Research workshop in 2006.17 If progenitors derived from a cancer stem cell lose the capacity of self-renewal by the induction of differentiation, the cancer stem cell population would be depleted and the tumor would subsequently shrink, according to the conventional cancer stem cell model. So this new model pays equal attention to self-renewal program and asymmetric division. Differentiation is just one of the mechanisms of this process.
Previous studies have identified a new population of cancer stem cells in ovarian cancer with different phenotypes. These cancer stem cells represented a fraction of the total cells comprising the tumor, and they were identified by CD133,18,19 CD44,7,20 CD24,21 ALDH,20,22 and other expressions. In our previous study, we identified that CD24(−) cells from adenocarcinoma cell line 3AO by serum-free starvation culture possessed the characteristics of CSC-LCs with higher capacity of tumorigenesis, drug resistance, self-renewal, and differentiation abilities. Here, we purified successfully ovarian CSC-LCs by the same method applied in previous studies.8,9 The free-serum culture switched the percentage of the expression of CD24, a small and heavily glycosylated cell surface protein linked to the membrane. We found that stem cell markers OCT4 and SOX2 were overexpressed in ovarian CSC-LCs, then decreased strikingly when they were recultured in medium with 10% FBS. Simultaneously, the morphology of cells changed into differentiation status accompanying the loss of stem cell markers. Salvatori et al23 reported that prostatic adenocarcinoma cancer stem cells cocultured with neuroendocrine cells in FBS medium differentiated toward a more aggressive phenotype. Just as the report of Fan et al24 in which the migration capability was low in sphere prostate cells, but gradually increased during the sphere cells differentiation, we also found the markedly increased invasion capacity of ovarian CSC-LCs when they differentiated from tumor spheroids to the fourth day, suggesting that ovarian cancer cells obtain aggressive behavior during their differentiation from cancer stem cells, which may facilitate the cancer metastasis.
IQGAP1, located in 15q26, is a member of the IQGAP family of multidomain proteins expressed in normal tissues and cancers. As a scaffold protein, IQGAP1 contains the following binding domains: CHD, calponin-homology domain; coiled coil, predicted α-helical structure; WW, polyproline protein-protein domain; IQ, 4 IQ motifs; GRD, Ras GTPase-activating protein-related domain; and RGCt, RasGAP C-terminus. The broad range of interacting partners places IQGAP1 as a key mediator of numerous cellular processes and signaling pathways, such as MAPK25 and Wnt signalings.26,27 In human epithelial cells in culture, endogenous IQGAP1 is distributed throughout the cytoplasm and accumulates at cell-cell junctions where it colocalizes with E-cadherin.28 Increased expression of IQGAP1 has also been observed in several human neoplasms, and IQGAP1 has been proposed to be an oncogene.14 Here, we demonstrated that IQGAP1 expression was significantly lower in ovarian cancer stem cells than that in their parental cells and increased de novo when cells were recultured in medium culture with 10% FBS, suggesting that IQGAP1 is mainly expressed in mature cancer cells.
In consideration of IQGAP1 action in cell movement as a cytoskeleton-associated protein, we knocked down IQGAP1 expression by a specific siRNA in ovarian CSC-LCs readhered for 2 days and found that invasion capacity of cells was attenuated, suggesting that IQGAP1 is involved in enhanced aggressive behavior of ovarian CSC-LCs during differentiation. When ovarian cancer stem cells differentiate toward mature cancer cells, they up-regulate IQGAP1 expression and may contribute to the metastasis of ovarian cancer.
ACKNOWLEDGMENTS
The authors would like to thank the Zhejiang Provincial Natural Science Foundation of China. The authors would also like to thank Professor Cheng, Professor Xie, Professor Lu, Mrs Hu, Mrs Xu, and Mrs Huang, who have all contributed directly to this article. The authors would also like to thank the editor who gave our article a chance to be published.
Footnotes
The authors declare no conflicts of interest.
Supported by the Zhejiang Provincial Natural Science Foundation of China (grant no. LY12H16024).
REFERENCES
- 1. Foster R, Buckanovich RJ, Rueda BR. Ovarian cancer stem cells: working towards the root of stemness. Cancer Lett. 2013; 338: 147– 157. [DOI] [PubMed] [Google Scholar]
- 2. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997; 3: 730– 737. [DOI] [PubMed] [Google Scholar]
- 3. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003; 100: 3983– 3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003; 63: 5821– 5828. [PubMed] [Google Scholar]
- 5. Ricci-Vitiani L, Pagliuca A, Palio E, et al. Colon cancer stem cells. Gut. 2008; 57: 538– 548. [DOI] [PubMed] [Google Scholar]
- 6. Ricci-Vitiani L, Fabrizi E, Palio E, et al. Colon cancer stem cells. J Mol Med (Berl). 2009; 87: 1097– 1104. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008; 68: 4311– 4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi MF, Jiao J, Lu WG, et al. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol Life Sci. 2010; 67: 3915– 3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiao J, Huang L, Ye F, et al. Cyclin D1 affects epithelial-mesenchymal transition in epithelial ovarian cancer stem cell-like cells. Onco Targets Ther. 2013; 6: 667– 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009; 583: 1817– 1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006; 16: 242– 249. [DOI] [PubMed] [Google Scholar]
- 12. Noritake J, Watanabe T, Sato K, et al. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005; 118: 2085– 2092. [DOI] [PubMed] [Google Scholar]
- 13. Nabeshima K, Shimao Y, Inoue T, et al. Immunohistochemical analysis of IQGAP1 expression in human colorectal carcinomas: its overexpression in carcinomas and association with invasion fronts. Cancer Lett. 2002; 176: 101– 109. [DOI] [PubMed] [Google Scholar]
- 14. Jadeski L, Mataraza JM, Jeong HW, et al. IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J Biol Chem. 2008; 283: 1008– 1017. [DOI] [PubMed] [Google Scholar]
- 15. Zhou R, Skalli O. Identification of cadherin-11 down-regulation as a common response of astrocytoma cells to transforming growth factor-alpha. Differentiation. 2000; 66: 165– 172. [DOI] [PubMed] [Google Scholar]
- 16. Patel V, Hood BL, Molinolo AA, et al. Proteomic analysis of laser-captured paraffin-embedded tissues: a molecular portrait of head and neck cancer progression. Clin Cancer Res. 2008; 14: 1002– 1014. [DOI] [PubMed] [Google Scholar]
- 17. Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006; 66: 9339– 9344. [DOI] [PubMed] [Google Scholar]
- 18. Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009; 28: 209– 218. [DOI] [PubMed] [Google Scholar]
- 19. Ferrandina G, Bonanno G, Pierelli L, et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008; 18: 506– 514. [DOI] [PubMed] [Google Scholar]
- 20. Landen CN, Jr, Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010; 9: 3186– 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng E, Long B, Sullivan P, et al. CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012; 29: 939– 948. [DOI] [PubMed] [Google Scholar]
- 22. Kryczek I, Liu S, Roh M, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2012; 130: 29– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salvatori L, Caporuscio F, Verdina A, et al. Cell-to-cell signaling influences the fate of prostate cancer stem cells and their potential to generate more aggressive tumors. PLoS One. 2012; 7: e31467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan X, Chen X, Deng W, et al. Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer. 2013; 13: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Cell Mol Biol. 2005; 25: 7940– 7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briggs MW, Li ZG, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem. 2002; 277: 7453– 7465. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Wang A, Wang F, et al. IQGAP1 activates Tcf signal independent of Rac1 and Cdc42 in injury and repair of bronchial epithelial cells. Exp Mol Pathol. 2008; 85: 122– 128. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Kim SH, Higgins JM, et al. IQGAP1 and calmodulin modulate E-cadherin function. J Biol Chem. 1999; 274: 37885– 37892. [DOI] [PubMed] [Google Scholar]


