Abstract
Vacuoles were isolated from protoplasts of Nicotiana glutinosa by the method of Mettler and Leonard (Plant Physiol 1979 64: 1114-1120) with minor modifications so that the number of intact protoplasts contaminating the vacuole preparation was reduced to less than 1% (by number). Isopycnic centrifugation of a [3H]choline-labeled, sonicated vacuole preparation on linear 5 to 40% sucrose gradients indicated that tonoplast vesicles equilibrated at a density of about 1.12 grams per cubic centimeter. When tonoplast vesicles were isolated on discontinuous sucrose density gradients substrate specific ATPase activity was not found to be associated with this membrane fraction. These results are discussed in terms of the energetics of ion transport through the tonoplast membrane.
Full text
PDF
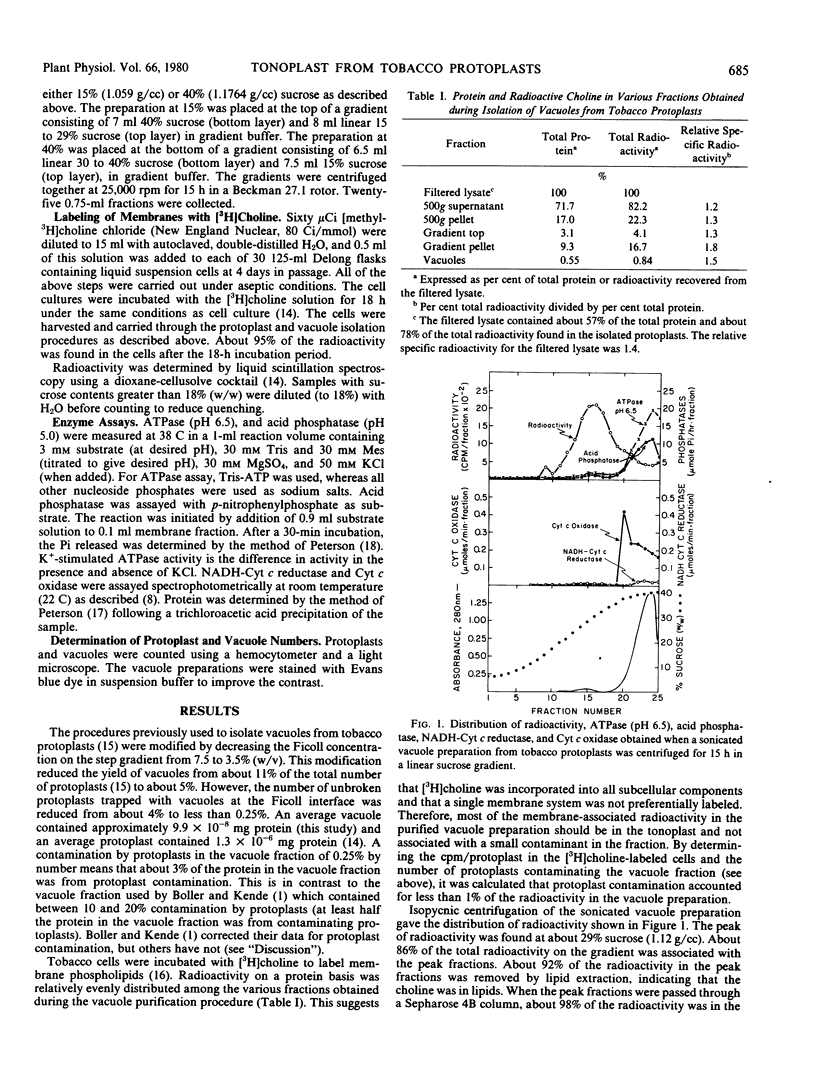
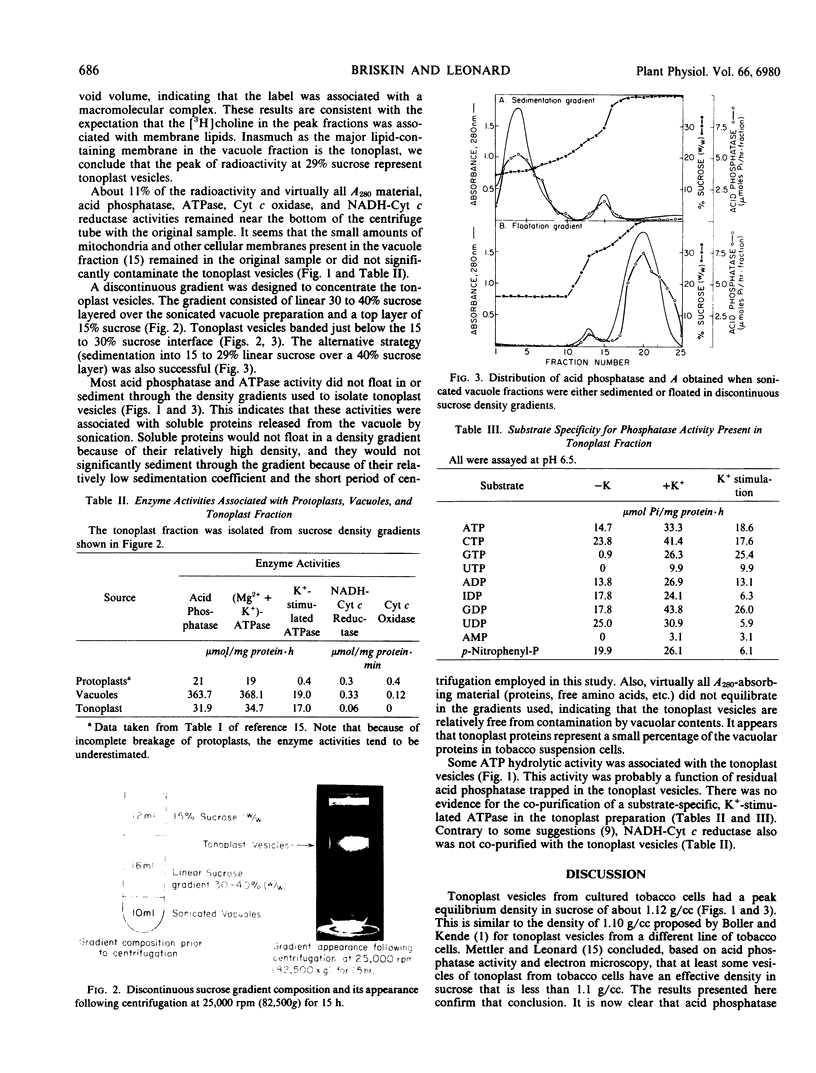

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubmeyer C., Melanson D., Duncan I., Spencer M. Oxidative phosphorylation in pea cotyledon submitochondrial particles. Plant Physiol. 1979 Nov;64(5):757–762. doi: 10.1104/pp.64.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L. Membrane transport of sugars and amino acids in isolated protoplasts. Plant Physiol. 1978 Apr;61(4):593–596. doi: 10.1104/pp.61.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M., Reinhold L., Michaeli D. Direct evidence for a sugar transport mechanism in isolated vacuoles. Plant Physiol. 1979 Jul;64(1):61–64. doi: 10.1104/pp.64.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplified method for analysis of inorganic phosphate in the presence of interfering substances. Anal Biochem. 1978 Jan;84(1):164–172. doi: 10.1016/0003-2697(78)90495-5. [DOI] [PubMed] [Google Scholar]
- Saunders J. A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979 Jul;64(1):74–78. doi: 10.1104/pp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]




