Abstract
Previous wound healing studies have failed to define a role for either α1β1 or α2β1 integrin in fibroblast-mediated wound contraction, suggesting the involvement of another collagen receptor in this process. Our previous work demonstrated that the integrin subunit α11 is highly induced during wound healing both at the mRNA and protein level, prompting us to investigate and dissect the role of the integrin α11β1 during this process. Therefore, we used mice with a global ablation of either α2 or α11 or both integrin subunits and investigated the repair of excisional wounds. Analyses of wounds demonstrated that α11β1 deficiency results in reduced granulation tissue formation and impaired wound contraction, independently of the presence of α2β1. Our combined in vivo and in vitro data further demonstrate that dermal fibroblasts lacking α11β1 are unable to efficiently convert to myofibroblasts, resulting in scar tissue with compromised tensile strength. Moreover, we suggest that the reduced stability of the scar is a consequence of poor collagen remodeling in α11−/− wounds associated with defective transforming growth factor-β–dependent JNK signaling.
Introduction
In the dermis, fibroblast interactions with the collagen network are important in maintaining skin homeostasis (Sorrell and Caplan, 2004) and become essential during repair of skin lesions (Liu et al., 2010). During wound healing, numerous complex cell–matrix interactions occur. Keratinocytes migrate on fibronectin in the provisional matrix to seal the wound toward the outside (Margadant et al., 2009). Dermal fibroblasts migrate into the wound, proliferate, and differentiate into myofibroblasts, which produce granulation tissue (GT) rich in collagen I (Hinz, 2007; Driskell et al., 2013). Myofibroblasts, which express α-smooth muscle actin (α-SMA), use cytoskeleton-driven forces to contract the wound (Tomasek et al., 2002; Wipff et al., 2007). Finally, the contracted GT is remodeled to restore normal tissue architecture. Although the importance of cell–matrix interactions for wound contraction seems to be deductive, the identity of participating receptors in the dermis has been ambiguous.
In the skin, the collagen-binding integrins α1β1 and α2β1 are present on fibroblasts and on microvasculature, but only α2β1 is expressed on basal keratinocytes (Gardner et al., 1999; Grenache et al., 2007; Zweers et al., 2007). Wound healing studies in α1β1 or α2β1 integrin–deficient mice have surprisingly failed to define a role for either receptor in fibroblast-mediated wound contraction (Gardner et al., 1999; Grenache et al., 2007; Zweers et al., 2007), suggesting that additional collagen receptor(s) are involved in this process. One possible candidate is α11β1, which in the skin is exclusively expressed on fibroblasts (Velling et al., 1999; Zhang et al., 2006).
Early remodeling of collagen matrices mediated by fibroblasts in vitro has been shown to be essentially an arginine-glycine-aspartic acid-independent but β1 integrin–dependent process (Gullberg et al., 1990). Data collected so far on fibroblasts have identified integrins α2β1 (Klein et al., 1991; Zhang et al., 2006) and α11β1 (Popova et al., 2007) as being involved in collagen remodeling. α2β1 has been shown to contribute to collagen gel remodeling in fibroblasts of different origin, including the dermis, whereas functional studies on α11β1 have so far been limited to mouse embryonic fibroblasts (MEFs) and periodontal ligament fibroblasts (Popova et al., 2007; Barczyk et al., 2013).
In the present study, we demonstrate that repair of skin wounds is compromised in mice lacking integrin α11β1, characterized by diminished wound contraction, reduced formation of GT, and altered scar stability. Our combined in vivo and in vitro data show that myofibroblast differentiation and collagen remodeling are impaired in the absence of α11β1. Hence, we demonstrate that efficient collagen remodeling requires both α11β1 and non-canonical transforming growth factor-β1 (TGF-β1)-dependent JNK signaling.
Results
Impaired wound healing in α11β1-deficient mice
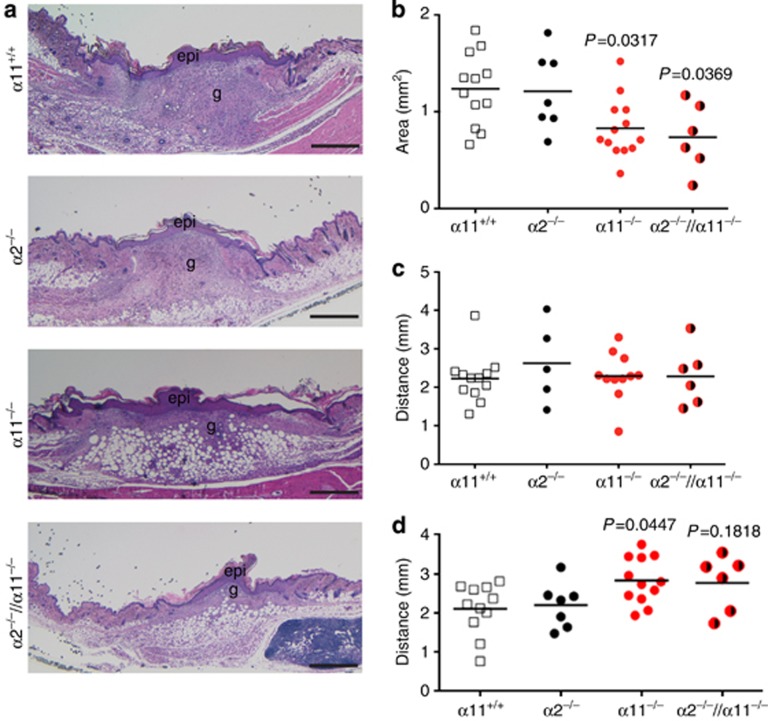
In previous studies, we showed that expression levels of the integrin subunits α2 and α11 are elevated during the course of wound healing, with a peak of integrin α11 at 7 days after injury (Zweers et al., 2007). To dissect whether these two integrins have distinguishable functions during tissue regeneration, we investigated wound repair in mice that are deficient of α2β1, α11β1 or both integrins. Specifically, we analyzed the formation of GT and contraction of full-thickness skin wounds 7 days after lesion. Sections through the middle of such wounds are illustrated in Figure 1a, showing GT of C57BL/6 wild-type mice and their integrin–deficient littermates. As expected, the amount of GT developed in α2-null mice did not differ from wild-type mice (Zweers et al., 2007); however, significantly less GT was developed by α11-null mice (P=0.0317) and by double mutants (P=0.0369; Figure 1b). As there was no difference between the single α11-null and the double α2/α11-null mutant wounds, we conclude that the effect seen in the double mutant is attributed to the absence of α11β1 integrin. Differences in wound area were not reflected by the scab size, which was comparable in all 3 mutants and wild-type mice (Supplementary Figure S1A and B online). However, histology revealed that the distances between wound edges–which serve as an indicator of wound contraction–were significantly increased in integrin α11β1-deficient wounds (P=0.0447; Figure 1d). The reduced wound contraction was not caused by an impaired function of the panniculus carnosus muscle that participates in the contraction, as it was equally contracted in all genotypes (Figure 1c). As we identified the lack of α11 as responsible for reduced GT formation and impaired wound contraction, we limited our further analysis to comparison of wounds in α11β1-deficient versus littermate control mice.
Figure 1.
Ablation of α11β1 impairs wound healing. (a) Hematoxylin and eosin staining of mid-wound sections from α11+/+, α2−/−, α11−/− and α2−/−//α11−/− mice at day 7 after wounding. (b) Histomorphometry of granulation tissues. (c) Distance between panniculus carnosus edges and (d) left and right wound edges. Each symbol represents one wound and 3–5 sections/wound were analyzed. Data were evaluated by one-way analysis of variance and Tukey's multiple comparison test. epi, new epidermis; g, granulation tissue. Scale bar=500 μm.
Impaired migration of fibroblasts lacking α11β1
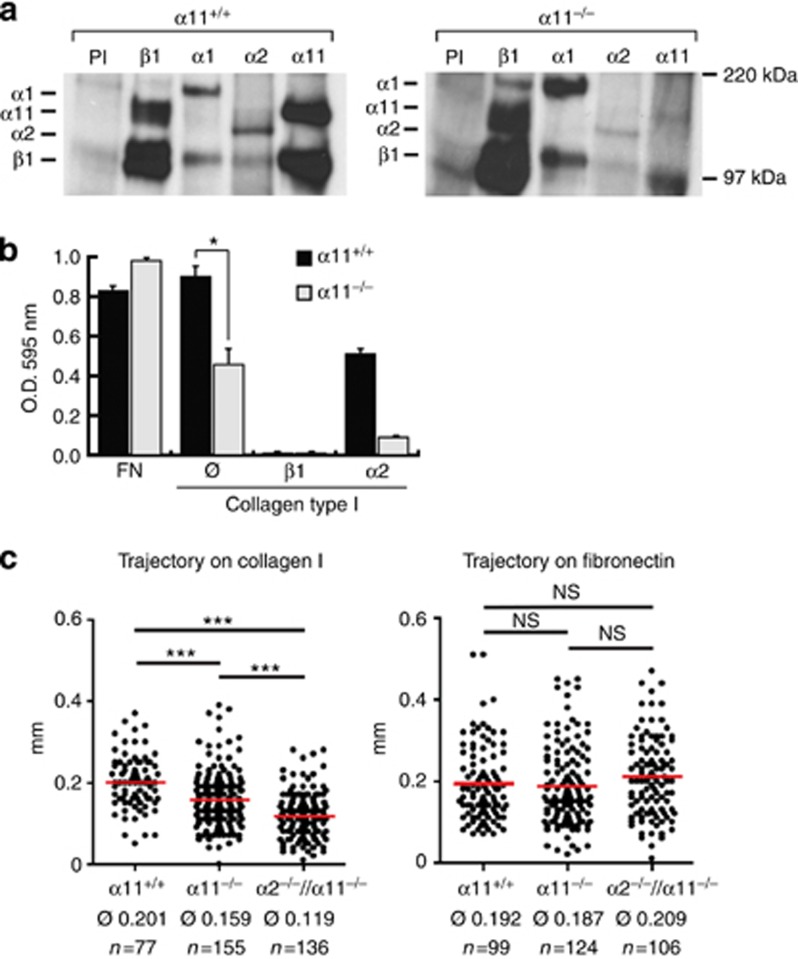
As fibroblasts are the main cells that produce GT during wound healing, we aimed to determine the impaired fibroblast function that could be responsible for the α11−/− wound healing phenotype. For this purpose, we isolated dermal fibroblasts from murine newborn skin. We determined expression levels of collagen-binding integrins by co-precipitation with the β1 subunit and indeed detected high levels of co-precipitating α11 but not of α2 integrin subunit (Figure 2a). Dermal fibroblast interactions with collagen I appeared to exclusively depend on integrins, as antibodies to integrin β1 completely blocked adhesion of wild-type cells on collagen I. Interestingly, adhesion to collagen I was strongly reduced in dermal α11−/− fibroblasts, whereas antibodies to α2 reduced attachment of α11+/+ dermal fibroblasts by only 50% (Figure 2b).
Figure 2.
Cell migration is impaired in α11+/+ fibroblasts. (a) 35S metabolic labeling and immunoprecipitation of integrins in murine dermal fibroblasts. (b) Contribution of α11 integrin to dermal fibroblast adhesion. Integrin blocking antibodies (anti-β1 or anti-α2) were added (10 μg ml−1). ∅ represents untreated cells. Adhesion to fibronectin was used as control. (c) Random migration of dermal fibroblasts was analyzed on type I collagen or fibronectin. Cell motility was quantified by measuring the cell trajectory of single cells; each symbol represents one cells. Average trajectories (∅) were calculated for each condition. (*P<0.05; ***P<0.001; NS, not significantly different; mean ±SD). O.D. optical density.
As an effective healing process relies on fibroblast migration, we investigated the importance of α11β1 and α2β1 for cell migration on collagen I and fibronectin (Figure 2c). Trajectory path length of individual fibroblast was measured, revealing a significant reduction in migration when α11 was absent. This effect was even more pronounced when α2−/−//α11−/− fibroblasts were analyzed. As expected, migration on fibronectin was not affected by the absence of both α2β1 and α11β1 (Figure 2c).
To test whether compromised migration of fibroblasts into the wound might be responsible for the impaired formation of GT, the total number of cells in the GT was estimated by assessment of DAPI-stained nuclei per unit area. However, the total number of cells in α11β1-deficient GTs was not reduced, indicating that impaired migration is not responsible for the diminished GT area (Supplementary Figure S1C and E online). Furthermore, we were able to exclude the possibility that α11 deficiency might impair proliferation and thus offer an explanation to the comprised formation of GT, as we did not observe a proliferation defect in the absence of α11 either in the wounds (Ki67 staining, Supplementary Figure S1F and G online) or in the cultured dermal fibroblasts (Supplementary Figure S1H online).
Reduced myofibroblast differentiation in the absence of α11β1
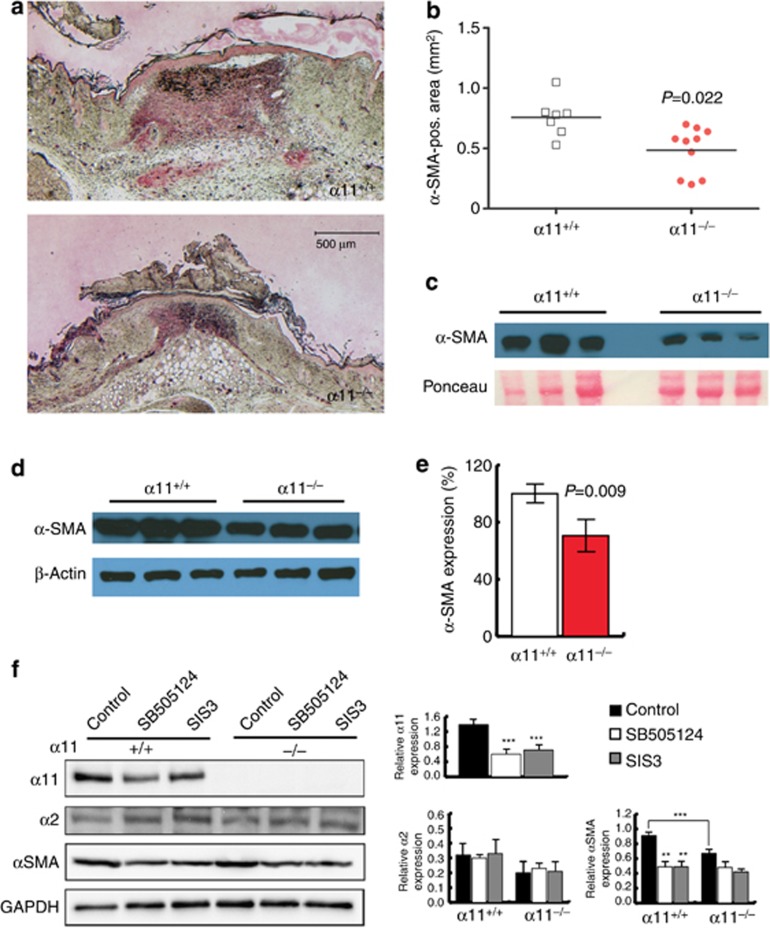
During wound healing, fibroblasts can become activated by mechanical tension and differentiate into α-SMA-expressing myofibroblasts, which secrete ECM proteins to form the GT and contract the wound (Van De Water et al., 2013). Immunohistochemical staining revealed that the α-SMA-positive area was significantly smaller in α11−/− wounds compared with control littermates (P=0.022; Figure 3a and b). In the skin, α-SMA is expressed by myofibroblasts and vascular smooth muscle cells. As vascularization was not affected (Supplementary Figure S1C and D online), we concluded that reduced levels of α-SMA in GTs of α11-deficient mice were caused by a reduced number of myofibroblasts. Western blotting of wound extracts confirmed reduced α-SMA levels in α11−/− wound lysates (Figure 3c).
Figure 3.
Ablation of α11β1 results in significant reduction in myofibroblast differentiation. (a) Immunohistochemical staining of mid-wound sections at 7 days after injury with antibodies directed to α-SMA (red). Scale bar = 500 μm. (b) α-SMA-positive area was quantified by histomorphometry. Each symbol represents one wound, and 3–5 sections per wound were evaluated. (c) Western immunoblotting of wound extracts harvested 7 days after injury (4 mm punch biopsies from the wound center) indicating levels of α-SMA. (d) α-SMA expression by primary fibroblasts embedded in attached collagen lattices was analyzed by western immunoblotting. (e) Quantification of α-SMA signals in (d). (f) Primary fibroblasts on collagen I (100 μg ml−1) were treated with SB505124 (10 μM) or SIS3 (10 μM) for 48 hours. Protein expression was analyzed by western immunoblotting and quantified by densitometry. α-SMA, α-smooth muscle actin.
In vitro, myofibroblast differentiation can be induced by using attached collagen lattices in which embedded fibroblasts experience mechanical resistance (Tomasek et al., 2002; Grinnell and Petroll, 2010). Hence, α11+/+ dermal fibroblasts expressed high levels of α-SMA when they were subjected to the mechanical strain of an attached collagen gel, indicating that they had differentiated into myofibroblasts. However, α11−/− fibroblasts embedded in attached collagen lattices under identical conditions displayed reduced α-SMA induction, clearly pointing to defective myofibroblast differentiation (Figure 3d and e).
Myofibroblast differentiation is not only dependent on mechanical forces but also requires TGF-β (Van De Water et al., 2013). When we treated fibroblasts in vitro with either an TGF-β receptor type I inhibitor or a reagent inhibiting Smad3 signaling, we found that––similar to α-SMA––expression of the integrin subunit α11 depends on TGF-β and downstream Smad signaling (Figure 3f). It is notable that in turn TGF-β-dependent Smad3 activation is not impaired by α11-deficiency (Supplementary Figure S2D online). Although treatment of fibroblasts with TGF-β resulted in elevated levels of the integrin α11-subunit, it did not influence expression of α2 (Supplementary Figure S2B online), which was also unaffected by inhibition of TGF-βRI and Smad3 (Figure 3f). To exclude that impaired myofibroblast differentiation of α11−/− fibroblasts could be due to an impaired ability to activate TGF-β, fibroblasts were co-cultured with mink lung endothelial cells (TMLC), which express luciferase when they are exposed to active TGF-β. Using this approach, we showed that activation of TGF-β is not affected by the presence of integrin α11β1 (Supplementary Figure S2C online).
Defect of collagen remodeling in α11-null mice
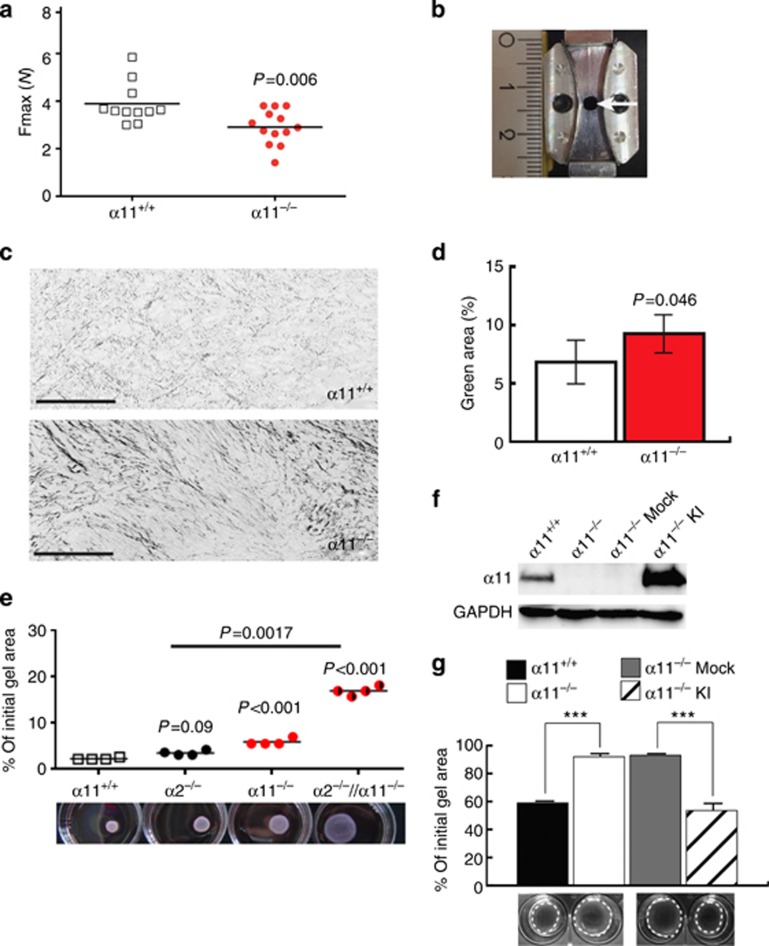
α-SMA-expressing myofibroblasts are the major source of fibrillar collagens, which are essential for producing a functional scar, thereby terminating the repair process (Klingberg et al., 2013). Therefore, we next addressed the question whether the impaired formation of GT would still give rise to a fully functional scar. For this purpose, we inflicted incisional wounds on the backs of α11−/− and control mice, which heal at a faster rate compared with excisional wounds and allowed us to assess the tensile properties of the developed scar (Wu et al., 2003). Thus, incisional wounds were removed together with cranial and caudal skin (Figure 4b) at 16 days after injury and placed in a material testing device, which determines the tensile strength. Scars of α11−/− mice ruptured at lower force applied (P=0.0063), indicating that expression of α11β1 integrin confers stability to cutaneous scars (Figure 4a). To investigate whether reduced scar stability was a consequence of an impaired formation of the reconstituted collagen matrix, collagens within the GT were stained with Sirius red. Polarized light microscopy allowed allocation of the anisotropic thin (green) and thick (red) collagen fibrils (Junqueira et al., 1979). This analysis revealed a significant increase in thinner fibrils in the GT of integrin α11-deficient mice (Figures 4c and d), demonstrating an abnormal composition of the provisional collagen matrix.
Figure 4.
Ablation of α11β1 impairs collagen remodeling. (a) Tensile strength of incisional wounds determined at 16d after injury. Fmax depicts ultimate force applied to skin strips before the scar ruptured. Each symbol represents one incisional wound. (b) Skin strips were excised using the punch with the indicated shape. Arrow indicates position of the scar. (c) Sirius red staining of GTs analyzed by polarized light microscopy. Colors were inverted and split into single channels to separatly assess the amount of green (thin) fibrils. (d) Quantification of the green fibrils from (c). (e) Fibroblasts were allowed to remodel collagen for 72 h. (f) Western immunoblotting of α11 expression after α11 knock-in (KI) in α11−/− fibroblasts. (g) Effect of α11 knock-in on collagen remodeling (***P<0.001; mean ±SD). GT, granulation tissue; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To verify that α11−/− fibroblasts had reduced capacity to reorganize the collagen environment, floating collagen lattices were used as a suitable model for early GT contraction (Dallon and Ehrlich, 2008; Grinnell and Petroll, 2010). Fibroblasts were embedded in collagen lattices and allowed to reorganize the matrix, which was quantified by measuring gel diameters over time and calculating gel area. As illustrated in Figure 4e, over a time course of 72 h of contraction, α11−/− fibroblasts displayed significantly less remodeling of the collagen matrix compared with α11+/+ and α2−/− cells. To exclude the involvement of collagen integrin bridging that might assist in integrin collagen interaction (Zeltz et al., 2013), we limited the following contraction experiments to 24 hours. Under these conditions, antibodies to the β1 integrin subunit almost completely blocked fibroblast-mediated collagen gel contraction and α11-deficiency considerably reduced collagen remodeling (Supplementary Figure S2E online), implicating the integrin α11β1 as a crucial receptor in this process. Importantly, overexpression of α11 in α11−/− fibroblasts (α11−/−KI, Figure 4f) rescued the α11−/− phenotype (Figure 4g).
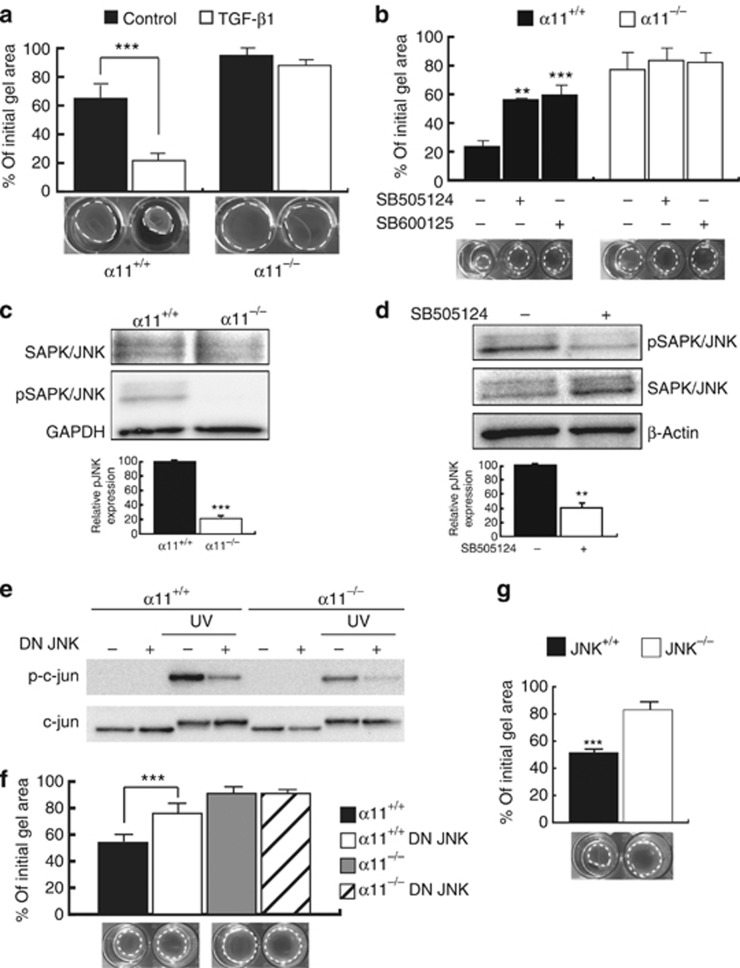
Collagen remodeling requires α11β1 and TGF-β-dependent JNK signaling
Our previous collagen remodeling experiments were assessed in the presence of 2% serum, as unstimulated murine dermal fibroblasts (no serum) displayed minimal basal remodeling activity (Figure 5a). To understand why α11−/− cells fail to reorganize collagen lattices, we investigated the soluble autocrine factor(s) responsible for initiation of collagen remodeling. On the basis of its documented importance for collagen remodeling, TGF-β1 was again a good candidate (Montesano and Orci, 1988). The cytokine TGF-β1 is known to enhance the capacity of fibroblasts to remodel collagen matrices (Poon et al., 2009), which was confirmed when the cytokine was applied to collagen gels with embedded α11+/+ dermal fibroblasts. Interestingly, the TGF-β1 stimulating effect on collagen remodeling was lost when added to collagen gels containing α11−/− fibroblasts (Figure 5a).
Figure 5.
Collagen remodeling is dependent on TGF-β-mediated JNK signaling. (a) Fibroblasts were incorporated into collagen lattices and allowed to contract for 24 hours in serum-free DMEM or in the presence of TGF-β1 (5 ng ml−1). (b) Effect of signaling inhibitors (SB505124, 10 μM or SP600125, 25 μM) on collagen remodeling stimulated with 2% serum. (c) JNK activation was evaluated during collagen remodeling. (d) Effect of SB505124 on JNK phosphorylation in α11+/+ fibroblasts during collagen remodeling. (e) c-jun activition was assessed after fibroblast co-transfection with DN JNK constructs. UV light was used to activate the JNK pathway. (f) Effect of DN JNK on collagen remodeling stimulated with 2% serum. (g) JNK−/− MEFs were allowed to remodel floating collagen gels for 24 hours in the presence of 2% serum (**P<0.01; ***P<0.001; mean ±SD). DN, dominant negative; TGF-β, transforming growth factor-β.
TGF-β exerts its effects via two major signaling pathways, the canonical pathway, which involves Smad signaling, or alternatively by a non-canonical pathway, which leads to activation of MAPK signaling by a mechanism that includes JNK and p38. As our data obtained with chemical inhibitors indicated that α11 expression was regulated via canonical Smad signaling, we investigated whether this pathway was also involved in the α11-dependent collagen remodeling. For this purpose, we silenced Smad4, a cofactor of Smad2 and Smad3, using siRNA (Supplementary Figure S3A online). Knock-down efficiency was confirmed by western blotting showing reduced α11 expression by 50%, similar to the reduction that we previously obtained with the Smad3 inhibitor. However, the Smad4 knock-down did not impair collagen reorganization (Supplementary Figure S3B online), indicating that the canonical Smad pathway was not involved in this process.
We next investigated the non-canonical pathway. Inhibition of p38-MAPK by SD203580 did not impair collagen reorganization (Supplementary Figure S3C online). In contrast, inhibition of JNK by SP600125 and of the upstream MKK1 using PD098059 significantly delayed α11-mediated collagen remodeling (Figure 5b and Supplementary Figure S3C online). We furthermore observed a defect of JNK phosphorylation in α11-deficient cells during collagen gel contraction (Figure 5c). However, JNK could also be activated through the soluble integrin- and growth factor–activated tyrosine kinase, FAK. To discriminate between JNK signaling as result of integrin/FAK activation versus non-canonical TGF-β signaling in α11+/+ fibroblasts, phospho-JNK levels were determined in fibroblasts embedded in contracting collagen gels following treatment with FAK inhibitor PF-573228 or TGF-β receptor type I inhibitor SB505124. Despite inhibition of collagen remodeling, PF-573228 did not affect JNK phosphorylation (Supplementary Figure S3D and E online). Conversely, SB505124 significantly inhibited both JNK phosphorylation (Figure 5d) and collagen remodeling (Figure 5b), indicating involvement of the non-canonical TGF-β signaling pathway in α11-mediated collagen remodeling.
To further ascertain the role of JNK in collagen remodeling, fibroblasts were co-transfected with the dominant negative form of JNK1 and JNK2. The efficiency of the dominant negative JNK variants was confirmed by reduced levels of activated c-jun, a downstream effector of JNK (Figure 5e). Dominant negative JNK–transfected fibroblasts embedded in collagen lattices showed a reduced ability to reorganize the matrix (Figure 5f). Moreover, MEFs isolated from JNK1/2-null mouse embryos also presented a defect in collagen remodeling (Figure 5g). Together these results clearly demonstrate that fibroblast-mediated collagen remodeling requires α11β1 integrin–mediated contact with collagen and the presence of TGF-β that elicits a non-canonical signal pathway involving JNK.
Discussion
In the present study, we addressed the functional relevance of the α11β1 integrin during the repair process of skin wounds and described specific functions exerted by α11β1 but not by other collagen-binding integrins such as α2β1. Using targeted deletion of α11, we show that development of GT and formation of mechanically stable scar tissue critically rely on the presence of α11β1. Our in vitro results strongly suggest that α11β1-deficient fibroblasts exhibit a reduced ability to differentiate into myofibroblasts. The resulting reduction in myofibroblast number causes an impaired tissue restoration and compromised wound contraction in vivo. Finally, remodeling of collagenous tissues to a mature and strong scar depends almost exclusively on α11β1, supported by the in vitro finding that lattice contraction by α11−/− fibroblasts is similarly abolished as reported for fibroblasts deficient in β1 integrins (Liu et al., 2010).
Our in vitro data further show that α2β1 and α11β1 cooperate in murine fibroblast adhesion and cell migration on collagen I-coated two-dimensional surfaces and confirm our earlier results obtained using α2−/− dermal fibroblasts (Zhang et al., 2006). Despite these findings, the overall cell number in wounds did not differ from controls, maybe reflecting the ability of α11-null fibroblasts to migrate on fibrin and other components of the provisional wound matrix (Reyhani et al., 2014). We recently suggested compensatory/complementary cellular interactions with collagen integrin bridgings molecules (Zeltz et al., 2013). A prominent collagen integrin bridging molecule in the dermis is periostin (Egbert et al., 2014), which is important for fibroblast migration into the wounds (Elliott et al., 2012). The integrin-mediated periostin-dependent adhesion might provide an indirect link of fibroblasts to fibrillar collagen and is one potential mechanism to compensate for the lack of α11β1 (Zeltz et al., 2013).
Integrin α11β1 can regulate myofibroblast differentiation in corneal and cardiac fibroblasts (Carracedo et al., 2010; Talior-Volodarsky et al., 2012). Here we showed that α11 and α-SMA are regulated in a similar manner by TGF-β signaling in primary dermal fibroblasts and that the absence of α11 leads to reduced myofibroblast numbers in GT in vivo and impaired ability to remodel collagen lattices in vitro. Addition of recombinant TGF-β1 failed to rescue the ability of α11−/− fibroblasts to contract/remodel lattices.
Although both canonical and non-canonical TGF-β receptor–mediated induction of myofibroblast differentiation has been described, α-SMA can be induced in wounds in the absence of TGF-β type II receptor (Martinez-Ferrer et al., 2010), indicating that fibroblasts can activate parallel/compensatory mechanisms.
Integrins are also dynamically regulated in both two-dimensional and three-dimensional contexts. In the three-dimensional environment, integrin α2 is upregulated in floating collagen lattices (Klein et al., 1991) and both α2 and α11 are upregulated in attached collagen gels (Klein et al., 1991; Carracedo et al., 2010). We think that the low levels of α2 expression in mouse dermal fibroblasts are, however, sufficient to provide cell attachment and migration but not to efficiently remodel collagen at early time points (24 hours). Expression of α2 is upregulated over time by fibroblasts in collagen lattices, which leads to more efficient collagen remodeling, as we observed at 72hours. As only α11 appears to induce α-SMA levels, we assume that differential signaling is active during myofibroblast differentiation and also during collagen remodeling.
Our results demonstrate that both TGF-β and α11β1 integrin have important roles in collagen matrix reorganization by activation of the JNK signaling pathway. Blocking JNK has been shown to inhibit TGF-β-induced collagen remodeling (Shi-Wen et al., 2009) and myofibroblast differentiation (Shi-Wen et al., 2006). A previous report showed that FAK is essential for TGF-β-induced JNK activation in MEFs (Liu et al., 2007). Although FAK is also necessary for collagen reorganization in our model, we could not demonstrate its role in JNK phosphorylation. Cross talk between non-Smad TGF-β signaling and integrins has already been described (Mu et al., 2012). A tempting speculation is that part of the non-canonical TGF-β signaling might depend on α11β1 but not on FAK.
In summary, our data demonstrate that α11β1 is the major collagen receptor present on dermal fibroblasts essential in collagen remodeling. We also demonstrate that α11β1 is involved in myofibroblast differentiation and GT formation following injury to ensure scar tissue strength. As myofibroblast differentiation and collagen dynamics are at the core of fibrotic processes, α11β1 is an interesting collagen receptor to investigate further in pathological fibrosis.
Materials and Methods
Detailed materials and methods are provided in Supplementary Data online.
Mice
C57BL/6 mice with targeted deletion of Itga2 or of Itga11 gene as previously described (Holtkotter et al., 2002; Popova et al., 2007) were used in this study. Compound α2−/−//α11−/− mutant mice were generated by intercrossing the single mutants. Wild-type and compound α2−/−//α11−/− mutant mice were crossed with the immorto mouse carrying the Simian virus 40 large T antigen under control of the temperature-sensitive H-2Kb-tsA58 promoter (Jat et al., 1991; kindly provided by U Mayer, University of East Anglia, UK), generating immorto mice carrying at least one copy of the H-2Kb-tsA58 transgene. Animals were housed in specific pathogen-free facilities. PCR genotyping was performed on DNA extracted from tail tip biopsies as described (Holtkotter et al., 2002; Popova et al., 2007). All animal experiments were approved by the local veterinary authorities (LANUV NRW, Germany or the Norwegian Animal Research Authority).
Cell culture
Mouse dermal fibroblasts were isolated as previously described and used in experiments up to passage 2 (Zweers et al., 2007). Briefly, the entire trunk skin of postnatal day 3 C57BL/6 mice, α11−/− mutant mice or compound α2−/−//α11−/− mutant mice was removed and incubated overnight at 4 °C with trypsin-EDTA (0.05%-0.02%, PAA Laboratories, Pasching, Austria). Upon manual detachment from the epidermis, the dermis was minced and incubated with Dulbecco's modified essential medium (DMEM, Gibco Invitrogen, Oslo, Norway) containing 400 U ml−1 of collagenase I (CLS-1, Worthington, Lakewood, NJ) for 1 hour at 37 °C. Dermal fibroblasts were cultured in DMEM supplemented with 10% fetal calf serum (PAA Laboratories) and 1% penicillin and streptomycin (Sigma-Aldrich, Oslo, Norway). Immorto mouse dermal fibroblasts were generated as previously described (Whitehead and Joseph, 1994). Immortalized cells showed collagen-binding integrin expression levels similar to primary cells (Supplementary Figure S2A online). Immortalized fibroblasts were used in experiments leading to the following results: Figure 2c, 4f and g, 5e and f. Integrin α11 was rescued in Simian virus 40-immortalized α11−/− fibroblasts (α11−/−KI) by viral transfection with the full-length of mouse Itga11 cDNA (Lu et al., 2014). jnk1−/−//jnk2−/− MEFs are described in Schumacher et al. (Schumacher et al., 2014), originally isolated by Prof. Erwin Wagner (Javelaud et al., 2003).
Wounding and staining of wound tissues
Excisional and incisional full-thickness wounds were inflicted on the shaved backs of 10-week-old female mice as described (Zweers et al., 2007). Incisional wounds of 2.5 cm were placed on the lower back crossing the midline, sutured in the center, and harvested at 16 days after injury.
For histology, wounds were bisected, and either fixed for 2 hours in 4% paraformaldehyde and then processed for paraffin embedding or frozen unfixed in O.C.T. compound (Sakura, Staufen, Germany). Paraffin sections were stained with hematoxylin and eosin or Sirius red according to standard procedures. Vascular structures were visualized by immunofluorescence staining of cryosections, acetone fixed, and incubated overnight at 4 °C with an antibody against CD31 (MEC13.3, BD Biosciences, Heidelberg, Germany; 1:1,000 in (phosphate buffered saline) PBS/1% bovine serum albumin (BSA)), followed by incubation with a Cy3-conjugated antibody directed against α-SMA (4A1, Sigma-Aldrich; 1:500 in PBS/1% BSA). Secondary IgG1κ, Alexa 488-conjugated goat anti-mouse antibody (Molecular Probes, Darmstadt, Germany; 1:500 in PBS/1% BSA) was applied for 1 hour at room temperature. Sections were counterstained with DAPI and mounted.
Myofibroblasts were visualized by immunohistochemical staining with an antibody directed to α-SMA (Tomasek et al., 2005). Paraffin sections were dewaxed in xylol and rehydrated, blocked in 10% normal goat serum for 30 minutes (endogenous biotin was blocked using the Biotin Blocking System (Dako, Hamburg, Germany)), incubated with primary fluorescein-conjugated antibody against α-SMA (1:250 in 1% BSA), followed by rabbit anti-fluorescein antibody (1:750, Molecular Probes A889) and subsequently by biotinylated goat anti-rabbit IgG (Vectastain ABC kit, Vector Laboratories, Peterborough, UK). Complex was detected by incubation with the ABC-alkaline phosphatase complex (Vector Laboratories), and color was developed with Vector red alkaline phosphatase substrate (Vector Laboratories).
Collagen type I gel contraction
Collagen gel contraction was performed as previously described (Barczyk et al., 2013). Briefly, each ml contained the following: 500 μl of 2 × DMEM containing 1.6 × 105 cells per ml, 10 μl 200 mM Glutamine (Cambrex Bioscience, Stockholm, Sweden), 10 μl antibiotics, 100 μl 0.2M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich) pH 8.0, and 400 μl collagen type I (PureCol, Advanced Biomatrix, Carlsbad, CA). A total of 300 μl of this mixture was added into each well of a 24-well plate and were allowed to polymerize (∼90 minutes) at 37 °C. In order to obtain floating conditions, gels were poured into wells that had been previously coated overnight with 5% BSA in sterile PBS. Once the cell-containing collagen mixture had polymerized, DMEM supplemented with 2% fetal calf serum was added. Recombinant human TGF-β1 (PeproTech, Stockholm, Sweden; 5 ng ml−1), SB505124 (Sigma-Aldrich; 10 μM), SP600125 (Sigma-Aldrich; 25 μM), SB203580 (Sigma-Aldrich; 10 μM), PD098059 (Sigma-Aldrich; 5 μM), PF-573228 (Sigma-Aldrich; 10 μM) or β1 integrin antibody Ha 2/5 (BD Biosciences) were used as indicated. After 24 hours of collagen remodeling, we did not notice significant differences in cell number between α11+/+ and α11−/− fibroblasts. Results are expressed as the mean from three independent experiments±SD, each condition at least performed in triplicates per experiment.
Statistical analysis
Results are expressed as the mean±SD of at least three replicates and are representative of three independent experiments. Statistical significance was assessed using unpaired Student's t-tests unless stated differently, with P<0.05 being considered significant. Gaussian distribution was verified by the Kolmogorov-Smirnov test. Calculations were performed using GraphPad Prism (GraphPad software, La Jolla, CA).
Acknowledgments
We thank Ning Lu (Bergen) for help with viral transfection and Katrin Blumbach (Cologne) for help with cell tracking. We are grateful to Peter Angel (DFKZ, Heidelberg) for kindly providing jnk1−/−//jnk2−/− mouse embryonic fibroblasts. This project was supported by EU Marie Curie Early Stage Training Contract (MEST-CT-2004-514483; SC), a bi-lateral German-Norwegian DAADppp grant from the Research council of Norway (to DG), by grants from Research Council of Norway (197066; to DG), EEA grant Poland Norway MOMENTO (ID 202952: to DG) Western Norway Regional Health Authority (ID 911899: to DG), by Deutsche Forschungsgemeinschaft through EC140/5 and SFB 829 (to BE) and by the Koeln Fortune Program/Faculty of Medicine, University of Cologne (to J-NS and BE).
Glossary
- α-SMA
α-smooth muscle actin
- GT
granulation tissue
- MEF
mouse embryonic fibroblast
- TGF-β
transforming growth factor-β
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Barczyk MM, Lu N, Popova SN, et al. alpha11beta1 integrin-mediated MMP-13-dependent collagen lattice contraction by fibroblasts: Evidence for integrin-coordinated collagen proteolysis. J Cell Physiol. 2013;228:1108–1119. doi: 10.1002/jcp.24261. [DOI] [PubMed] [Google Scholar]
- Carracedo S, Lu N, Popova SN, et al. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434–10445. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472–479. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert M, Ruetze M, Sattler M, et al. The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J Dermatol Sci. 2014;73:40–48. doi: 10.1016/j.jdermsci.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Elliott CG, Wang J, Guo X, et al. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, et al. Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, et al. Wound healing in the α2β1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–466. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tingstrom A, Thuresson AC, et al. b1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Holtkotter O, Nieswandt B, Smyth N, et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Laboureau J, Gabison E, et al. Disruption of basal JNK activity differentially affects key fibroblast functions important for wound healing. J Biol Chem. 2003;278:24624–24628. doi: 10.1074/jbc.M301942200. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, et al. Integrin a2b1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xu SW, Blumbach K, et al. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- Liu S, Xu SW, Kennedy L, et al. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Karlsen TV, Reed RK, et al. Fibroblast alpha11beta1 integrin regulates tensional homeostasis in fibroblast/A549 carcinoma heterospheroids. PLoS One. 2014;9:e103173. doi: 10.1371/journal.pone.0103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Raymond K, Kreft M, et al. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, et al. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98–107. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85:4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- Poon R, Nik SA, Ahn J, et al. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol. 2009;10:38. doi: 10.1186/1471-2121-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova SN, Barczyk M, Tiger CF, et al. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27:4306–4316. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyhani V, Seddigh P, Guss B, et al. Fibrin binds to collagen and provides a bridge for alphaVbeta3 integrin-dependent contraction of collagen gels. Biochem J. 2014;462:113–123. doi: 10.1042/BJ20140201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Schuster C, Rogon ZM, et al. Efficient keratinocyte differentiation strictly depends on JNK-induced soluble factors in fibroblasts. J Invest Dermatol. 2014;134:1332–1341. doi: 10.1038/jid.2013.535. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Parapuram SK, Pala D, et al. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60:234–241. doi: 10.1002/art.24223. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Rodriguez-Pascual F, Lamas S, et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol. 2006;26:5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- Talior-Volodarsky I, Connelly KA, Arora PD, et al. alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96:265–275. doi: 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, McRae J, Owens GK, et al. Regulation of alpha-smooth muscle actin expression in granulation tissue myofibroblasts is dependent on the intronic CArG element and the transforming growth factor-beta1 control element. Am J Pathol. 2005;166:1343–1351. doi: 10.1016/s0002-9440(10)62353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care. 2013;2:122–141. doi: 10.1089/wound.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T, Kusche-Gullberg M, Sejersen T, et al. cDNA cloning and chromosomal localization of human alpha11 integrin. A collagen-binding, I domain-containing, beta1-associated integrin alpha-chain present in muscle tissues. J Biol Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, Joseph JL. Derivation of conditionally immortalized cell lines containing the Min mutation from the normal colonic mucosa and other tissues of an "Immortomouse"/Min hybrid. Epithelial Cell Biol. 1994;3:119–125. [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, et al. Myofibroblast contraction activates latent TGF- 1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–932. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- Zeltz C, Orgel J, Gullberg D. Molecular composition and function of integrin-based collagen glues-Introducing COLINBRIs. Biochim Biophys Acta. 2013;1840:2533–2548. doi: 10.1016/j.bbagen.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Bothe I, Hirche F, et al. Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of a2b1 integrin. J Cell Sci. 2006;119:1886–1895. doi: 10.1242/jcs.02921. [DOI] [PubMed] [Google Scholar]
- Zweers MC, Davidson JM, Pozzi A, et al. Integrin a2b1 Is required for regulation of murine wound angiogenesis but Is dispensable for reepithelialization. J Invest Dermatol. 2007;127:467–478. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.