Abstract
Objective
To (1) model growth in anxiety and depressive symptoms from late school age through young adulthood in individuals with autism spectrum disorder (ASD) and controls with developmental delay (DD); and (2) assess relationships between internalizing growth patterns, participant characteristics, baseline predictors, and distal outcomes.
Method
Data were collected between 6-24 years of age in 165 participants (n=109 with ASD; n=56 with nonspectrum DD), most of whom received diagnostic evaluations in both childhood and early adulthood. Questionnaires were collected approximately every 3-6 months between ages 9-24. Parent-rated Child and Adult Behavior Checklists (CBCL; ABCL) and Developmental Behavior Checklist anxiety- and depression-related subscale distributions were modeled with mixed-effects Poisson models, covarying diagnosis, age, verbal IQ (VIQ), gender, and significant two- and three-way interactions.
Results
Anxiety was positively associated with VIQ, and controlling for VIQ, both anxiety and depressive symptoms were greater in ASD than nonspectrum participants. Female gender predicted greater increases over time in anxiety and depressive symptoms for both diagnostic groups. Lower maternal education was associated with increasing internalizing symptoms in a subset of less verbal individuals with ASD. In exploratory post-hoc analyses, internalizing symptoms were associated with poorer emotional regulation in school age, and with lower life satisfaction and greater social difficulties in early adulthood.
Conclusion
Findings support previous claims that individuals with ASD are at particular risk for affect- and anxiety-specific problems. While symptom levels in females increase at a faster rate throughout adolescence, males with ASD appear to have elevated levels of depressive symptoms in school age that are maintained into young adulthood.
Keywords: autism spectrum disorder, depression, anxiety, growth curve, Child Behavior Checklist
Introduction
Mood and anxiety problems are common comorbidities in individuals with autism spectrum disorder (ASD), with data suggesting rates that exceed both the lifetime prevalence in the general population and the elevated levels seen in children with intellectual disability (ID) without autism.1–5 Subclinical symptom levels may be even more widespread and similarly impairing among people with ASD.6,7 The specific focus of this paper is how mood and anxiety symptoms develop over time in ASD and nonspectrum developmentally delayed (NSDD) samples comparatively.
Adolescence is an obvious time in which to focus on the course of internalizing problems.8-10 The prevalence of depression increases substantially during this period for typically developing youth,4,8 with approximately 14% meeting criteria for major depressive disorder at some point before age 18.9 To this point, research suggests that internalizing problems in ASD samples either improve or remain stable across adolescence; however, most studies are based on measures that lump depressive and anxiety symptoms, include very few items about either construct, or include items not specific to affect or anxiety that may be confounded with ASD symptoms (e.g., inattention, repetitive behavior, social withdrawal) 11–15 (see Supplement 1, available online, for a more detailed overview). In this study, we are interested in the developmental courses of cognitive-emotional symptoms of depression and anxiety distinctly. We model longitudinal change in individuals with ASD and NSDD controls on the specific depression and anxiety subscales of the well-validated parent-rated measures, the Child Behavior Checklist 6-18 (CBCL)16 and Adult Behavior Checklist (ABCL).17
The CBCL has been shown to be sensitive in detecting behavioral and emotional problems in adolescents with mild and moderate ID, which encompasses youth with IQ scores ranging from 35-69 18–20, c.f.21. In a sample of 8- to 20-year-olds with mild to profound ID (including IQ<20), one team18 observed that CBCL Internalizing factors were essentially the same as those derived from the instrument's norming sample.22 However, researchers18,19 have noted that for both the Internalizing scale and its component Anxious/Depressed subscale, lower IQ was associated with fewer emotional health problems (see conflicting evidence in ASD14,15). To avoid potential measurement artifact in this population, we covaried verbal IQ (VIQ) when using the well-validated CBCL as an outcome measure for our entire sample. Then, for those participants who had less than fluent speech (measured by clinician's module choice on the Autism Diagnostic Observation Schedule [ADOS]23), we also present trends over time in anxiety and depression subscales of the parent-rated Developmental Behavior Checklist (DBC)24, a measure specifically created for and validated in the intellectually and developmentally-delayed population.
Predictors and Outcome of Affective Distress in Populations With DD
Shattuck et al.14 and Gray et al.15 found that lower IQ was associated with greater emotional and behavioral problems in ASD. The impact of socioeconomic status (SES) on depression in ASD is unclear, with findings that lower SES predicts emotional problems at age 16,25 is not associated with emotional problems,15 predicts less improvement over time,11 and is associated with overall level of psychopathology but not with changes over time.26 Gender is another important covariate due to the complicated relations of higher rates of depression/anxiety in females, higher rates of ASD in males, yet purportedly higher rates of depression/anxiety in ASD than in typical samples. Finally, recent commentary suggests that emotion regulation27 and autism traits12 warrant study as potential mechanisms underlying affect and anxiety problems.
The current objectives were to (1) model growth in anxiety and depressive symptoms from late school age through young adulthood in a sample with heterogeneous developmental disorders; (2) evaluate differences in developmental patterns of internalizing symptoms based on diagnosis (ASD versus NSDD), verbal IQ, gender, and maternal education (as a proxy for SES); (3) assess relations between internalizing growth patterns, baseline covariates (e.g., the Emotional Control subscale of the Behavior Rating Inventory of Executive Function [BRIEF]28 and autism severity scores based on the ADOS symptom domains29), and distal outcomes (e.g., quality of life ratings).
Method
Participants and Procedures
Our sample included 165 participants with data collected between the ages of 6 and 24 years. Of these, n=148 had between 2 and 7 repeat iterations of the parent-rated CBCL and/or ABCL (n=99 with ASD; n=49 with heterogeneous nonspectrum disorders or early delays). A subset of n=44 less verbal participants, all of whom had ASD and n=32 with 2-4 iterations of repeat data, were also included in analyses of the DBC. See Table 1 for sample description by diagnostic cohort.
Table 1. Sample Demographics by Diagnostic Group.
| ASD | Nonspectrum DD | |
|---|---|---|
| N | 109 | 56 |
| Number assessments | 1 – 7 | 1 – 7 |
| 3.5 (1.32) | 3.4(1.37) | |
| Verbal IQ | 10 – 141 | 14 – 139 |
| 56.3(40.1) | 79.6(33.5) | |
| Age at First Assessment | 5.9 – 19.8 | 7.6 – 16.7 |
| (in years) | 10.7(2.5) | 12.3(2.3) |
| Age at Last Assessment | 10.8 – 24.5 | 12.0 – 23.9 |
| (in years) | 19.3(3.1) | 19.0(2.9) |
| Female | 12% | 39% |
| Non-White | 20% | 13% |
| Maternal: High School/Less | 9% | 23% |
| ADOS Social Affect | 1-20 | 0 – 15 |
| 10.3(4.9) | 3.9(3.4) | |
| ADI-R Social | 0-30 | 0-24 |
| 20.6(8.2) | 7.6(6.4) |
Note. Italicized text represents significant differences by diagnostic group at p<.05. ADI-R=Autism Diagnostic Interview-Revised; ADOS=Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; DD = developmental delay.
There was great variability in IQ in the overall sample, with VIQ scores of 10-20 at the low end to approximately 140 for each diagnostic group. The group with ASD tended to have lower VIQ (t[130]=3.94, p<.001) and chronological age at first data point (t[121]=4.11, p<.001) than the group with NSDD, and was more likely to be male (χ2=13.03; p<.001). Groups with ASD and NSDD did not differ significantly on number of assessments, age at last assessment, race, or maternal education. Both ASD and NSDD participants came from two related recruitment cohorts: (1) participants in the Early Diagnosis of ASD (EDX) study (n=131) had been consecutive referrals at age 2 to autism specialty clinics. The group with NSDD consisted of 22 children with developmental disability who were recruited from specialists who referred to the autism clinics, as well as those consecutive clinic referrals who did not receive an ASD diagnosis (n=18); these children presented with some degree of intellectual disability or language delay (91%) or issues such as severe attention-deficit/hyperactivity disorder (ADHD). Most EDX participants received multiple diagnostic evaluations between ages 2-9 and 18-22 years, including the ADOS23 and Autism Diagnostic Interview-Revised (ADI-R)30 by research-reliable clinicians. See Lord et al.31 and Anderson et al.13 for detailed descriptions of recruitment and diagnostic procedures in the EDX subsample. (2) An additional group (n=34) of “new recruit” participants with ASD and NSDD of similar ages were evaluated at the time the EDX participants were approximately 7-10 years old in order to increase the number of participants followed longitudinally through adolescence and beyond. These children were recruited from the community, had received at least one diagnostic evaluation prior to age 5, and received a comprehensive evaluation upon enrollment to confirm diagnosis.
Exclusion criteria for all participants included moderate to severe visual, hearing, or motor impairments. Families in both recruitment cohorts completed parent- and self-report questionnaires at regular intervals of 3–6 months between ages 9 and 24.
Measures
See Supplement 1, available online, for descriptions of diagnostic measures and assessment protocol, as well as measures used to define baseline and distal covariates.
Emotional Health
We used the CBCL and ABCL as primary outcome instruments because they have evidence of validation across samples both typical and with ASD, as well as the greatest consistency across the wide age period assessed here. We chose the “Anxiety” and depression-specific (CBCL: “Affective”; ABCL: “Depressive”) subscales as dependent variables given our interest in cognitive and emotional symptoms of depression and anxiety; by contrast, the commonly-used Internalizing scale includes symptoms of social interest and withdrawal that are likely confounded with ASD symptoms, as well as a greater number of items about somatic symptoms that are not specific to affective and anxiety problems.
Because the CBCL and ABCL are companion measures across age ranges within the Achenbach System of Empirically-Based Assessment (ASEBA),16,17 we will refer to the two collectively as “ASEBA” scales. These instruments generally have been found to have high sensitivity but low specificity for emotional health disorders in some samples with ASD,32,33 and, by contrast, to under-report emotional health problems in comparison to self- and teacher-report ASEBA versions in ASD.34 We modeled ASEBA variables for all participants, regardless of language level, after our preliminary findings indicated that neither verbal IQ nor ADOS module was related to rate of anxious/depressive symptom growth, our primary outcome of interest.
For participants with less than fluent speech, we also analyzed the Communication and Anxiety Disturbance (hereafter, DBC-Anxiety) and Depressive (DBC-Depressive) subtotals of the Developmental Behavior Checklist-Adult, a 107-item parent/caregiver-rated questionnaire focusing on emotional and behavioral problems in adults 18 years and older with intellectual and/or developmental disabilities (IDD). In a study of 1,538 adults with IDD, both of these subscales had excellent internal consistency.35
Design and Analysis
Generalized mixed-effects models (GMM) were used to capture growth in the dependent variables (DVs; ASEBA Anxiety and Depressive subscales, DBC-Anxiety and -Depressive subscales). Because ASEBA items have a restricted response range (0 to 2) and 0 scores were common, distributions of the DVs were positively skewed, with the vast majority of participants scoring on the low end of the symptom scales. Thus, DV distributions better resembled count (Poisson) than Gaussian distributions, so mixed-effects Poisson models with a logarithmic link function were used to model growth in the outcome variables using the lme4 package36 in R version 3.0.1.37 Model parameters were estimated using Laplace approximation, with CIs calculated using basic, parametric bootstrap with 1,000 random draws. The Bayesian Information Criterion (BIC) and likelihood ratio tests aided comparison of competing model specifications. See Supplement 1, available online, for additional analytic details.
To account for potential discontinuity in growth (specifically, a sudden increase in symptom levels due to the transition from the CBCL to the ABCL at age 18), we included in all ASEBA analyses a dummy variable (measure) coding whether scores were based on the CBCL or ABCL. The effect of measure was positive and significant in all cases, indicating an increase in scores associated with the transition from the CBCL to ABCL. Significant effects of age (i.e., time) on symptom levels were thus indicative of increases in symptom levels over and above increases attributable to a change in instruments. Additionally, we compared linear growth models to piecewise growth models that allowed for a change in the rate of growth in the ASEBA subscales concomitant with the transition from the CBCL to the ABCL; linear growth models were preferable for both the Anxiety and Depression subscales.
To aid in interpretation, we report exponentiated estimates of model parameters. In Poisson models, these can be interpreted as incident rate ratios: What percentage increase or decrease in the rate of the outcome variable is expected for a one unit increase in the predictor, holding constant the effects of model covariates? Thus, an exponentiated estimate of γ̂ =2.00 associated with the regression of a count variable on sex (0=male, 1=female) means that females are expected to have double the rate of the outcome variable compared to males. Exponentiated values less than 1 reflect an expected decrease in rate with a one unit increase in the predictor variable, and values greater than 1 reflect an expected increase in rate.
Finally, in exploratory efforts, Pearson bivariate correlations were used to assess associations between predicted ASEBA anxiety and depressive levels and rates of growth over time with the following baseline and distal predictors (see Supplement 1, available online, for measure details): (1) the BRIEF-Emotional Control subscale as close to age 9 as it was available; (2) the ADOS Social Affect and Restricted Repetitive Behavior calibrated severity scores as close to age 9 as available; (3) Vineland Adaptive Behavior38 domain standard scores at age 18; and (4) the last available iteration of the Quality of Life Questionnaire (QLQ)39 Satisfaction, Competence, Independence, and Social Belonging domains, the Well-Being Questionnaire Total40, and Family Environment Scale (FES)41 Cohesion, Expressive, and Conflict domains (age was variable but most data came from age 18 for all three questionnaires).
Results
ASEBA Anxiety Subscale
A linear growth model was preferable to a quadratic growth model in the ASEBA Anxiety subscale (BIClinear = 1,779.6 vs. BICquadratic = 1,812.3). We observed main effects of both VIQ and diagnosis: Higher VIQ was associated with higher levels of anxiety (γ̂ =1.005, 95% CI [1.001, 1.009]), and ASD was associated with higher levels of anxiety than NSDD (γ̂ =1.392, 95% CI [1.010, 1.885]). In the competition for variance, the effect of higher VIQ was ultimately quite small, whereas ASD predicted a 39% increased rate even after holding constant model covariates. There was also a significant interaction between age and gender (γ̂=0.898, 95% CI [0.816, 0.978]). At study outset in late school age, males had higher levels of anxiety than females (γ̂ =1.881, 95% CI [1.069, 3.490]), but females showed greater increases in symptoms over time throughout adolescence, with no significant gender difference at age 21 (γ̂=0.792, 95% CI [0.529, 1.187]). See Figure 1, including comparative data by gender from the ASEBA norming samples.
Figure 1.
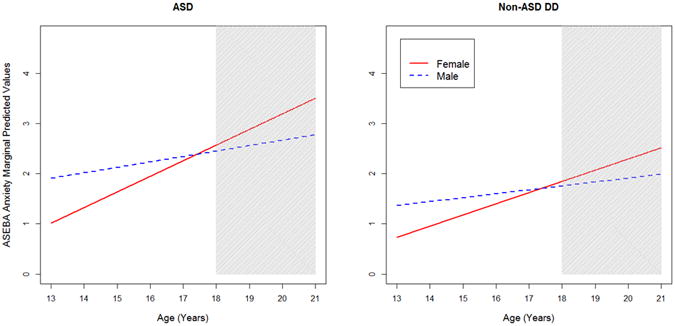
Anxiety subscale predicted scores by diagnosis and gender; by comparison, Achenbach System of Empirically-Based Assessment (ASEBA) norming sample16,17 raw score averages on the Anxiety subscale are as follows: Child Behavior Checklist (CBCL) ages 6-11: 1.4 (boys, green square), 1.7 (girls, green circle); ages 12-18: 1.2 (boys), 1.4 (girls); Adult Behavior Checklist (ABCL) ages 18-35: 3.8 (men), 4.3 (women). Note: The gray shaded area reflects ages associated with the ABCL as opposed to the CBCL. ASD = autism spectrum disorder; DD = developmental delay.
ASEBA Depressive Subscale
The ASEBA Combined Depressive subscale (again, labeled “Affective” on the CBCL and “Depressive” on the ABCL) was best described by a linear model: (BIClinear = 2,019.5 vs. BICquadratic = 2,039.3). We observed main effects of both diagnosis and age. ASD was associated with higher levels of depressive symptoms than NSDD (γ̂ =1.588, 95% CI [1.100, 2.287]) (see Figure 2).
Figure 2.
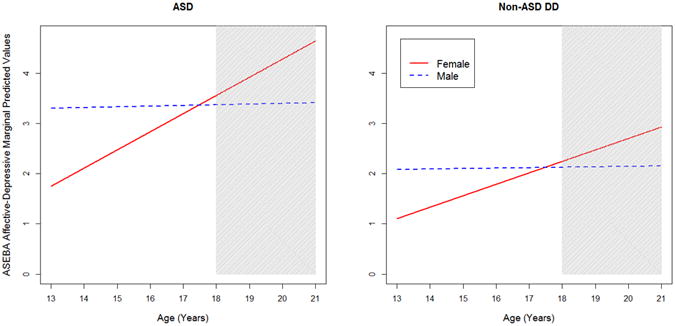
Depressive subscale predicted scores by diagnosis and gender; by comparison, Achenbach System of Empirically-Based Assessment (ASEBA) norming sample16,17 raw score averages on the Affective or Depressive subscales are as follows: Child Behavior Checklist (CBCL) ages 6-11: 1.4 (boys), 1.4 (girls); ages 12-18: 1.6 (boys), 1.9 (girls); Adult Behavior Checklist (ABCL) ages 18-35: 3.5 (men), 3.9 (women). Note: The gray shaded area reflects ages associated with the ABCL as opposed to the CBCL. ASD = autism spectrum disorder; DD = developmental delay.
Depressive symptoms tended to increase over time in both diagnostic groups (γ̂=1.130, 95% CI [1.044, 1.227]) (see Figure 2); however, this must be interpreted in light of the significant interaction between age and gender (γ̂=0.889, 95% CI [0.820, 0.964]). As with the Anxiety subscale, males tended to have higher levels of depression than females at age 13 (γ̂=1.888, 95% CI [1.089, 3.279]), and, on average for boys with ASD, more than double the norming sample mean for that age period and gender (see Figure 2 caption). However, females showed greater symptom increases throughout adolescence (an approximately 13% increase per year over the previous year's count), resulting in no gender differences by age 21 (γ̂=0.736, 95% CI [0.460, 1.178]). Of note, we observed no significant effects of VIQ on depressive symptoms (γ̂=1.002, 95% CI [0.998, 1.006]).
Supplemental Figures S1 and S2 (available online) show observed ASEBA-Anxiety and ASEBA-Depression scores plotted against age and broken down by gender. General patterns of growth over time in the observed scores are similar to the patterns in the model predicted scores (i.e., Figures 1 and 2). Additionally, we included loess (locally weighted regression) lines to better capture the “jump” in ASEBA scores that coincides with the transition from CBCL to ABCL (i.e., the transition from the white background to the gray shaded region). The jump appears to be more dramatic among females; however, in post-hoc analyses, the interaction between the dummy variable coding CBCL vs. ABCL and gender was not significant.
Anxiety and Depression Subscales in Less- Verbal Participants
We also modeled changes over time in the DBC Anxiety and Depression subscales for participants with less than fluent speech. This smaller subset of 44 participants all had ASD and were in or nearing young adulthood at the point at which their parents began completing DBCs (first iteration: M=19.5 years, SD=0.76).
On the DBC-Anxiety subscale, we observed no effects of VIQ or gender and no significant change over time in symptom levels. There was a significant interaction between age (time) and maternal education (our proxy for SES). Mothers without college degrees tended to report greater increases over time in anxiety symptoms in their children (γ̂=0.974, 95% CI [0.948, 0.999]).
On the DBC-Depression subscale, a main effect of age was noted in this ASD sample, with depressive symptoms apparently increasing from model onset around age 18 (γ̂=1.079, 95% CI [1.020, 1.141]). We also observed an interaction between age and gender, such that depressive symptom levels did not differ significantly across gender at the model outset, but increased for females over time at a greater rate (γ̂=0.950, 95% CI [0.911, 0.991]). Finally, a similar SES effect was noted for the Depressive subscale: Mothers without college degrees reported greater increases in their children's symptoms over the modeled time period (γ̂=0.952, 95% CI [0.921, 0.984]).
Potential predictors of anxiety/depressive symptoms
In both of the groups with ASD and NSDD, ASEBA symptom levels at model intercept were highly associated with the BRIEF-Emotional Control rating around age 9, such that higher initial depressive and anxiety symptoms were associated with more impaired emotional control (see Table 2). Most of the post-hoc correlations to the BRIEF were surprisingly robust, around the p <.01 or p <.001 level. Only those associations that met at least trend-level significance (p <.10) are displayed in Table 2 (full correlation matrix available from the authors).
Table 2. Significant Correlations Between Achenbach System for Empirically-Based Assessment measures (ASEBA) Intercepts and Slopes and Baseline and Distal Correlates.
| ASEBA modeled variable | ASD (n=70-88) | Nonspectrum DD (n=36-48) |
|---|---|---|
| Anxiety intercept | BRIEF (r=.41, p=.001) QLQ-Satisfaction (r=-.29, p=.01) Vineland Social (r=-.27, p=.02) Vineland Communication (r=-.25, p=.03) Vineland Daily Living (r=-.31, p=.007) |
BRIEF (r=.49, p=.003) |
| Anxiety slope | BRIEF (r=-.35, p=.003) QLQ-Satisfaction (r=-.26, p=.02) Vineland Social (r=.29, p=.01) Vineland Communication (r=-.27, p=.02) Vineland Daily Living (r=.31, p=.005) |
BRIEF (r=-.48, p=.003) |
| Depressive intercept | BRIEF (r=.47, p<.001) QLQ-Satisfaction (r=-.37, p=.001) Vineland Social (r=-.24, p=.03) Vineland Daily Living (r=-.21, p=.06) Well-Being Total (r=-.22, p=.05) FES Conflict (r=-.23, p=.06) |
BRIEF (r=.56, p<.001) QLQ-Satisfaction (r=-.45, p=.004) Vineland Communication (r=-.31, p=.05) |
| Depressive slope | BRIEF (r=-.37, p=.002) QLQ-Satisfaction (r=.27, p=.02) Vineland Social (r=.22, p=.05) |
BRIEF (r=-.48, p=.003) QLQ-Satisfaction (r=.29, p=.07) ADOS Social Affect Severity Score (r=.29, p=.05) |
Note: Italicized text represents variables approaching significance at p=0.05-0.07. ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; BRIEF = Behavioral Rating Inventory for Executive Functioning-Emotional Control subscale; DD = developmental delay; FES = Family Environment Scale; QLQ = Quality of Life Questionnaire; Vineland = Vineland Adaptive Behavior Scales.
Note that slopes of depression and anxiety subscales, indicating change over time, often had opposite associations with these correlates than did the intercepts. For example, greater impairment on the BRIEF-Emotional Control subscale was associated with greater anxiety and depression intercepts but lower slope values (i.e., slower-growing symptoms). We interpret this as natural regression to the mean, in which those with initially higher scores would be more likely to either get better over time or at least increase less rapidly due to ceiling effects.
Potential outcomes influenced by anxiety/depressive symptoms
Higher baseline anxious/depressive symptom levels (model intercepts) tended to be associated with lower life satisfaction on the QLQ-Satisfaction completed in early adulthood. Several anxious/depressive variables were also associated post hoc with lower scores on Vineland Adaptive Behavior domains around age 18 (see Table 2).
Discussion
We modeled growth in anxiety and depressive symptoms from school age through young adulthood in participants with ASD and nonspectrum DD, focusing on differences in trajectory across diagnostic groups and by VIQ and gender. We found that VIQ was a positive predictor of anxiety but not depressive symptoms in the CBCL/ABCL models. Both anxiety and depressive symptoms tended to begin and remain higher in ASD compared to NSDD. For both types of symptoms in both diagnostic groups, females showed greater increases throughout adolescence on ASEBA anxious/depressive variables than did males, with no significant gender differences by young adulthood, although in the autism subgroup, this was based on a very small sample of 13 girls. Taken as a whole, these findings support previous claims that individuals with ASD are at particular risk for affect- and anxiety-specific problems, with females following the course in the general population of increasing growth during adolescence, while males with ASD had elevated levels of depressive symptoms in school age that remained stable through young adulthood.
Another study objective was to assess relations between internalizing growth patterns and baseline and distal covariates. Higher anxious/depressive symptoms tended to be associated with poorer emotional regulation in school age, and lower life satisfaction and greater social difficulties in early adulthood.
Whereas many of our significant findings had small effect sizes, the main effects of diagnosis were clearly the most robust, with the group with ASD averaging anxiety totals nearly 1.4 times the NSDD average and depressive totals 1.6 times the NSDD average. It is also noteworthy that this was largely a community sample of consecutive referrals for ASD at age 2, and therefore these were not individuals presenting for ongoing clinical assessment or intervention, in which case we might expect to see higher rates of psychiatric comorbidities.
Our findings provide preliminary support for increases in anxiety and depressive symptoms in adolescent girls with developmental disabilities (including ASD), consistent with the course of both generalized anxiety and depression reported for typically developing (TD) adolescents.10,42 Particularly interesting, however, is how these results diverge from TD samples: In the general population, boys and girls exhibit approximately equivalent internalizing symptom levels until adolescence, at which point girls' growth outpaces boys' to result in higher overall levels, whereas in this ASD sample, anxious/depressive symptom levels in boys were higher than in girls at study outset and remained relatively stable over time, with faster female symptom growth in adolescence causing the sexes to converge by young adulthood. Of particular note, the average model-predicted depressive symptom level in school-age boys with ASD was more than double the TD norming sample mean (see Figure 2 caption), indicating these problems should be a focus of future study in this gender-by-age group.
While our sample may not be representative of the population with ASD as diagnosed today because of its relatively low mean IQ, VIQ was not significantly associated with growth or overall magnitude of parent-reported depressive symptoms in this study. By contrast, higher VIQ was related to greater anxiety symptoms on the CBCL/ABCL (though with a small effect size), and no growth over time in anxiety was noted in the less verbal, less cognitively able participants on the DBC analyses. It is unclear whether the relation between anxiety and higher verbal IQ represents an important aspect of the phenomenology of anxiety in ASD, or its relative ease of measurement in individuals with stronger verbal fluency or insight. In either case, it is interesting that the same did not apply to depressive symptoms in our sample.
Just as the role of SES is mixed in previous literature (see Introduction), so too did we observe an unusual effect in which lower maternal education was associated with greater internalizing symptom increases over time, but only for the analyses on less verbal individuals with ASD. Though we were not able to test mediation, if we assume that lower SES is associated with greater psychopathology due to stress-related mechanisms, we might have expected to see this effect in our sample regardless of verbal ability.
Our post-hoc analyses of baseline correlates were preliminary in the sense that the study was not designed prospectively with this in mind, and therefore somewhat differing ages at baseline and missing data prevented us from including emotional regulation and ASD severity variables as covariates within the longitudinal models themselves. However, associations were notably robust between internalizing model variables and the BRIEF-Emotional Control subscale (via parent report around age 9). This suggests that emotional control and affective state around baseline measurement point were related and provides more evidence in favor of considering executive functioning, and specifically emotion regulation, as a mechanism underlying elevated rates of anxiety/depressive symptoms in ASD. These post-hoc correlations may be influenced by the use of a single informant (parent report); thus, it will be important to replicate in future studies using multiple-informant designs.
The relation of anxious/depressive symptom levels and growth to the QLQ-Satisfaction subscale in our group with ASD (above other quality of life subscales, e.g. independence and competence) suggests that these symptoms impact life satisfaction. Specific to our sample with ASD, several predicted modeling variables also were associated with Vineland Adaptive Behavior domain scores around age 18, particularly Social scores. This suggests effects of internalizing problems on social and other adaptive behavior deficits beyond those associated with ASD alone, which may shed light on findings of particularly high service need in individuals with ASD and comorbid psychiatric disorder.43 It also points to the need for thoughtful development of targeted treatments for internalizing problems in these populations.
This comprised an analysis of longitudinal data from what was largely a community sample of a special population (i.e., consecutive referrals as toddlers, rather than a convenience sample of those presenting clinically at the ages of focus). The present sample included a comparison group with NSDD, and encompassed a wide IQ range in both diagnostic groups. The fact that approximately one-third of the group with NSDD originally had been referred for ASD evaluations suggests that the current findings may be a conservative comparison between these groups. Further, the low average mean IQ in the group with ASD reduces the representativeness of these data to more recently diagnosed samples with ASD. Our models are based on the unverifiable assumption (missing-at-random) that the probability of missingness was a function of the observed data alone (i.e., would not be altered by values of missing data). Finally, we did not include externalizing variables in these models but refer readers to a discussion of the same in a related sample.13
Psychiatric comorbidity has been tied to higher service and medication use and lower quality of life in the segment of the population with ASD.44,45 The availability of better-tailored services could decrease lengthier (thus more costly) service use. We need instrument validation for depression assessment in this population,33 and it likely will be valuable to widen our scope beyond categorical comorbidity to the pervasive issue of affective distress in ASD. We know little about internalizing phenomenology or prevalence in the early and late phases of development within DD populations. Finally, it will be important to explore the current gender findings to replicate and understand high rates of depressive symptoms specifically in school-age boys with ASD, and to see whether females' symptoms continue to increase in adulthood and eventually surpass males' as they age.
Clinical Guidance
Health care providers should be aware of the need to screen for anxiety and depression in children and adolescents with autism spectrum disorder, particularly in those who exhibit emotion regulation difficulties.
As noted in the general population, girls with ASD may have greater increases in anxious/depressive symptoms across adolescence than boys.
Unlike the general population, school-age boys with ASD may present with higher levels of anxious/depressive symptoms than girls with ASD or same-age peers of either sex.
Supplementary Material
S1. This figure shows observed Anxiety scores from the Achenbach System of Empirically-Based Assessment (ASEBA) measures plotted against participant age. Female data are plotted as black circles, and male data as red triangles. The figure contains linear regression lines (straight dashed) and smoothed non-parametric loess lines (curved solid) for both females (black) and males (red). Points within the gray shaded area reflect Adult Behavior Checklist (ABCL) scores as opposed to Child Behavior Checklist (CBCL) scores. Anxiety scores tended to increase with age, particularly among female participants.
S2. This figure shows observed Depressive symptom scores from the Achenbach System of Empirically-Based Assessment (ASEBA) measures plotted against participant age. Female data are plotted as black circles, and male data as red triangles. The figure contains linear regression lines (straight dashed) and smoothed non-parametric loess lines (curved solid) for both females (black) and males (red). Points within the gray shaded area reflect Adult Behavior Checklist (ABCL) scores as opposed to Child Behavior Checklist (CBCL) scores. Depressive scores tended to increase with age, particularly among female participants.
Acknowledgments
This work was supported by funding from the National Institute of Mental Health (T32-MH18921; R01-MH57167; R01-MH066469; R01-MH081873-01A1; K01-MH103500-01A1).
Dr. Brunwasser served as the statistical expert for this research.
The authors gratefully acknowledge the families that participated in this research; Shanping Qiu, MA, Somer Bishop, PhD, and Marisela Huerta, PhD, of the Center for Autism and the Developing Brain, New York-Presbyterian Hospital; Whitney Guthrie, MA, Florida State University; Melissa Maye, MA, University of Massachusetts-Boston; Kylie Gray, PhD, and John Taffe, PhD, of Monash University; and Andrew Tomarken, PhD, and Warren Lambert, PhD, of Vanderbilt University.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Gotham has received royalties from the publisher of the Autism Diagnostic Observation Schedule-2 (ADOS-2). Dr. Lord has received royalties from the publisher of the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS; ADOS-2). These measures were used for establishing diagnosis in this sample and were not primary to outcome in this paper; further, Drs. Lord and Gotham donate to charity all royalties from clinics and projects in which they are involved. Dr. Brunwasser reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Katherine Gotham, Vanderbilt University School of Medicine, Nashville.
Dr. Steven M. Brunwasser, Vanderbilt University Kennedy Center, Nashville.
Dr. Catherine Lord, Center for Autism and the Developing Brain, Weill-Cornell Medical College, New York.
References
- 1.Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917. doi: 10.1016/j.ridd.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Mayes SD, Calhoun SL, Murray MJ, Ahuja M, Smith LA. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Res Autism Spectr Disord. 2011;5(1):474–485. [Google Scholar]
- 3.Brereton AV, Tonge BJ, Einfeld SL. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. J Autism Dev Disord. 2006;36(7):863–870. doi: 10.1007/s10803-006-0125-y. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder. Jama J Am Med Assoc. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 5.Einfeld SL, Tonge BJ, Gray K, Taffe J. Evolution of symptoms and syndromes of psychopathology in young people with mental retardation. Int Rev Res Ment Retard. 2006;33:247–265. doi: 10.1016/S0074-7750(06)33010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(8):694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL. Socioeconomic burden of subsyndromal depressive symptoms and major depression in a sample of the general population. Am J Psychiatry. 1996;153(11):1411–1417. doi: 10.1176/ajp.153.11.1411. [DOI] [PubMed] [Google Scholar]
- 8.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Merikangas KR, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hale WW, Raaijmakers Q, Muris P, van Hoof A, Meeus W. Developmental trajectories of adolescent anxiety disorder symptoms: A 5-Year prospective community study. J Am Acad Child Adolesc Psychiatry. 2008;47(5):556–564. doi: 10.1097/CHI.0b013e3181676583. [DOI] [PubMed] [Google Scholar]
- 11.Taylor JL, Seltzer MM. Changes in the autism behavioral phenotype during the transition to adulthood. J Autism Dev Disord. 2010;40(12):1431–1446. doi: 10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett V, Ronald A, Rijsdijk F, Happé F. Association of autistic-like and internalizing traits during childhood: A longitudinal twin study. Am J Psychiatry. 2010;167(7):809–817. doi: 10.1176/appi.ajp.2009.09070990. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DK, Maye MP, Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in Autism Spectrum Disorder. Am J Intellect Dev Disabil. 2011;116(5):381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an Autism Spectrum Disorder. J Autism Dev Disord. 2007;37(9):1735–1747. doi: 10.1007/s10803-006-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray K, Keating C, Taffe J, Brereton A, Einfeld S, Tonge B. Trajectory of behavior and emotional problems in autism. Am J Intellect Dev Disabil. 2012;117(2):121–133. doi: 10.1352/1944-7588-117-2.121. [DOI] [PubMed] [Google Scholar]
- 16.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlingt Vt Univ Vt Res Cent Child Youth Fam. 2001 [Google Scholar]
- 17.Achenbach TM, Rescorla LA. Manual for the ASEBA adult forms and profiles. Burlington, VT: University of Vermont; 2003. [Google Scholar]
- 18.Borthwick-Duffy SA, Lane KL, Widaman KF. Measuring problem behaviors in children with mental retardation: Dimensions and predictors. Res Dev Disabil. 1997;18(6):415–433. doi: 10.1016/s0891-4222(97)00020-6. [DOI] [PubMed] [Google Scholar]
- 19.Dekker MC, Koot HM, van der Ende J, Verhulst FC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. J Child Psychol Psychiatry. 2002;43(8):1087–1098. doi: 10.1111/1469-7610.00235. [DOI] [PubMed] [Google Scholar]
- 20.Wallander JL, Dekker MC, Koot HM. Psychopathology in children and adolescents with intellectual disability: measurement, prevalence, course, and risk. In: Glidden L, editor. International Review of Research in Mental Retardation. San Diego: Academic Press; 2003. pp. 93–134. [Google Scholar]
- 21.Embregts PJCM. Reliability of the Child Behavior Checklist for the assessment of behavioral problems of children and youth with mild mental retardation. Res Dev Disabil. 2000;21(1):31–41. doi: 10.1016/s0891-4222(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach TM, Edelbrock C. Child behavior checklist. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 23.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule— Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 24.Einfeld SL, Tonge BJ. The Developmental Behavior Checklist: The development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. J Autism Dev Disord. 1995;25(2):81–104. doi: 10.1007/BF02178498. [DOI] [PubMed] [Google Scholar]
- 25.Simonoff E, Jones CRG, Baird G, Pickles A, Happé F, Charman T. The persistence and stability of psychiatric problems in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2013;54(2):186–194. doi: 10.1111/j.1469-7610.2012.02606.x. [DOI] [PubMed] [Google Scholar]
- 26.Midouhas E, Yogaratnam A, Flouri E, Charman T. Psychopathology trajectories of children with Autism Spectrum Disorder: The role of family poverty and parenting. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1057–1065.e1. doi: 10.1016/j.jaac.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Mazefsky CA, Herrington J, Siegel M, et al. The role of emotion regulation in Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(7):679–688. doi: 10.1016/j.jaac.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Test Review: Behavior Rating Inventory of Executive Function. Child Neuropsychol. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 29.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2012:1–13. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 31.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63(6):694. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 32.Pandolfi V, Magyar CI, Dill CA. An initial psychometric evaluation of the CBCL 6–18 in a sample of youth with autism spectrum disorders. Res Autism Spectr Disord. 2012;6(1):96–108. doi: 10.1016/j.rasd.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotham K, Unruh K, Lord C. Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism. doi: 10.1177/1362361314536625. Published online ahead of print June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurtig T, Kuusikko S, Mattila ML, et al. Multi-informant reports of psychiatric symptoms among high-functioning adolescents with Asperger syndrome or autism. Autism Int J Res Pr. 2009;13(6):583–598. doi: 10.1177/1362361309335719. [DOI] [PubMed] [Google Scholar]
- 35.Einfeld S, Tonge B. [Accessed June 2014];Developmental Behavior Checklist information package. http://www.med.monash.edu.au/spppm/research/devpsych/download/dbc-info-package.pdf.
- 36.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4 (Version 1.1-6) [Accessed July 20, 2014]; http://CRAN.R-project.org/package=lme4. Published 2014.
- 37.R Core Team. R: A language and environment for statistical computing (Version 3.0.1) R Foundation for Statistical Computing website; [Accessed January 3, 2014]. http://www.R-project.org/ [Google Scholar]
- 38.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales: (Vineland II), Survey interview form/caregiver rating form. Livonia, MN: Pearson Assessments; 2005. [Google Scholar]
- 39.Schalock RL, Keith KD. Quality of Life Questionnaire. Columbus: IDS Publishing Corporation; 1993. [Google Scholar]
- 40.Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J Pers Soc Psychol. 1989;57(6):1069–1081. [Google Scholar]
- 41.Moos RH, Moos BS. Family environment scale manual. Washington, D.C.: Consulting Psychologists Press; 1994. [Google Scholar]
- 42.Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor JL, Henninger NA. Frequency and correlates of service access among youth with autism transitioning to adulthood. J Autism Dev Disord. 2015;45(1):179–191. doi: 10.1007/s10803-014-2203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi G, Wozniak J, Petty C, et al. Psychiatric comorbidity and functioning in a clinically referred population of adults with Autism Spectrum Disorders: A comparative study. J Autism Dev Disord. 2013;43(6):1314–1325. doi: 10.1007/s10803-012-1679-5. [DOI] [PubMed] [Google Scholar]
- 45.Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry. 2012;51(9):879–888. doi: 10.1016/j.jaac.2012.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. This figure shows observed Anxiety scores from the Achenbach System of Empirically-Based Assessment (ASEBA) measures plotted against participant age. Female data are plotted as black circles, and male data as red triangles. The figure contains linear regression lines (straight dashed) and smoothed non-parametric loess lines (curved solid) for both females (black) and males (red). Points within the gray shaded area reflect Adult Behavior Checklist (ABCL) scores as opposed to Child Behavior Checklist (CBCL) scores. Anxiety scores tended to increase with age, particularly among female participants.
S2. This figure shows observed Depressive symptom scores from the Achenbach System of Empirically-Based Assessment (ASEBA) measures plotted against participant age. Female data are plotted as black circles, and male data as red triangles. The figure contains linear regression lines (straight dashed) and smoothed non-parametric loess lines (curved solid) for both females (black) and males (red). Points within the gray shaded area reflect Adult Behavior Checklist (ABCL) scores as opposed to Child Behavior Checklist (CBCL) scores. Depressive scores tended to increase with age, particularly among female participants.


