Abstract
Background and Aims Conservation of the genetic diversity afforded by recalcitrant seeds is achieved by cryopreservation, in which excised embryonic axes (or, where possible, embryos) are treated and stored at temperatures lower than −180 °C using liquid nitrogen. It has previously been shown that intracellular ice forms in rapidly cooled embryonic axes of Acer saccharinum (silver maple) but this is not necessarily lethal when ice crystals are small. This study seeks to understand the nature and extent of damage from intracellular ice, and the course of recovery and regrowth in surviving tissues.
Methods Embryonic axes of A. saccharinum, not subjected to dehydration or cryoprotection treatments (water content was 1·9 g H2O g−1 dry mass), were cooled to liquid nitrogen temperatures using two methods: plunging into nitrogen slush to achieve a cooling rate of 97 °C s−1 or programmed cooling at 3·3 °C s−1. Samples were thawed rapidly (177 °C s−1) and cell structure was examined microscopically immediately, and at intervals up to 72 h in vitro. Survival was assessed after 4 weeks in vitro. Axes were processed conventionally for optical microscopy and ultrastructural examination.
Key Results Immediately following thaw after cryogenic exposure, cells from axes did not show signs of damage at an ultrastructural level. Signs that cells had been damaged were apparent after several hours of in vitro culture and appeared as autophagic decomposition. In surviving tissues, dead cells were sloughed off and pockets of living cells were the origin of regrowth. In roots, regrowth occurred from the ground meristem and procambium, not the distal meristem, which became lethally damaged. Regrowth of shoots occurred from isolated pockets of surviving cells of peripheral and pith meristems. The size of these pockets may determine the possibility for, the extent of and the vigour of regrowth.
Conclusions Autophagic degradation and ultimately autolysis of cells following cryo-exposure and formation of small (0·2–0·4 µm) intracellular ice crystals challenges current ideas that ice causes immediate physical damage to cells. Instead, freezing stress may induce a signal for programmed cell death (PCD). Cells that form more ice crystals during cooling have faster PCD responses.
Keywords: Acer saccharinum, Aceraceae, autophagy, cooling rate, cryopreservation, embryonic axes, intracellular ice, light microscopy, mechanical stress, programmed cell death, recalcitrant seed, silver maple, transmission electron microscopy, TEM
INTRODUCTION
Ex situ conservation of genetic diversity within species or ecotypes is most efficiently accomplished by preserving sexual propagules – seeds and pollen – from populations (Tanksley and McCouch, 1997). The recalcitrant nature of seeds from some species precludes ex situ conservation using ‘conventional’ storage practices currently recommended for dry desiccation-tolerant (orthodox) seeds (FAO, 2013). Conservation of the genetic diversity afforded by recalcitrant seeds is achieved by cryopreservation, in which excised embryonic axes (or, where possible, embryos) are treated and stored at temperatures lower than −180 °C using liquid nitrogen (LN) as the cryogen (Engelmann, 2011; Berjak and Pammenter, 2014; Wesley-Smith et al., 2014).
The large size, irregular geometry and high water content of recalcitrant-seeded explants promote intracellular ice formation at subfreezing temperatures, an event regarded as invariably lethal (Wesley-Smith et al., 2014). Why ice formation is lethal remains an unanswered question in cryobiological research, with two major schools of thought relating to membrane damage (Steponkus et al., 1993) or enigmatic damage associated with compression of cellular constituents reminiscent of desiccation stress (Ishiguro and Rubinsky, 1994, 1998; Hubel et al., 2007; Saragusty et al., 2009).
This paper represents the second in a two-part series that investigates the damaging nature of intracellular ice. In the previous paper, we demonstrated that ice crystal formation was unavoidable when hydrated cells of embryonic axes of Acer saccharinum (silver maple) were cooled to LN temperatures at 97 or 3 °C s−1 (Wesley-Smith et al., 2014). When crystals were small (≤0·4 µm in diameter), preservation of cellular structures was excellent, belying the notion that ice crystals disrupted cell organization and ultrastructure. Despite excellent structural preservation immediately after cooling, survival of axes ranged between 50 and 90 % and growth of shoot tissues was severely impaired, indicating significant and tissue-specific damage (Wesley-Smith et al., 2014). We suggested that damage occurred upon thawing cells and that the low recovery of the plumule was associated with the smaller but more numerous ice crystals that formed there compared with in the radicle, which showed relatively constant size and density of ice crystals regardless of cooling rate (Wesley-Smith et al., 2014).
In this paper, we investigate the thawing and recovery process of embryonic axes of A. saccharinum to determine the stage at which cellular damage could be detected and the nature of the damage. We use microscopy studies to track the progress – or fate – of hydrated tissues and cells that experienced known levels of ice formation. We expected to see evidence of cellular disruption immediately upon thawing and greater structural damage in shoot tissues compared with root tissues (Wesley-Smith et al., 2014).
MATERIALS AND METHODS
Seed material
Browning samaras (a sign of maturity) of Acer saccharinum L. were harvested from a single tree in mid-May in Fort Collins, Colorado, and stored at 4 °C in vented plastic bags for up to 1 week. Batches of embryonic axes were excised from seeds for water content determinations, cryogenic cooling and post-cryogenic survival assessment, as previously described (Wesley-Smith et al., 2014). This set of experiments used axes that received no drying treatments; mean water content was 1·9 ± 0·3 g H2O g−1 d. wt. Water content of individual axes was determined gravimetrically after oven-drying at 95 °C to constant weight and expressed as g H2O per g dry mass (g g−1).
Survival and growth of axes was assessed following 4 weeks in vitro using at least 40 axes per treatment, as reported previously (Wesley-Smith et al., 2001b, 2014). Briefly, thawed axes were held on moist filter paper for 30–60 min in the dark prior to surface decontamination and plating onto Woody Plant Medium (Lloyd and McCown, 1980) solidified with 0·7 % (w/v) agar. Petri plates were maintained in the dark at 25 °C for 2 d, and gradually introduced to a 16:8-h light/dark photoperiod over 2 d. Surviving axes were scored as ‘normal’ when the radicles doubled in length and shoots expanded and turned green, or ‘abnormal’ whenever axes failed to develop shoots, roots appeared stunted or developed callus.
Cryo-exposure treatments
Hydrated embryonic axes were cooled at 97 ± 41 °C s−1 by plunging individual axes, each affixed to a small metal specimen support, into sub-cooled nitrogen (−210 °C) using a spring-loaded device (Wesley-Smith et al., 2004a). A slower cooling treatment of 3·3 °C s−1 used the sample holder of a differential scanning calorimeter (DSC7; Perkin Elmer, Norwalk CT, USA); axes cooled to −160 °C were immediately transferred to LN. In both cooling treatments, axes were maintained in LN for at least 30 min.
Axes retrieved from LN were immediately thawed in water at 40 °C for 1 min (177 ± 52 °C s−1) (survival and microscopy studies) or using a programmed warming rate of 0·08 °C s−1 (5 °C min−1) in the DSC7 (survival studies only).
Cooling and warming rates were calculated from time-temperature data recorded for a subset of axes impaled with fine-gauge (0·075 mm × 0·12 mm) bare wire, type T thermocouples (Omega Engineering Inc., Stamford, CT, USA). Temperature was recorded at millisecond intervals.
Processing for microscopy
For each recovery time, five randomly selected axes were sampled immediately after 1 min in the rapid warming treatment (in 40 °C water) (0 min), after 30 and 60 min on filter paper, and after 2·5, 6, 12, 24, 48 and 72 h in culture. Axes were halved transversely into root and shoot segments which were immersed with agitation in a solution of 2·5 % (v/v) glutaraldehyde in 0·1 m Sørensen phosphate buffer, pH 7·2, at room temperature. Specimens were rinsed in the same buffer, then post-fixed in 0·5 % (w/v) aqueous osmium tetroxide for 1 h, rinsed and dehydrated in a graded acetone series after which they were gradually infiltrated with a low-viscosity epoxy resin (Spurr, 1969) for 16 h and finally polymerized in fresh resin at 70 °C for 10 h. Semi-thin (1 µm) and ultra-thin sections were cut using an Ultracut E ultramicrotome (Leica, Vienna, Austria), the former being mounted on slides and stained with toluidine blue (1 %, w/v, in a 60:40 mixture of 1 % sodium bicarbonate and glycerol) and the latter collected on 200-mesh copper grids and contrasted by consecutive exposure to saturated aqueous uranyl acetate and lead citrate (Reynolds, 1963). Semi-thin sections were viewed with a Nikon Biophot microscope (Nikon, Tokyo, Japan) and ultra-thin sections with a JEOL JEM 1010 (JEOL, Tokyo, Japan). All images were captured photographically and digitally. In addition to specimens processed after cryo-exposure, newly excised axes, not subjected to experimental manipulation, were similarly processed and viewed.
RESULTS
Newly harvested axes had high water contents (1·9 g H2O g−1 d. wt) and germinated within 2–3 d of culture (data not shown). The ultrastructure of the distal cells of the root and shoot poles of axes provided evidence of high metabolic activity, with abundant polysomes, endoplasmic reticulum, mitochondria and plastids (Fig. 1A, B). Root cells contained many plastids and few vacuoles; shoot cells had fewer plastids and prominent large vacuoles.
Fig. 1.

Features of cells typical of newly excised embryonic axes of Acer saccharinum (water content 1·9 ± 0·3 g g−1). (A) Ultrastructural detail of the cytomatrix of a meristematic cell of the radicle showing abundant polysomes (arrow) and a typical plastid containing both starch (s) and plastoglobuli (pg). m, mitochondrion. (B) Cells within the corpus region of the shoot showing a prominent vacuole (v). ER, endoplasmic reticulum; m, mitochondrion; Nu, nucleus; p, plastid.
Undried embryonic axes of A. saccharinum were cooled to LN temperatures using faster and slower methods (97 and 3·3 °C s−1, respectively) and then warmed using faster and slower methods (177 and 0·08 °C s−1, respectively). Upon thawing, 50–90 % of radicles survived and up to 75 % grew; in contrast, fewer than 10 % of plumules survived (Fig. 2). The combination of slower cooling and faster warming gave greatest recovery, while faster cooling and slower warming was invariably lethal to all tissues and axes became necrotic within hours after thawing. Microscopical assessments were not conducted on the slowly warmed axes.
Fig. 2.

Survival and growth of undried (water content 1·9 ± 0·3 g g−1) A. saccharinum axes that were exposed to LN using rapid [plunge-cooled into nitrogen slush (−210 °C) at 97 °C s−1] and slower (programmed cooling using the Perkin Elmer DSC7; 3 °C s−1) cooling methods and rapid (plunge into 40 °C water; 177 °C s−1) and slower (programmed warming using the Perkin Elmer DSC7; 0·08 °C s−1) warming methods. Normal regrowth is distinguished from stunted growth or callus formation. Data for rapid warming treatments are repeated from Wesley-Smith et al. (2014).
Recovery in axes cooled at 97 °C s−1 (plunge-cooled)
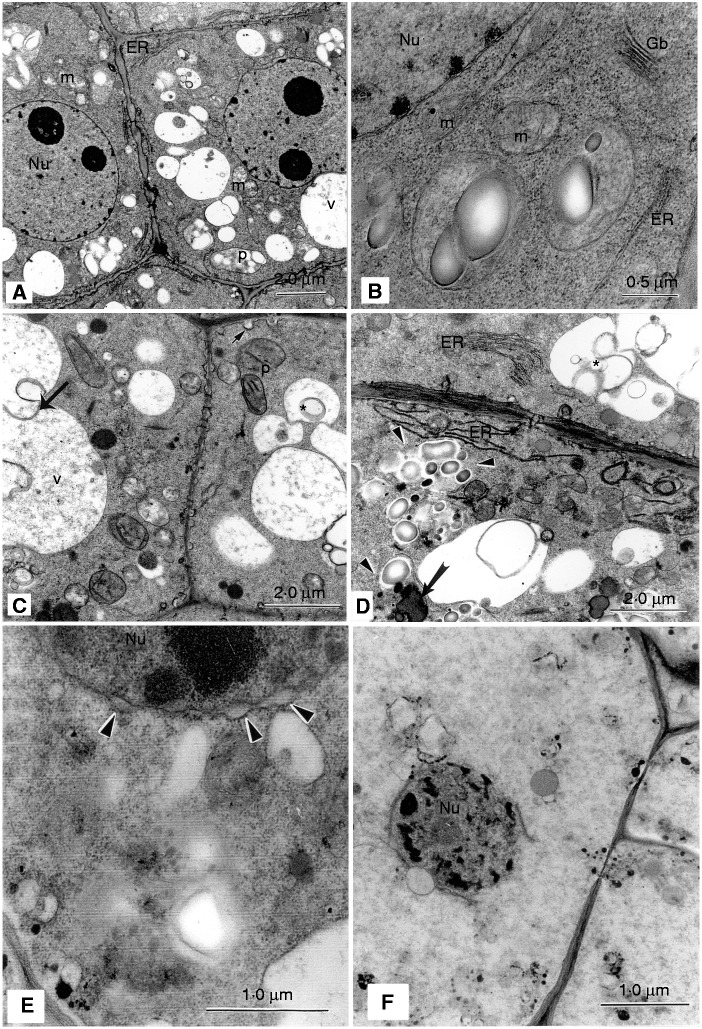
Seconds after rapid thawing, radicle meristem cells from axes that had been plunge-cooled into nitrogen slush (faster cooling) appeared little changed (Fig. 3A) from their counterparts in newly excised axes. The cells were characterized by structurally intact starch-containing plastids, mitochondria showing retention of cristae, profiles of endoplasmic reticulum, well-defined nuclear envelopes, Golgi bodies and intact plasmalemma closely associated with the cell wall (Fig. 3A, B). However, unlike radicle cells from control axes that had few and small vacuoles, vacuolation appeared more substantial in radicle cells of cryo-exposed axes. The expanded vacuoles in cryo-exposed axes showed no signs of tonoplast damage (Fig. 3A). In a few cases, radicle cells contained curiously drawn out mitochondria, perhaps indicating a level of compression (Fig. 3B). After 2·5 h in vitro, distal radicle cells from thawed axes showed a marked degree of vacuolar fusion; the occurrence of intravacuolar membranous elements and granular material provided evidence of autophagic activity (Fig. 3C). A further feature of these cells was the formation of many plasmalemma invaginations (Fig. 3C). When sampled after 6 h in vitro, ongoing autophagic activity and proliferation of endoplasmic reticulum were evident in radicle meristem cells (Fig. 3D). There was also concomitant evidence of deterioration, as shown by advanced dissolution of plastid membranes and coalescence of previously discrete lipid bodies (Fig. 3D).
Fig. 3.
Transmission electron micrographs of cells of undried (water content 1·9 ± 0·3 g g−1) A. saccharinum axes that were rapidly plunge-cooled into nitrogen slush (−210 °C), then rapidly warmed and sampled after various times in vitro. (A–D) Radicle (distal) meristem; (E, F) corpus cells of the shoot meristem. (A, B) 0 min: when sampled immediately after warming, cells of the distal meristem showed little evidence of damage consequent upon the widespread ice crystallization (evident in freeze-fractured and freeze-substituted material sampled in the frozen state; fig. 4A-C in Wesley-Smith et al., 2014). Change was apparent in the marked extent of vacuolation (V; A) and occasionally drawn-out mitochondria (asterisk; B). ER, endoplasmic reticulum; Gb, Golgi body; m, mitochondrion; p, plastid. (C) 2·5 h: distal cells of the radicle appeared intact 2·5 h after rapid warming while showing fusion of vacuoles (large arrow) which contained granular and membranous material (asterisk) indicating autophagic activity. Invagination of the plasmalemma (small arrow, top right) was a further feature of these cells. p, plastid. (D) 6 h: elongated profiles of endoplasmic reticulum (ER) were a marked characteristic of cells of the distal meristem 6 h after warming, as was continuing evidence of autophagy (asterisk). However, deteriorative changes were also apparent, as indicated by plastid breakdown (arrowheads) and fusion of lipid bodies (arrow). (E) 30 min: a cell of the shoot meristem showing marked signs of deterioration which was a general feature within 30 min of warming. Localized distension of the nuclear envelope (arrowheads) is evident as is the diffuse appearance of the plasmalemma and intracellular membranes generally. (F) 2·5 h: extensive lysis of many cells had occurred where virtually no intracellular structures could be identified, other than severely degraded nuclei (Nu) as illustrated.
Like radicle cells, those of the shoot apical meristem from plunged-cooled axes appeared intact and undamaged immediately after thawing (not illustrated). However, within just 30 min, many cells of the shoot apical meristem showed marked signs of deterioration (Fig. 3E). The nuclear envelope was locally distended, and membranes, including the plasmalemma, had become diffuse. After 2·5 h, many cells within the shoot apex had completely lysed (Fig. 3F).
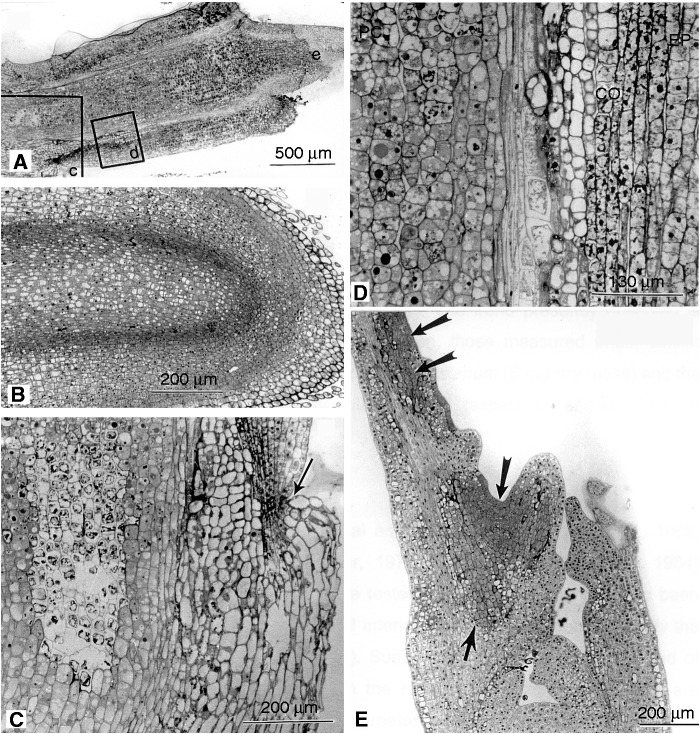
After 24 and 48 h of culture, surviving axes that had been plunge-cooled and rapidly thawed were viewed using light microscopy to reveal the fate of distal cells of the radicle and origin of new growth, which culminated in root development [noting that there was no shoot development (Fig. 2) in view of the early lysis of apical shoot cells (Fig. 3F)]. Within 24 h in vitro, while the distal meristem cells appeared compressed and inactive, there was marked activity of the pre-existing ground meristem and procambium (Fig. 4A). Examination of the more proximal regions of the hypocotyl 24 h after warming showed that many pith cells appeared deteriorated, but cells of the cortex were relatively intact (Fig. 4E). After 48 h in vitro, voids were observed in the hypocotyl of axes presumably in regions where cells had been extensively damage (Fig. 4B, C). Toward the distal end of the root, new growth displaced the root cap and distil meristem initials (Fig. 4B, C) and a schism developed in the plane of the shrunken, necrotic cells of the meristem (Fig. 4D).
Fig. 4.
Light micrographs of germinating axes after plunge-cooling as indicated by initiation of radicle growth in vitro 24 and 48 h after warming. (A) 24 h: median longitudinal sections revealed that growth was initiated by cells of the ground meristem (GM) and procambium (PC) but not from the distal meristem (DM), which appeared moribund. (B) 48 h: displacement of the root cap and some cells of the distal meristem (region of darkened tissue, lower right) by the renewed growth was evident within 48 h after warming, while distinct voids (asterisk) could be seen in other tissues. (C) 48 h: regional damage as evidenced by voids (asterisks) was present in the pith, indicating that localized irreparable tissue damage had occurred, but not to the extent that affected active growth of the root as evidenced by the intensely stained region proximal to the root cap (boxed region labelled d, shown at higher magnification in D). (D) 48 h: details showing displacement of the root cap and cells of the distal meristem by growth originating from the proximal ground meristem and procambium. Note the distended cells (upper right) which become disassociated (lower right) from the regenerating root tip. (E) 24 h: examination of the hypocotyl revealed that the pith cells (Pi) were more prone to freezing damage than were those of the cortex (CO), presumably leading to the voids illustrated above. EP, epidermis.
Recovery in axes cooled at 3 °C s−1 (programmed cooling rate)
Development of damage and regrowth of cells of axes that had been cooled at about 3 °C s−1 using the DSC7 followed similar patterns to the plunge-cooled cells. Cells of the distal radicle appeared little changed, compared with cells from untreated axes, when sampled immediately after warming (Fig. 5A). Slight abnormalities included the drawn-out mitochondria, which were also observed in radicle cells of the plunge-cooled axes (Fig. 5A). After 2·5 h in vitro, autophagic vacuoles sequestering miscellaneous structures and somewhat diluted ground cytoplasm were observed in distal radicle cells (Fig. 5B). After 6 h in vitro, damaged intracellular components and enhanced autophagic activity were evident in these cells (Fig. 5C).
Fig. 5.
Ultrastructure of A. saccharinum axes (water content 1·9 ± 0·3 g g−1) after cooling at 3·3 °C s−1 and rapid warming, sampled at various times in vitro. (A–C) Radicle, (D, E) shoot. (A) 0 min: radicle cells generally appeared little changed by cryo-exposure, relative to those of newly excised axes processed for microscopy (cf. Fig. 1). One curious feature was the drawn-out appearance of some mitochondria (asterisks). ER, endoplasmic reticulum; m, mitochondrion; Nu, nucleus; p, plastid; v, vacuole. (B) 2·5 h: early phases of autophagy, suggested to be initiated by cytolysome formation, were evidenced by the occurrence of autophagic vacuoles (AV). ER, endoplasmic reticulum; m, mitochondrion. (C) 6 h: enhanced removal of damaged intracellular components (arrowed) was evident by the appearance of cytolysomes/autophagic vacuoles such as the multilamellar body illustrated (asterisk). Nu, nucleus; p, plastid. (D) 2·5 h: the extent of damage within a short interval after warming varied among cells in shoot tissue, where apparently surviving cells were contiguous with lysing and lysed neighbours. (E) 6 h: localized distension of the nuclear envelope (arrowheads), tonoplast damage (arrow) and the diffuse appearance of the plasmalemma were features in corpus cells which, although manifested later, showed commonality with such cells in plunge-cooled axes (cf. Fig. 3E). cw, cell wall; Nu, nucleus; v, vacuole.
In contrast to plumule cells from plunged-cooled axes, some cells of shoot apices of axes that were cooled at the slower rate showed signs of integrity. After 2·5 h in vitro, there was a mosaic of variably degraded and relatively intact cells (Fig. 5D). After 6 h in vitro, many corpus cells of the shoot apex showed localized distension of the nuclear envelope, and tonoplast and plasmalemma abnormality (Fig. 5E). This level of cell disruption was apparent in faster cooled counterparts after just 30 min.
Histological examination of shoot and hypocotyl tissues (Fig. 6A, C–E) and developing roots (Fig. 6B) of germinating axes after 72 h in vitro following warming revealed variable responses to LN exposure among the cells. Root development proceeded normally (Fig. 6B), with no apparent ill-effects to cells of the distal meristem, in contrast to the situation following rapid cooling in which subcellular damage and autophagic activity was observed (Fig. 3C, D). In pith cells, there were localized regions of necrosis surrounded by intact tissue and occasional constrictions of the hypocotyl surface were observed (Fig. 6C). Cortical cells appeared to have been less resilient to damage as a consequence of slower cooling (Fig. 6D) than were their counterparts in axes that were plunge-cooled. The histology of shoot apices revealed pockets of surviving cells apparently originating from peripheral and pith meristems (Fig. 6E). If there were a sufficient number of neighbouring surviving cells, we suggest shoot development would be possible.
Fig. 6.
Light micrographs of germinating axes (water content 1·9 ± 0·3 g g−1) that were cooled at 3·3 °C s−1, rapidly warmed and maintained in vitro for 72 h. (A) Median longitudinal section of the shoot segment of an axis. The demarcated regions (c, d and e) are shown at high magnification in C, D and E. (B) The distal segment of a germinating axis showing apparently normal root development, unlike the situation in plunge-cooled axes. (C) Necrotic regions within the pith of slowly cooled axes were a feature of commonality with those that had been plunge-cooled. Occasional superficial constrictions (arrow) were associated with uneven surface expansion. (D) Cells comprising the procambial cylinder (PC) appeared more resistant to damage upon slow cooling than did those of the cortex (CO). EP, epidermis. (E) Pockets of cells (arrowed) in the shoot, seemingly of peripheral and pith meristem origin, appeared to have retained viability and, if constituting a critical mass, would have had the capacity for new shoot growth in the small proportion of axes that survived to form apparently normal seedlings.
DISCUSSION
We report the process of recovery or the development of damage following cryo-exposure of mature embryonic axes excised from Acer saccharinum seeds. Cells that were not exposed to LN appeared typical for mature, unstressed recalcitrant embryonic axes; plentiful organelles gave the appearance of high metabolic activity (Fig 1), with little evidence of intracellular de-differentiation that is generally observed in cells of mature orthodox seeds (Farrant et al., 1997). Our observations are consistent with the concept of unpunctuated metabolic activity in recalcitrant embryos between the maturation and germination stages (Berjak and Pammenter, 2008; Pammenter and Berjak, 2014).
A previously published electron microscopy study of freeze-fractured and freeze-substituted hydrated A. saccharinum axes showed that intracellular ice formation was unavoidable in the cells during cooling (Wesley-Smith et al., 2014). The excellent preservation of cell structures, despite the widely dispersed small ice crystals, suggests that the lethal effects of LN exposure are manifested during and after warming. Thawing was, indeed, a critical step with high tissue and axis mortality occurring in tissues warmed at a slower rate (Fig. 2), probably because there was more time for the ice crystals formed upon cooling to grow to larger, lethal dimensions during warming. The faster-cooled cells of the shoot apical meristem would be particularly prone to damage during thawing because they had many more crystals (Wesley-Smith et al., 2014). It is important to emphasize that the ‘slower’ cooling treatment used in this study (3·3 °C s−1 or about 200 °C min−1, which reflects the upper limits of cooling rate to LN temperatures possible in the Perkin Elmer DSC7) is fast cooling by most cryobiology standards.
Based on the survival data (Fig. 2), we can divide recovery and growth of cells following cryoexposure into three categories: (1) radicle cells cooled at 3 °C s−1 showing high recovery; (2) radicle cells cooled at 97 °C s−1 or plumule cells cooled at 3 °C s−1 – both showing some survival but a high incidence of abnormal growth; and (3) plumule cells cooled at 97 °C s−1 showing no signs of survival. In all three categories immediately after thawing cell structures appeared intact and similar to cells of fresh axes (Figs 3A, B and 5A). Increased degrees of vacuolation and slightly misshapen mitochondria appeared as the only detectable symptoms of stressed cells.
Affected cells in cryo-exposed axes developed symptoms of stress, progressing towards mortality in a series of canonical steps. First, vacuoles appeared enlarged and mitochondria compressed (Fig. 3A, B) and concomitant occurrences of small, but frequent invaginations of the plasmalemma could indicate elimination of damaged areas (Figs 3C and 5E), although freeze-fracture electron microscopy of cryo-exposed cells revealed minor and infrequent irregularities in the membrane (Wesley-Smith et al., 2014). The nuclear envelope also appeared regionally distended and chromatin became highly condensed (Fig. 5E). Further swelling and fusion of vacuoles and accumulation of intravacuolar material indicated active autophagy (Figs 3C, D and 5B, C). The endoplasmic reticulum appeared to increase in extent, probably a precursor of cytolysome formation, where specialized endoplasmic reticulum comes to surround and sequester organelles and cytoplasm (Matile, 1975; Lamb and Berjak, 1981). Eventually cell organelles appeared to degrade: plastid membranes (as observed in radicle cells) disintegrated leaving starch grains ‘scattered’ in the ground cytoplasm, and lipid bodies appeared to coalesce (Fig. 3D). Sooner or later autolysis occurred (Fig. 3F). Although the pattern of intracellular dismantling was similar across root and shoot cells cooled to LN temperature at different rates, the onset of cell lysis and number of cells involved varied. Most cells of shoot apices in rapidly cooled axes (no survival) showed extensive autophagy within 30 min and lysed within 2·5 h, while progress towards cell lysis in the distal radical cells or shoot cells of slower cooled axes took between 6 and 12 h.
Similar patterns of increased vacuolation and proliferation of endoplasmic reticulum during recovery have been reported among diversely stressed specimens and have been interpreted as increasing the capacity for autophagic removal of damaged intracellular constituents (Wesley-Smith et al., 2001a; Sershen et al., 2012a, b). Freezing injury has more typically been characterized as a necrotic response resulting from massive cell injury (van Doorn et al., 2011). However, the self-mediated lysis observed here has similar characteristics to programmed cell death (PCD). Desiccation-stressed cells also express metabolism, suggesting oxidative cascades or PCD upon rehydration (Kranner et al., 2010). In addition, mechanical compression within cells, which is an hypothesized effect of freezing stress, can trigger PCD in animal tissues (Loening et al., 2000; Hunter et al., 2002) and affects morphology of plants (Robinson et al., 2013). Consolidation of cytoplasm by water removal or an advancing ice front may result in compressive stresses (Ishiguro and Rubinsky, 1994, 1998; Hubel et al., 2007; Saragusty et al., 2009), which induce subtle disruption of the spatial organization within the cytoplasm to elicit signals for rapid metabolism in response to environmental cues (Hyman and Simons, 2012). Permealization of the outer mitochondrial membrane (MOMP) appears to be an initial checkpoint that irreversibly induces PCD in animal cells (Danial and Korsmeyer, 2004). Altered mitochondria were noted immediately after thawing A. saccharinum axes (Fig 3B); but it is difficult to attribute this subtle change to a profound effect or to say the drawn-out shape is symptomatic of MOMP.
Delayed expression of cell trauma in cryo-exposed axes is a major discovery consistent with PCD-like responses. In animal cells, time from stress to MOMP and from MOMP to autolysis can vary from hours to days; but the cell’s fate is sealed within 10 min after MOMP (Green, 2005). The time sequence observed in this study is consistent with general reports and suggests that the speed of activation of the PCD signal corresponds to the number of intracellular ice crystals formed upon cooling. Following the signal, A. saccharinum embryo cells appeared to be actively involved in dismantling constituents, and the speed at which this occurs is inversely correlated with the degree of recovery.
Susceptibility to damage seemed to be cell-type specific and may be a consequence of high vacuolation or differentiation of some cells, such as those constituting parenchyma (Volk and Caspersen, 2007). Light microscopy of surviving A. saccharinum axes showed voids (Fig. 4C) or groups of necrotic cells in the pith (Fig. 6C) of the hypocotyls. Similarly, the pith parenchyma was shown to be a major site of injury of somatic embryos of Theobroma cacao processed for cryopreservation by encapsulation-dehydration (Fang and Wetten, 2011). The markedly greater sensitivity of cells in shoot apices compared with those of the root tips has been noted in cryopreservation trials with recalcitrant embryonic axes of many species (Wesley-Smith et al., 2004b; Perán et al., 2006; Engelmann, 2011; Hajari et al., 2011; Normah et al., 2011; Berjak and Pammenter, 2014). The propensity for shoot formation in A. saccharinum axes depended on survival of groups of the relatively smaller cells of the peripheral and pith meristems (Fig. 6E) rather than those of the tunica or central corpus of the shoot apical meristem.
Within a few hours after thawing, meristems of cryo-exposed A. saccharinum axes appeared as a mosaic of damaged cells contiguous with others in which deterioration seemed minimal (Fig. 5D). As shown here and in other studies, the topography of cell survival in shoot and root apices is not necessarily uniform and resumption of growth appears to depend on a critical mass of contiguous surviving cells. New root growth in A. saccharinum embryonic axes originated from the ground meristem and procambium cells (Fig. 4A), displacing the root cap along a scission zone afforded by the moribund distal meristem (Fig. 4B–D). A similar pattern of renewed growth and sloughing of distal tissues was described in roots of Pisum sativum seedlings following cryo-exposure (Berjak et al., 1995; Wesley-Smith et al., 1995). Regeneration was mainly from leaf primordium regions in shoot apices of Solanum tuberosum (Kaczmarczyk et al., 2008) and from small groups of cells in the meristematic dome and primordia in PVS2-exposed meristems of Musa species (Helliot et al., 2003). In moderately damaged shoot tips of Dioscorea alata cryo-exposed following encapsulation-dehydration, basal cells did not survive, but other cells of the meristematic zone did (Barraco et al., 2014). Survival of groups of small meristematic cells, each functioning independently, may explain development of multiple shoots in recovering Mentha × piperita plants (Volk and Caspersen, 2007).
CONCLUDING COMMENTS
Cells of fully hydrated embryonic axes of Acer saccharinum showed virtually no signs of stress immediately following exposure to LN at relatively fast cooling that resulted in small intracellular ice crystals. However, within hours after a rapid thawing treatment, cells underwent canonical steps of autophagic dismantling and ultimately autolysis, with faster deterioration of larger blocks of cells observed in shoot, compared with root, apices. Cell lysis resulted in isolated regions of living cells from which regrowth occurred if a threshold number of cells survived. Regrowth was rarely from the original apical meristems, but usually from the ground meristem and procambium in roots and occurred only infrequently from pockets of surviving peripheral and pith meristems in shoots. Our study suggests that the small intracellular ice crystals did not cause massive damage to cell structures, but somehow signalled metabolically driven PCD leading to tissue atrophy.
ACKNOWLEDGEMENTS
This work was supported by funding from the United States Department of Agriculture-Agricultural Research Service (USDA-ARS) and the National Research Foundation (South Africa). We thank Lisa Hill and Jennifer Crane for expert technical assistance. We acknowledge the insightful and knowledgeable comments made by the anonymous reviewers. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Barraco G, Sylvestre I, Collin M, Escoute J, et al. 2014. Histological analysis of yam (Dioscorea alata) shoot tips cryopreserved by encapsulation-dehydration. Protoplasma 251: 177–189. [DOI] [PubMed] [Google Scholar]
- Berjak P, Pammenter NW. 2008. From Avicennia to Zizania: seed recalcitrance in perspective. Annals of Botany 101: 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjak P, Pammenter NW. 2014. Cryostorage of germplasm of tropical recalcitrant-seeded species: approaches and problems. International Journal of Plant Sciences 175: 29–39. [Google Scholar]
- Berjak P, Mycock DJ, Watt P, Wesley-Smith J, Hope B. 1995. Cryostorage of pea (Pisum sativum L.). In: Bajaj YPS, ed. Biotechnology in agriculture and forestry. Berlin: Springer Verlag, 292–307. [Google Scholar]
- Danial NN, Korsmeyer SJ. 2004. Cell death: critical control points. Cell 116: 205–219. [DOI] [PubMed] [Google Scholar]
- Engelmann F. 2011. Cryopreservation of embryos: an overview. In: Thorpe TA, Yeung EC, eds. Plant embryo culture: methods and protocols. Methods in Molecular Biology Series. Totowa NJ: Humana Press, 155–184. [DOI] [PubMed] [Google Scholar]
- Fang J-Y, Wetten A. 2011. Importance of structural integrity of somatic embryos for long-term cryopreservation of cocoa (Theobroma cacao L.) germplasm. African Journal of Agricultural Research 6: 3954–3961. [Google Scholar]
- FAO. 2013. Genebank standards for plant genetic resources for food and agriculture . Rome: FAO. [Google Scholar]
- Farrant JM, Pammenter NW, Berjak P, Walters C. 1997. Subcellular organization and metabolic activity during the development of seeds that attain different levels of desiccation tolerance. Seed Science Research 7: 135–144. [Google Scholar]
- Green DR. 2005. Apoptotic pathways: ten minutes to dead. Cell 121: 671–674. [DOI] [PubMed] [Google Scholar]
- Hajari E, Berjak P, Pammenter NW, Watt MP. 2011. A novel means for cryopreservation of the recalcitrant-seeded species, Ekebergia capensis. CryoLetters 32: 308–316. [PubMed] [Google Scholar]
- Helliot B, Swennen R, Poumay Y, Frison E, Lepoivre P, Panis B. 2003. Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Reports 21: 690–698. [DOI] [PubMed] [Google Scholar]
- Hubel A, Darr TB, Chang A, Dantzig J. 2007. Cell partitioning during directional solidification of trehalose solutions. Cryobiology 55: 182–188. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Imler SM, Malaviya P, Nerem RM, Levenston ME. 2002. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials 23: 1249–1259. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Simons K, 2012. Beyond oil and water – phase transitions in cells. Science 337: 1047–1049. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Rubinsky B. 1994. Mechanical interactions between ice crystals and red blood cells during directional solidification . Cryobiology 31: 483–500. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Rubinsky B. 1998. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. International Journal of Heat and Mass Transfer 41: 1907–1915. [Google Scholar]
- Kaczmarczyk A, Rutten T, Melzer M, Keller ERJ. 2008. Ultrastructural changes associated with cryopreservation of potato (Solanum tuberosum L.) shoot tips. CryoLetters 29: 145–156. [PubMed] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. 2010. What is stress? Concepts, definitions and applications in seed science. New Phytologist 188: 655–673. [DOI] [PubMed] [Google Scholar]
- Lamb JM, Berjak P. 1981. A unifying view of vacuolar ontogeny from studies on the root cap of Zea mays L. South African Journal of Science 77: 120–125. [Google Scholar]
- Lloyd G, McCown B. 1980. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proceedings of the International Plant Propagator's Society 30: 421–427. [Google Scholar]
- Loening AM, James IE, Levenston ME, et al. 2000. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Archives of Biochemistry and Biophysics 381: 205–212. [DOI] [PubMed] [Google Scholar]
- Matile Ph. 1975. The lytic compartment in plant cells. In: Alfert M, Beermann W, Rudkin G, Sandritter W, Sitte P. eds. Cell Biology Monongraphs, Vol. 1 Berlin: Springer Verlag, 40–84. [Google Scholar]
- Normah MN, Choo WK, Vun YI, Mohamed-Hussein ZA. 2011. In vitro conservation of Malaysian biodiversity – achievements, challenges and future directions. In vitro Cellular and Developmental Biology – Plant 47: 26–36. [Google Scholar]
- Pammenter NW, Berjak P. 2014. Physiology of desiccation-sensitive (recalcitrant) seeds and implications for cryopreservation. International Journal of Plant Sciences 175: 21–28. [Google Scholar]
- Perán R, Berjak P, Pammenter NW, Kioko JI. 2006. Cryopreservation, encapsulation and promotion of shoot production in embryonic axes of a recalcitrant species, Ekebergia capensis Sparrm. CryoLetters 27: 1–12. [PubMed] [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Burian A, Couturier E, et al. 2013. Mechanical control of morphogenesis at the shoot apex. Journal of Experimental Botany 64: 4729–4744. [DOI] [PubMed] [Google Scholar]
- Saragusty J, Gacitua H, Rozenboim I, Arav A. 2009. Do physical forces contribute to cryodamage? Biotechnology and Bioengineering 104: 719–728. [DOI] [PubMed] [Google Scholar]
- Sershen, Berjak P, Pammenter NW, Wesley-Smith J. 2012a. The effects of various parameters during processing for cryopreservation on the ultrastructure and viability of recalcitrant zygotic embryos of Amaryllis belladonna. Protoplasma 249: 155–169. [DOI] [PubMed] [Google Scholar]
- Sershen, Berjak P, Pammenter NW, Wesley-Smith J. 2012b. Rate of dehydration, state of sub-cellular organisation and nature of cryoprotection are critical factors contributing to the variable success of cryopreservation: studies on recalcitrant embryos of Haemanthus montanus. Protoplasma 249: 171–186. [DOI] [PubMed] [Google Scholar]
- Spurr AR. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Steponkus PL, Uemura M, Webb MS. 1993. The contrast of the cryostability of the plasma membrane of winter rye and spring oat – two species that widely differ in their freezing tolerance and plasma membrane lipid composition. In: Steponkus PL, ed. Advances in low temperature biology, Vol. 2 London: JAI Press, 211–312. [Google Scholar]
- Tanksley SD, McCouch SR. 1997. Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277: 1063–1066. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. 2011. Morphological classification of plant cell deaths. Cell Death and Differentiation 18: 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk GM, Caspersen AM. 2007. Plasmolysis and recovery of different cell types in cryoprotected shoot tips of Mentha × piperita. Protoplasma 231: 215–226. [DOI] [PubMed] [Google Scholar]
- Wesley-Smith J, Berjak P, Pammenter NW, Vertucci CW. 1995. Ultrastructural evidence for the effects of freezing in embryonic axes of Pisum sativum L. at various water contents. Annals of Botany 76: 59–64. [Google Scholar]
- Wesley-Smith J, Pammenter NW, Walters C, Berjak P. 2001a. The effects of two drying rates on the desiccation tolerance of embryonic axes of recalcitrant jackfruit (Artocarpus heterophyllus Lamk.) seeds. Annals of Botany 88: 653–664. [Google Scholar]
- Wesley-Smith J, Walters C, Pammenter NW, Berjak P. 2001b. Interactions of water content, rapid (non-equilibrium) cooling to −196 °C and survival of embryonic axes of Aesculus hippocastanum L. seeds. Cryobiology 42: 196–206. [DOI] [PubMed] [Google Scholar]
- Wesley-Smith J, Walters C, Berjak P, Pammenter NW. 2004a. Non-equilibrium cooling of Poncirus trifoliata L. embryonic axes at various water contents. CryoLetters 25: 121–128. [PubMed] [Google Scholar]
- Wesley-Smith J, Walters C, Berjak P, Pammenter NW. 2004b. The influence of water content, cooling and warming rates upon survival of embryonic axes of Poncirus trifoliata. CryoLetters 25: 129–138. [PubMed] [Google Scholar]
- Wesley-Smith J, Berjak P, Pammenter NW, Walters C. 2014. Intracellular ice and cell survival in cryo-exposed embryonic axes of Acer saccharinum: an ultrastructural study of factors affecting cell and ice structures. Annals of Botany 113: 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]