Abstract
Background:
The main purpose of the study was to establish whether essential components of the renin-angiotensin system (RAS) exist in the human aqueous humor.
Methods:
Forty-five patients ≥ 60 (74±7) years of age undergoing cataract surgery at Tampere University Hospital were randomly selected for the prospective study. The exclusion criterion was the use of oral antihypertensive medicine acting via renin-angiotensin system. Aqueous humor samples were taken at the beginning of normal cataract extraction. The samples were frozen and stored at -80 °C. The concentrations of intraocular endogenous RAS components Ang(1-7), ACE2, and ACE1 were measured using ELISA.
Results:
Concentration medians of Ang(1-7), ACE2, and ACE1 in the aqueous humor were: Ang(1-7) 4.08 ng/ml, ACE2 2.32 ng/ml and ACE1 0.35 ng/ml. The concentrations were significantly higher in glaucomatous than in non-glaucomatous eyes, ACE1 (p=0.014) and Ang(1-7) (p=0.026) vs non-glaucomatous eyes.
Conclusions:
Ang(1-7), ACE2 and ACE1 are found in the human aqueous humor. The observations are consistent with the conception that local tissue-RAS exists in the human eye and it might have a role in the control of intraocular pressure.
Keywords: Angiotensin (1-7), angiotensin converting enzyme 2, angiotensin II, angiotensin converting enzyme 1, aqueous humor, glaucoma, renin-angiotensin system
INTRODUCTION
The systemic renin-angiotensin system (RAS) controls fluid volume, electrolyte balance and blood pressure (BP) homeostasis [1]. RAS is also regarded as a tissue-specific regulatory system accounting for local effects and long-term changes in different organs [2, 3]. Many peptides and enzymes of RAS have already been detected in the human eye [2, 4, 5] and they are even suggested to have a role in the pathogenesis of different ocular diseases including glaucoma [6]. One of the major known risk factors for glaucoma is increased intraocular pressure (IOP) [7-9] which is a net sum of homeostatic balance between aqueous humor formation and outflow.
It has recently been reported that orally administered antihypertensive drugs can also reduce IOP [10];for example, oral angiotensin converting enzyme (ACE) inhibitor (captopril) [11] and the AT1- receptor blocker (ARB) [12] (losartan) have been shown to lower IOP in both non-glaucomatous and glaucomatous patients. In animal studies, ACE inhibitors [13, 14] ARBs [15, 16], renin inhibitors [17] and angiotensin (1-7) [18] have been reported to lower IOP, even when they are locally administered. These findings imply that a local intraocular RAS may beinvolved in the regulation of IOP [19, 20]. Recently, other broader theories have been published on RAS involvement in the pathogenesis of glaucoma [6, 21].So far, only AngII and ACE1 have been identified in the human aqueous humor [6, 22]. RAS is known to consist of over twenty peptidases, close to twenty angiotensin peptides, and at least six receptors [23]. AngII-ACE1-angiotensin 1 receptor type (AT1R)-axis together with Ang(1-7)-ACE2-Mas-receptor (MasR)-axis are seen as the main pathways of RAS that may mediate therapeutic benefits. The main purpose of this study was to detect the levels of Ang(1-7), ACE2 and ACE1 qualitatively and quantitatively in the human aqueous humor.
MATERIALS AND METHODOLOGY
The study was undertaken in the Department of Ophthalmology in the University Hospital of Tampere, Finland between 28th February and 8th April 2014. The study conforms to the World Medical Association Declaration of Helsinki and the Ethical Principles for Medical Research Involving Human Subjects, and received approval from the Regional Ethics Committee at Tampere (ETL R14010). Informed consent was obtained from all participants. Patients over 60 years of age undergoing cataract surgery were included. The patients were selected randomly from the cataract operation list and they were operated upon by the same surgeon. The only exclusion criterion was the use of oral antihypertensive medicine acting via renin-angiotensin system. Together, 45 patients were operated upon; 15 of them had been diagnosed with glaucoma according to Finnish Evidence- Based Guidelines [9]. All the glaucomatous patients were ocular normotensive because of continuous antiglaucoma medication or previous glaucoma surgery. Thus all the eyes independently of medication were normotensive, and the division to glaucomatous/non-glaucomatous was based on the history of the patients. Medical records (age, gender, medications and IOP) were registered during the preoperative visit. IOP was measured using a rebound tonometer (Icare®, Icare Finland Oy, Vantaa, Finland). Blood pressure was measured three consecutive times prior to surgery in sitting position and the average of the measurements was calculated.
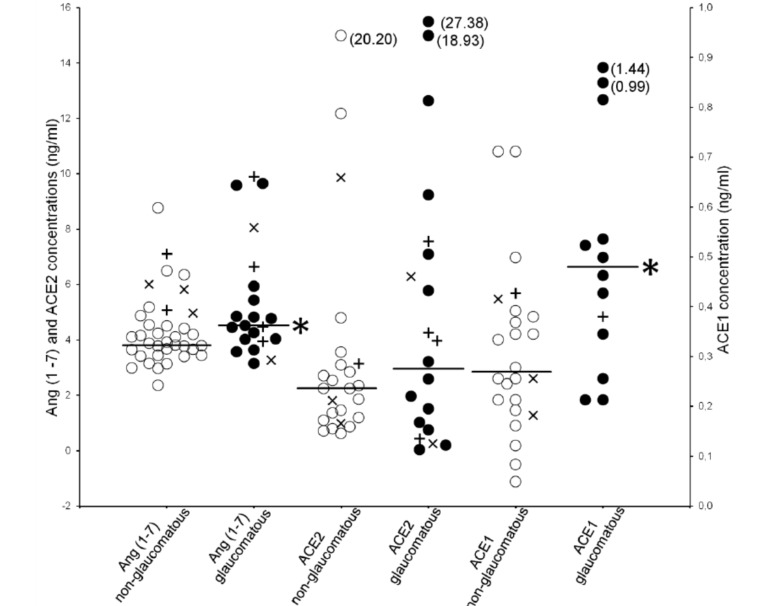
An additional eleven patients were operated upon; six of them were taking oral ACE inhibitor medication for high blood pressure and five of them were on ARBmedication. These patients were not included in the main study results, but their anterior chamber RAS component concentrations are shown in Fig. (1).
Fig. (1).
Individual aqueous humor concentrations of Ang(1-7), ACE2 and ACE1 in non-glaucomatous (white open circles) and glaucomatous (black spots) patients. Comparison with Mann-Whitney test, *p<0.05. Patients using ACE inhibitors (plus marks) or ATreceptor blockers (x marks) are also shown; these patients were excluded from the data in determination of the median concentrations (horizontal lines). For abbreviations see Table 1.
Aqueous humor samples (0.05-0.35 ml) were taken in the beginning of routine cataract surgery. The liquid was collected into Eppendorf tubes and immediately chilled in an icebox. After the operation, the collected samples were frozen and stored at -80°C within 2 h. No protease/peptidase inhibitors were added in the collection and storage of the samples. The samples were analyzed using commercially available enzyme-linked immunosorbent assays (ELISA) due to their applicability to simultaneously assess the target molecule concentrations in a large number of samples. Ang(1-7) was measured using the Human Angiotensin(1-7) Elisa kit (MyBioSource, San Diego, CA, USA) with a detection limit of 0.1 ng/ml. ACE2 was measured using the Human ACE2 ELISA kit (Boster Immunoleader, Pleasanton, CA, USA) with a detection limit of <10 pg/ml, ACE1 using the Human ACE ELISA kit (Boster Immunoleader) with a detection limit of <5 pg/ml. AngII was also measured using the Angiotensin II ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA) with a detection limit of 4.6 pg/ml. The kits were designed for usage with human serum, plasma, cell culture supernates, body fluid or tissue homogenates. The assay procedures and assays were conducted according to manufacturers’ instructions. Only Angiotensin II ELISA kit has been shown to have cross-reactivity, but it is of no relevance. In other ELISA kits cross-reactions were negligible. All assays were done blinded. Results are shown in ng/ml.
The data from the ELISA assays were analyzed using Microsoft Excel and SPSS Statistics for Windows ver. 21.0. (IBM Corp, Armonk, NY, USA). Nonparametric Mann-Whitney test and Spearman's Correlation were used for data with skewed distribution and the results are shown as median with upper and lower quartiles, whereas independent sample t-test and Pearson’s Correlation were used for normally distributed data. The results are shown as mean ±SD. The level of significance was set at ˂0.05 (two-tailed) in all statistical tests.
RESULTS
Forty-six aqueous humor samples were analyzed from 45 subjects with an average age of 74±7 (mean ± SD) years. Of these, 27 (60 %) were female and 18 (40 %) male. Fifteen were diagnosed with glaucoma and 30 were non-glaucomatous. Overall demographics of the glaucomatous and non-glaucomatous subgroups are presented in Table 1.
Table 1.
Demographics and intracameral concentrations of Ang(1-7), ACE2 and ACE1. For normally distributed data results are shown as mean ±SD, for skewly distributed data median with upper and lower quartiles. The small volume of some samples did not allow all measurements.
|
Non-Glaucomatous
(n = 30) † |
Glaucomatous
(n = 15) |
p-Value | |
|---|---|---|---|
| Age | 74 ± 7 | 75 ± 8 | 0.843 |
| Gender (M/W) | 10/20 | 8/7 | |
| IOP | 15 ± 4 | 16 ± 6 | 0.658 |
| Systolic BP | 163 ± 21 | 166 ± 26 | 0.692 |
| Diastolic BP | 88 ± 10 | 92 ± 11 | 0.335 |
| Ang(1-7) | 3.81 | 4.53 | 0.026* |
| (3.69-4.59) | (4.03-6.21) | ||
| (n = 31) | (n = 15) | ||
| ACE2 | 2.26 | 2.96 | 0.409 |
| (1.19-6.02) | (1.91-12.33) | ||
| (n = 20) | (n = 14) | ||
| ACE1 | 0.27 | 0.48 | 0.014* |
| (0.23-0.39) | (0.33-0.79) | ||
| (n = 20) | (n = 12) |
†Number of patients: n=30, number of eyes: n=31. * p-value<0.05.
Ang(1-7), angiotensin (1-7); ACE1, -2, angiotensin- converting enzyme 1, -2; BP, blood pressure; IOP, intraocular pressure; M, men; W, women.
Median aqueous humor (n=46) Ang(1-7), ACE2 and ACE1 concentrations were: 4.08 ng/ml (Q1-Q3; 4.00-4.92), 2.32 ng/ml (Q1-Q3; 2.58-7.53) and 0.35 ng/ml (Q1-Q3; 0.30-0.51), respectively. None of the samples showed measurable levels of AngII. The Ang(1-7) and ACE1 concentrations were significantly higher in glaucomatous than in non-glaucomatous eyes (p=0.026 and p=0.014). See Table 1. Individual aqueous humor concentrations of Ang(1-7), ACE2 and ACE1 in non-glaucomatous and glaucomatous patients are presented in Fig. (1).
Age, IOP- and BP-values did not differ between the two subgroups (Table 1). With one exception, no significant differences between men and women were found in Ang(1-7), ACE2 or ACE1 concentrations in the subgroups (Table 2). The significant correlations were in the non-glaucomatous group ACE1 vs age and ACE1 vs ACE2 both genders together. In glaucomatous patients no correlations were found.
Table 2.
Correlations between RAS components (ng/ml), sex, age, IOP and BP. The small volume of some samples did not allow all measurements. For abbreviations see Table 1.
| Non- Glaucomatous | Glaucomatous | ||||||
|---|---|---|---|---|---|---|---|
| Ang(l-7) n = 31 |
ACE2 n = 20 |
ACE1 n = 20 |
Ang(l-7) n = 15 |
ACE2 n = 14 |
ACE1 n = 12 |
||
| ACE2 | p-value | 0.156 | 0.770 | ||||
| Cc† | 0.330 | 0.100 | |||||
| ACE1 | p-value | 0.361 | 0.025* | 0.347 | 0.947 | ||
| Cc† | 0.216 | 0.595 | -0.298 | -0.023 | |||
| Gender | Women | 3.80 | 1.81 | 0.25 | 4.03 | 3.29 | 0.51 |
| Men | 4.05 | 2.88 | 0.40 | 4.82 | 3.22 | 0.41 | |
| p-value | 0.210 | 0.119 | 0.052 | 0.031* | 0.101 | 0.497 | |
| Age | p-value | 0.128 | 0.960 | 0.027* | 0.292 | 0.214 | 0.621 |
| Cc† | 0.259 | -0.011 | 0.901 | 0.241 | 0.290 | -0.152 | |
| lOP | p-value | 0.338 | 0.897 | 0.600 | 0.282 | 0.617 | 0.465 |
| Cc† | -0.178 | -0.029 | -0.125 | -0.297 | 0.170 | 0.234 | |
| Systolic BP | p-value | 0.823 | 0.875 | 0.502 | 0.794 | 0.519 | 0.829 |
| Cc† | -0.042 | -0.036 | 0.160 | 0.077 | 0.218 | -0.070 | |
| Diastolic BP | p-value | 0.769 | 0.452 | 0.332 | 0.567 | 0.729 | 0.444 |
| Cc† | -0.055 | 0.169 | 0.229 | -0.168 | -0.118 | 0.244 | |
†Cc. correlation coefficient.
*p-value < 0.05.
Glaucoma patients (n=15) used different topically administered antiglaucoma drugs. Patients who used prostaglandin analogues had higher Ang(1-7) and ACE1 concentrations vs patients with no medication (p=0.012 and p=0.028 respectively). Also, the use of a combination of beta blocker and carbonic anhydrase inhibitor was associated with higher ACE1 concentration (p=0.035). No statistically significant difference was detected between patients using other glaucoma medications.
DISCUSSION
The present study was aimed to detect the central components of RAS in the human aqueous humor. In addition possible differences between glaucomatous and non-glaucomatous eyes were studied. We showed that endogenous Ang(1-7) and ACE1 and ACE2 are present in the aqueous humor of the human eye. The glaucomatous eyes have higher levels of ACE1 vs non-glaucomatous eyes. This would suggest a role of ACE1 in IOP balance in the development of glaucoma. Furthermore, ACE1 levels were associated with higher ACE2 concentrations in non-glaucomatous eyes, this offsetting each other's effects on IOP. Interestingly, in the glaucomatous subjects age did not correlate with the aqueous humor concentrations of any of the RAS components, while in non-glaucomatous eyes increasing age was with higher ACE1 concentrations. Aqueous humor Ang(1-7), ACE1 and ACE2 levels did not differ between the genders in either of the subgroups. BP and IOP values were not associated with higher RAS component concentrations. High BP values measured just before surgery were likely to result from the anxiety and fear that patients usually feel prior to an operation. On the other hand, all glaucomatous patients were under treatment and therefore had normal IOP values.
The lack of measurable levels of AngII in aqueous humor samples may be explained by the absence of protease/peptidase inhibitors in the collection and storage of the samples. Thus AngI (DRVYIHPFHL) and AngII (DRVYIHPF) peptides can be cleaved to shorter angiotensin peptides [24]; to AngIII (RVYIHPF) or Ang(1-9) (DRVYIHPFH) or Ang(1-7) (DRVYIHP) [2]. For example, prolyl endopeptidase and prolyl carboxypeptidase can hydrolyze AngII to Ang(1-7). These alternative pathways of angiotensin degradation system are possible. In previous studies [4] intraocular AngII detection measurements were performed in pools consisting of different samples. It is also possible that the sensitivity of the used AngII assay was too weak in this study. Unfortunately, there is no reference to literature showing a rigorous assessment of these kits. Usually, ELISA methods are qualitative and quantitative but they need highly specific and sensitive antibodies. Because the sensitivity of the assays is strictly limited by the affinity between antibodies, peptides, and proteins, it is not possible in practice to accurately validate or tune assays.
Interestingly, subjects who used prostaglandin analogues as glaucoma medication had higher Ang(1-7) and ACE1 concentrations. In addition, the use of a combination of beta blocker + carbonic anhydrase inhibitor was associated with higher ACE1 concentrations. Due to the limited number of patients using glaucoma medications in the present study, these observations require further confirmation.
CONCLUSION
Angiotensin(1-7) and ACE2, the “hot spots” in the renin-angiotensin system, are found in the human aqueous humor. This supports the assumption that intraocular RAS may be involved in the regulation of IOP. This theory is further strongly supported by our very recent observation [25] on the expression of Mas-receptors in the retina and especially in the anterior part of the human eye.
ACKNOWLEDGEMENTS
The authors wish to thank the great team in the operating theater of the Eye Center at Tampere University Hospital, especially nurses Ms Anja Korpiaho, Ms Michiko Franzen and Ms Sirpa Arvonen. The authors thank the Päivikki and Sakari Sohlberg Foundation, the Eye Foundation, the Glaucoma Research Foundation Lux and the Foundation for Clinical Chemistry Research for supporting the study.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224–36. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin angiotensin systems. Rev Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 3.Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529–39. [PubMed] [Google Scholar]
- 4.Danser AHJ, Derkx FHM, Admiraal PJJ , et al. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35:1008–18. [PubMed] [Google Scholar]
- 5.Savaskan E, Loffler KU, Meier F , et al. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res. 2004;36:312–20. doi: 10.1159/000081633. [DOI] [PubMed] [Google Scholar]
- 6.Giese MJ, Speth RC. The ocular renin-angiotensin system A therapeutic target for the treatment of ocular disease. Pharmacol Ther. 2014;142:11–32. doi: 10.1016/j.pharmthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Wu SY, Nemesure B, Leske MC. Observed versus indirect estimates of incidence of open-angle glaucoma. Am J Epidemiol. 2001;15:184–7. doi: 10.1093/aje/153.2.184. [DOI] [PubMed] [Google Scholar]
- 8.Kroese M, Burton H, Vardy S, Rimmer T, McCarter D. Prevalence of primary open angle glaucoma in general ophthalmic practice in the United Kingdom. Br J Ophthalmol. 2002;86:978–80. doi: 10.1136/bjo.86.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuulonen A, Airaksinen PJ, Erola E , et al. The Finnish evidence-based guideline for open-angle glaucoma. Acta Ophthalmol Scand. 2003;81:3–18. doi: 10.1034/j.1600-0420.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 10.Khawaja AP, Chan MP, Broadway DC , et al. Systemic medication and intraocular pressure in a British population the EPIC-Norfolk Eye Study. Ophthalmology. 2014;121:1501–7. doi: 10.1016/j.ophtha.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costagliola C, Di Benedetto R, De Caprio L, Verde R, Mastropasqua L. Effect of oral captopril (SQ 14225):on intraocular pressure in man. Eur J Ophthalmol. 1995;5:19–25. doi: 10.1177/112067219500500104. [DOI] [PubMed] [Google Scholar]
- 12.Costagliola C, Verolino M, de Rosa ML , et al. Effect of oral losartan potassium on intraocular pressure in normotensive and glaucomatous human subjects. Exp Eye Res. 2000;71:167–71. doi: 10.1006/exer.2000.0866. [DOI] [PubMed] [Google Scholar]
- 13.Watkins RW, Baum T, Cedeno K , et al. Topical ocular hypotensive effects of the novel angiotensin converting enzyme inhibitor SCH 33861 in conscious rabbits. J Ocul Pharmacol. 1987;3:295–307. doi: 10.1089/jop.1987.3.295. [DOI] [PubMed] [Google Scholar]
- 14.Shah GB, Sharma S, Mehta AA, Goyal RK. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharmacol. 2000;36:169–75. doi: 10.1097/00005344-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Wang RF, Podos SM, Mittag TW, Yokoyoma T. Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp Eye Res. 2005;80:629–32. doi: 10.1016/j.exer.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Yokoyoma T, Mori Y , et al. The effect of topical CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure and aqueous humor dynamics in rabbits. Curr Eye Res. 2001;23:133–8. doi: 10.1076/ceyr.23.2.133.5473. [DOI] [PubMed] [Google Scholar]
- 17.Giardina WJ, Kleinert HD, Ebert DM , et al. Intraocular pressure lowering effects of the renin inhibitor ABBOTT-64662 diacetate in animals. J Ocul Pharmacol. 1990;6:75–83. doi: 10.1089/jop.1990.6.75. [DOI] [PubMed] [Google Scholar]
- 18.Vaajanen A, Vapaatalo H, Kautiainen H, Oksala O. Angiotensin (1-7):reduces intraocular pressure in the normotensive rabbit eye. Invest Ophthalmol Vis Sci. 2008;49:2557–62. doi: 10.1167/iovs.07-1399. [DOI] [PubMed] [Google Scholar]
- 19.Vaajanen A, Luhtala S, Oksala O, Vapaatalo H. Does the renin-angiotensin system also regulate intra-ocular pressureκ. Ann Med. 2008;40:418–27. doi: 10.1080/07853890802043924. [DOI] [PubMed] [Google Scholar]
- 20.Vaajanen A, Vapaatalo H. Local ocular renin-angiotensin system - a target for glaucoma therapyκ. Basic Clin Pharmacol Toxicol. 2011;109:217–24. doi: 10.1111/j.1742-7843.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuchtey J, Kuchtey RW. The microfibril hypothesis of glaucoma implications for treatment of elevated intraocular pressure. J Ocul Pharmacol Ther. 2014;30:170–80. doi: 10.1089/jop.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osusky R, Nussberger J, Amstutz C, Flammer J, Brunner HR. Individual measurements of angiotensin II concentrations in aqueous humor of the eye. Eur J Ophthalmol. 1994;4:228–33. doi: 10.1177/112067219400400407. [DOI] [PubMed] [Google Scholar]
- 23.Ferrão FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney What is newκ. World J Nephrol. 2014;3:64–76. doi: 10.5527/wjn.v3.i3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiota N, Saegusa Y, Nishimura K, Miyazaki M. Angiotensin II-generating system in dog and monkey ocular tissues. Clin Exp Pharmacol Physiol. 1997;24:243–8. doi: 10.1111/j.1440-1681.1997.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 25.Vaajanen A, Kalesnykas G, Vapaatalo H, Uusitalo H. The expression of Mas-receptor of the renin-angiotensin system in the human eye. Graefes Arch Clin Exp Ophthalmol. 2015;[Epub ahead of print] doi: 10.1007/s00417-015-2952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]