Abstract
OBJECTIVE
Cannabis use disorder is the most common co-occurring drug use disorder in people with schizophrenia and is associated with poor outcomes. We launched a randomized controlled trial to assess the impact of clozapine compared with treatment as usual on cannabis use in patients with schizophrenia and co-occurring cannabis use disorder.
METHODS
Thirty-one patients with schizophrenia and co-occurring cannabis use disorder were randomly assigned to switch to clozapine or to stay on their current antipsychotic and were then followed weekly for 12 weeks. Blinded raters assessed participants weekly with the Timeline Follow-back for substance use and the expanded Brief Psychiatric Rating Scale for symptoms. Longitudinal random effects models were used to investigate the time-varying differences in cannabis use and other outcomes between the treatment as usual and clozapine groups.
RESULTS
The two groups differed in average intensity of cannabis use by approximately 4.5 joints/week, with lesser use in the clozapine group (t = −1.77; df = 28.5; p=.086; effect size ~ 0.6). Symptoms and functioning were not different between the two groups.
CONCLUSIONS
Clozapine may reduce cannabis use among patients with schizophrenia and co-occurring cannabis use disorder. Further controlled trials are warranted.
Keywords: schizophrenia, cannabis, substance use disorder, clozapine, treatment, co-occurring
Up to 60% of people with schizophrenia have a lifetime co-occurring substance use disorder (Alterman, Erdlen, & Murphy, 1981; Ananth, Vandewater, & al, 1989; Freed, 1975; Ronald C. Kessler et al., 2005; R. C. Kessler et al., 1994; Mueser et al., 1990). Cannabis use disorder is the most common co-occurring drug use disorder in these individuals (Ronald C. Kessler et al., 2005; Mueser et al., 1990; Regier et al., 1990), with lifetime rates ranging from 13 to 42% (DeQuardo, Carpenter, & Tandon, 1994; Dixon, Haas, Weiden, Sweeney, & Frances, 1991; Hambrecht & Hafner, 1996; Koskinen, Lohonen, Koponen, Isohanni, & Miettunen, 2010; Mueser, Bennett, & Kushner, 1995; Peralta & Cuesta, 1992), a three to ten-fold increase over the lifetime prevalence of 4% in the general population (Regier et al., 1990).
Cannabis use disorder has been associated with clinical exacerbations, non-compliance with treatment, poor global functioning, and increased relapse and rehospitalization rates (DeQuardo et al., 1994; Knudsen & Vilmar, 1984; Linszen, Dingemans, & Lenior, 1994; Negrete, Knapp, Douglas, & Smith, 1986; Peralta & Cuesta, 1992; Treffert, 1978). It adds greatly to the financial costs and emotional toll that schizophrenia places on patients, families and the mental health system (e.g., Dickey & Azeni, 1996; Kivlahan, Heiman, Wright, Mundt, & Shupe, 1991).
Comprehensive psychosocial treatment programs that integrate treatments for schizophrenia and substance (including cannabis) use disorder have been developed and shown to be effective for this population (Drake, Mueser, Brunette, & McHugo, 2004). Nevertheless, even with abstinence, relapse is common (Xie, McHugo, Fox, & Drake, 2005); moreover, almost half of the patients with co-occurring disorders continue to use substances and suffer negative consequences after three to 12 months of psychosocial treatment. Hence, there continues to be a need for better treatments and for a better understanding of the role of pharmacologic treatments of co-occurring cannabis and other substance use disorders in patients with schizophrenia.
While typical antipsychotic medications do not appear to limit cannabis or other substance use in patients with schizophrenia (Noordsy, O’Keefe, Mueser, & Xie, 2001), preliminary data suggest that the atypical antipsychotic clozapine may be helpful not only in the treatment of their psychotic symptoms, but also in limiting their cannabis and other substance abuse. Case reports, retrospective studies and a small, open label, prospective study have reported reduced substance and tobacco use during treatment with clozapine (Albanese, Khantzian, Murphy, & Green, 1994; Buckley, McCarthy, Chapman, Richman, & Yamamoto, 1999; George, Sernyak, Ziedonis, & Woods, 1995; Lee, 1998; Marcus & Snyder, 1995; McEvoy, Freudenreich, Levin, & Rose, 1995; Yovell & Opler, 1994; Zimmet, Strous, Burgess, Kohnstamm, & Green, 2000).
A subsequent study by our group provided further evidence of the potential role of clozapine in dual diagnosis patients. This study, a naturalistic, clinical services survey, followed 151 patients with co-occurring psychotic and substance use disorders for three years (Drake, Xie, McHugo, & Green, 2000). Patients taking clozapine had higher rates of remission of cannabis abuse (6/9 = 67%) compared to those treated with typical antipsychotics (12/37 = 32%). Similarly, remission of alcohol abuse was higher in patients taking clozapine (15/19 = 79%) compared to those treated with typical antipsychotics (29/86 = 34%). Moreover, in a continuing assessment of those patients whose abuse remitted, only 8% of those treated with clozapine relapsed to abuse in the following six months, compared to 40% of those treated with other antipsychotics (M.F. Brunette, Drake, Xie, McHugo, & Green, 2006).
Given the many side effects of clozapine (which led to restriction of its Food and Drug Administration approval to use for patients with treatment-refractory psychosis), many investigators are currently assessing whether other atypical antipsychotics share with clozapine an ability to limit substance use in patients with schizophrenia. Unfortunately, available data are somewhat mixed and not overly promising (Green, Noordsy, Brunette, & O’Keefe, 2008) for risperidone (Green, Burgess, Dawson, Zimmet, & Strous, 2003; Smelson et al., 2002; Smelson et al., 2004), olanzapine (Akerele & Levin, 2007; M.F. Brunette et al., 2008; Noordsy et al., 2001; Petrakis, Leslie, Finney, & Rosenheck, 2006; Smelson et al., 2006), quetiapine (E. S. Brown, Nejtek, Perantie, Rajan Thomas, & Rush, 2003; M. F. Brunette, O’Keefe, Dawson, Buckley, & Green, 2009; Potvin et al., 2006), and aripiprazole (Beresford et al., 2005; E.S. Brown, Jeffress, Liggin, Garza, & Beard, 2005; Warsi, Sattar, Bhatia, & Petty, 2005). Thus, at present, clozapine remains the only antipsychotic medication where published research provides consistent evidence of a substantive decrease in cannabis and other substance use.
We have presented a neurobiological formulation, based on animal studies, suggesting that clozapine’s unusual clinical effects on substance abuse in patients with schizophrenia may relate to its varied pharmacologic actions on dopaminergic and, particularly, noradrenergic systems. Specifically, we have proposed that clozapine’s weak dopamine D2 receptor blockade coupled with its activation of the norepinephrine (NE) system (including its antagonistic effects at the NE α2 receptor and its ability to release NE) e.g.,(Antelman & Caggiula, 1977; Breier et al., 1994; Green et al., 1993; Lieberman et al., 1991; Rice, Smith, Silk, & Rosen, 1984; Sarafoff, Davis, & Ruther, 1979; Svensson et al., 1995; Thierry, Godbout, Mantz, & Glowinski, 1990; Van Kammen et al., 1990)may ameliorate dysfunction in the DA-mediated “brain reward system” that may underlie the common use of cannabis and other substances in patients with schizophrenia (Green, Zimmet, Strous, & Schildkraut, 1999).
Given the consistent, albeit still preliminary data about clozapine’s ability to decrease substance use in patients with schizophrenia, as well as our published rationale of the basis of this effect, our group launched a randomized controlled trial of clozapine (as compared with treatment as usual) of cannabis use in patients with schizophrenia and co-occurring cannabis use disorder.
Methods
The study was a three-month, randomized, open-label investigation (with blinded assessments) of the efficacy and safety of clozapine compared to continued current antipsychotic treatment (i.e., treatment as usual) in outpatients with schizophrenia or schizoaffective disorder and comorbid cannabis use disorder. After researchers established participants’ eligibility at baseline, blinded raters assessed participants weekly for substance use, symptoms, and side effects. Unblinded clinicians prescribed and adjusted study medications weekly for 12 weeks. The Institutional Review Boards at Dartmouth Medical School and at the University of South Carolina approved the study. All procedures were consistent with the Declaration of Helsinki and Good Clinical Practice according to the International Conference on Harmonization guidelines. There was a complete and thorough discussion of the study with potential participants, and all participants gave written informed consent prior to engaging in any study procedures.
Study participants
Participants were recruited, primarily through clinician referral, from two urban study sites, one in New Hampshire, affiliated with Dartmouth, and the second in South Carolina, affiliated with the University of South Carolina. Inclusion criteria were: Diagnostic and Statistical Manual (DSM-IV) diagnosis of schizophrenia or schizoaffective disorder and current cannabis use disorder; cannabis use on at least 5 days over the 3 weeks prior to screening; age 18–65; outpatient status prior to randomization; and current treatment with antipsychotic medication other than clozapine. Patients taking medications with possible effects on alcohol use (e.g., naltrexone, disulfiram or topiramate) were excluded, as were patients with active, serious medical illness, suicidality, or severe psychiatric instability. Patients for whom clozapine was contraindicated (e.g., white blood count < 3500/mm3 at screening, history of a seizure disorder) were also excluded. Fifty patients were screened. Nine did not meet eligibility criteria; seven withdrew consent; four were lost to follow up; and one left the area prior to baseline. Thirty-one patients proceeded with a baseline study visit and were randomized to a treatment group.
Study Procedures
Once patients were deemed eligible, they were randomly assigned to one of two treatment conditions: switching to treatment with clozapine or staying on their current antipsychotic medication. Randomization was blocked by site. For those patients switched to clozapine, study physicians increased the dose of clozapine in a flexible manner to reach a daily dose of 400 milligrams by the end of week four. During this titration period, the dose of participants’ initial antipsychotic medication was gradually reduced and then eliminated within four weeks. Dose adjustments of clozapine were allowed based on psychosis symptom response and side effects, but the maximum allowed dose of clozapine was 550 milligrams per day. Those patients randomized to stay on their current antipsychotic were kept on a steady dose, although adjustments were allowed to address newly emerging symptoms of psychosis or side effects. The group of research physicians and the principal investigator (AIG) reviewed the pharmacological treatment of all study participants at monthly intervals to ensure that antipsychotic treatment was consistent across the study.
Participants attended weekly study visits for 12 weeks. Unblinded study physicians assessed participants for psychosis treatment response and adverse events. Trained, blinded raters assessed participants for cannabis, alcohol and other substance use, for psychiatric symptoms and for medication side effects. Experienced study raters were trained and certified prior to rating study patients and were re-certified yearly. Psychosocial treatment for psychosis and substance use was measured monthly. Participants were reimbursed with a $25 gift card at the completion of each study visit for their time and travel expenses.
Measures
During the screening phase, raters used the Structured Clinical Interview for DSM-IV Axis-I Disorders - Patient Edition (SCID) (First, Spitzer, Gibbon, & Williams, 1996) to establish participant diagnoses of schizophrenia or schizoaffective disorder and cannabis and other substance use disorders. Study physicians completed a physical exam and laboratory assessment (complete blood cell count [CBC], glucose and lipid profile) to establish the participants’ medical stability. Patients randomized to clozapine had the CBC repeated weekly; participants who stayed on their baseline antipsychotic medication did not have blood drawn for weekly CBC assessments.
Blinded raters assessed participant substance use with the Timeline Follow-back (TLFB) procedure (L. C. Sobell & Sobell, 1992; M. B. Sobell et al., 1980) at screening, baseline, and weekly during the course of the study. In addition, at each visit, participants were given a breathalyzer for alcohol (with the alcoHAWK-Pro from Q3 Innovations) and a urine screen for drugs (with the ACON 2 Panel Multi-line Test Device for tetra-hydro-cannabinol [50 ng/ml] and cocaine [300 ng/ml]). Blinded interviewers conducted the TLFB procedure using calendars and data from urine drug screens and breathalyzer tests to facilitate recall of substance use over the past week and to increase the validity of self-report. Participants were asked to estimate the amount of cannabis, converted to “average sized joints,” they had used each day. Information from collateral informants was also obtained to increase the validity of self-report. Baseline level of addiction treatment engagement was measured with the eight-point Substance Abuse Treatment Scale – with higher scores indicating more progress in treatment (McHugo, Drake, Burton, & Ackerson, 1995). Motivation to stop using cannabis was measured with the single item Contemplation Ladder (0–10; with higher level indicating more motivation) (Amodei & Lamb, 2004).
Blinded raters assessed participants symptoms with weekly expanded Brief Psychiatric Rating Scales (BPRS) (Lukoff, Liberman, & Nuechterlein, 1986), weekly Clinical Global Inventories (CGI) (Guy, 1976), and biweekly Schedule for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982). The BPRS rated 24 symptom items with a score of 1–7 for each item (1 = not present and 7 = extremely severe). The CGI was used to assess general improvement with one item scored 1–7 (1 = very much improved to 7 = very much worse). The SANS was used to assess five negative symptom components on a scale of 0 = ‘not at all’ to 5 = ‘severe.’ Unblinded clinicians measured neurologic side effects weekly with the Simpson Angus (Simpson & Angus, 1970), the Abnormal Involuntary Movement Scale (Lane, Glazer, Hansen, Berman, & Kramer, 1985), and the Barnes Akathisia Rating Scales (Barnes, 1989). At baseline and monthly thereafter, blinded raters assessed the amount of services patients received to address their alcohol use disorder with a substance use treatment services questionnaire (Clark et al., 1994). Study physicians recorded medication dose at each visit. Dose was later converted to chlorpromazine equivalents (Gardner, Murphy, O’Donnell, Centorrino, & Baldessarini). Medication adherence was measured by weekly pill count (Byerly et al., 2007).
Data analysis
Chi square and t-tests were used to assess whether the characteristics of the participants were different between groups. For analyzing repeated measures outcomes, we used longitudinal random effects (LRE) models (Laird & Ware, 1982) to investigate the time-varying differences in cannabis use and other outcomes between the treatment as usual and clozapine groups. The main outcome measure was intensity of cannabis use, as measured by the number of joints per week. Baseline values of this measure were included in the models to adjust for possible differences between the two groups and to improve efficiency; fixed effects included group, linear and quadratic trends (retained only if they were significant at the alpha = 0.05 level), and group by trend interactions. The covariance structure was modeled using random effects for the intercept and any significant trends; first-order autocorrelation for residual errors was also specified.
For the primary explanatory analyses, data for the clozapine group were censored (set to missing) for deviations from treatment protocol, including greater than three days of missed study medication doses and early termination of study medication. Secondary explanatory analyses of cannabis use also involved censoring for poor adherence and early termination of medication in both groups. For the intent-to-treat analysis, weekly data on cannabis use were censored for hospitalization or confinement for more than four consecutive days to control for halo effects on use due to forced abstinence. (There was no need to censor for hospitalization/incarceration in the explanatory analyses because data for such subjects had already been censored due to poor adherence). Note that subjects who terminated study medication contributed data to the LRE models up to the time of censoring due to medication termination, whereas subjects who were poorly adherent contributed data for all weeks they took medication except for the week(s) of poor adherence, which were censored on a week by week basis.
Results
The characteristics of the 31 study participants are noted in Table 1. Overall, participants were primarily single, unemployed, Caucasian males whose average age was 35 years. Participants were moderately symptomatic and had been hospitalized multiple times. The group assigned to clozapine had more education and fewer lifetime psychiatric hospitalizations. Most participants were diagnosed with cannabis dependence (rather than abuse), about half also had an alcohol disorder, and over a third had another drug use disorder. Measures of substance use, motivation, and treatment did not differ between groups.
Table 1.
Baseline Characteristics
| Total group N=31 N (%) or Mean +/− SD |
Clozapine N=15 N (%) or Mean +/− SD |
Other N=16 N (%) or Mean +/− SD |
|
|---|---|---|---|
| Age | 36 ± 10.3 | 33.3 ± 10.0 | 39.0 ± 10.2 |
| Gender | 24 (77.4) | 12 (80) | 12 (75) |
| Race (White) | 26 (83.9) | 12 (80) | 14 (87.5) |
| Education* | 10.9 ± 1.7 | 11.5 ± .8 | 10.3 ± 2 |
| Employed | 26 (83.9) | 12 (80) | 14 (87.5) |
| Marital (single) | 20 (64.5) | 10 (66.7) | 10 (62.5) |
| Diagnosis Schiz vs schizaf | 12 (38.7) | 7 (46.7) | 5 (31.3) |
| BPRS Total | 25.3 ± 8.4 | 26.7 ± 5.9 | 24 ± 10.1 |
| BPRS Positive | 6.9 ± 4.5 | 7.4 ± 3.8 | 6.5 ± 5.1 |
| BPRS Negative* | 3.7 ± 2.3 | 4.6 ± 2.5 | 2.9 ± 1.8 |
| SANS total | 43.6 ±13.0 | 47.1 ±13.3 | 40.3 ±12.3 |
| Lifetime hospitalizations* | 5.9 ± 7.3 | 2.5 ± 1.7 | 9.1 ± 9.1 |
| Cannabis Dependence (vs. abuse) | 24 (77.4) | 12 (80) | 12 (75) |
| Days Use/week | 4.5 ± 2.1 | 4.4 ± 2.5 | 4.7 ± 1.7 |
| Number Joints/week | 12.3 ± 12.0 | 10.5 ±11.7 | 13.9 ± 12.4 |
| Diagnosis Alcohol Disorder | 16 (51.6) | 8 (53.3) | 8 (50) |
| Diagnosis Other Drug Disorder | 12 (38.7) | 6 (40) | 6 (37.5) |
| MH counselor sessions/week | .2 ± .2 | .4 ±.6 | .34 ±.4 |
| SA counselor sessions/week | .08 ± .2 | .07 ±.2 | .09 ± .2 |
| AA meetings/week | .01 ± .04 | 0 | .06 ±.02 |
| Contemplation Ladder | 4.2 ± 3.7 | 3.2 ± 3.9 | 5.2 ± 3.3 |
|
| |||
| SATS | 2.7 ± .8 | 2.9 ± .8 | 2.5 ± .6 |
p<.05;
Schiz=schizophrenia, schizaf=schizoaffective disorder, MH=mental health, SA=substance abuse, AA=Alcoholics Anonymous, SATS=substance abuse treatment scale
The characteristics of study medication use and other treatment use are shown in Table 2. Most participants remained on study medication and in the study for the entire 12 weeks. Study medication was well tolerated, but many patients reported side effects, which are shown in Table 3. The most frequent side effects were somnolence, hypersalivation and weight gain. Patients who were taking clozapine were more likely to report somnolence, hypersalivation, and constipation than patients who remained on treatment as usual.
Table 2.
Treatment Characteristics
| Total group N=31 N (%) or Mean +/− SD |
Clozapine N=15 N (%) or Mean +/− SD |
TAU N=16 N (%) or Mean +/− SD |
|
|---|---|---|---|
| Medication | |||
| Weeks on study med | 11.3±2.3 | 10.9 ±3.0 | 11.6 ± 1.3 |
| Dose (chlorpromazine equivalents) | 414 ± 283.2 | 319 ± 163.5 | 492 ± 337.6 |
| Number ending med early | 4 (12.9) | 2 (13.3) | 2 (12.5) |
| Good Adherence | 28 (90) | 15 (100) | 13 (81) |
| Other Treatment | |||
| MH counselor sessions/week | .20 ±.23 | .18 ± .18 | .23 ±.28 |
| SA counselor sessions/week | .08 ±.19 | .08 ± .18 | .08 ±.2 |
| AA meetings/week | .01 ± .04 | 0 | .02 ± .06 |
TAU = Treatment as usual
Table 3.
Adverse events among patients taking clozapine versus other antipsychotics for cannabis disorder in schizophrenia
| Total group N=31 |
Clozapine N=15 |
TAU N=16 |
|
|---|---|---|---|
|
| |||
| N (%) | N (%) | N (%) | |
| Somnolence* | 11 (35.5) | 9 (60) | 2 (12.5) |
| Hypersalivation* | 10 (32.3) | 10 (66.7) | 0 (0.0) |
| Weight Gain | 8 (25.8) | 6 (40.0) | 2 (12.5) |
| Dizziness | 6 (19.4) | 5 (33.3) | 1 (6.3) |
| Vomiting | 6 (19.4) | 3 (20.0) | 3 (18.8) |
| Constipation* | 4 (12.9) | 4 (26.7) | 0 (0.0) |
| Dry Mouth | 4 (12.9) | 2 (13.3) | 2 (12.5) |
| Hypertension | 4 (12.9) | 2 (13.3) | 2 (12.5) |
| Akathisia | 3 (9.7) | 1 (6.7) | 1 (6.3) |
| Depression | 3 (9.7) | 2 (13.3) | 1 (6.3) |
| Fatigue | 3 (9.7) | 3 (20.0) | 0 (0.0) |
| Headache | 3 (9.7) | 2 (13.3) | 1 (6.3) |
| Insomnia | 3 (9.7) | 2 (13.3) | 1 (6.3) |
| Nausea | 3 (9.7) | 2 (13.3) | 1 (6.3) |
| Noncardiac Chest Pain | 3 (9.7) | 1 (6.7) | 2 (12.5) |
| Increased Agitation | 2 (6.5) | 1 (6.7) | 1 (6.3) |
| Flu Like Symptoms | 2 (6.5) | 0 (0.0) | 2 (12.5) |
| Irritability | 2 (6.5) | 1 (6.7) | 1 (6.3) |
| Libido Decreased | 2 (6.5) | 1 (6.7) | 1 (6.3) |
| Muscle Spasms | 2 (6.5) | 2 (13.3) | 0 (0.0) |
| Nasal Congestion | 2 (6.5) | 0 (0.0) | 2 (12.5) |
| Unusual Dream Activity | 2 (6.5) | 0 (0.0) | 2 (12.5) |
| Suicide Attempt | 2 (6.5) | 1 (6.7) | 1 (6.3) |
| Urinary Incontinence | 2 (6.5) | 2 (13.3) | 0 (0.0) |
p<.05;
TAU = treatment as usual
Outcomes
For the primary explanatory analysis, the two groups differed in average intensity of cannabis use by approximately 4.5 joints/week (main effect in LRE due to group), with lesser use in the clozapine group; there were no significant within-group trends during study follow-up (see Figure 1). This result suggests that clozapine treatment (as compared to continuation of the current antipsychotic) is associated with a decrease in cannabis use (effect size ~ 0.6), although differences for the small sample did not reach statistical significance (t = −1.77, df = 28.5; p=.086). Similar results were found using the intent-to-treat analysis, with modest attenuation of treatment differences (t = −1.72, df = 28.1; p = .096). The secondary explanatory analysis, in which data for the treatment as usual group were also censored due to poor adherence, showed little change in the estimated difference in intensity of cannabis use (which increased slightly to about 5 joints/week) but had an increased standard error due to fewer observations (t = −1.63, df = 24.9; p = .115).
Figure 1.
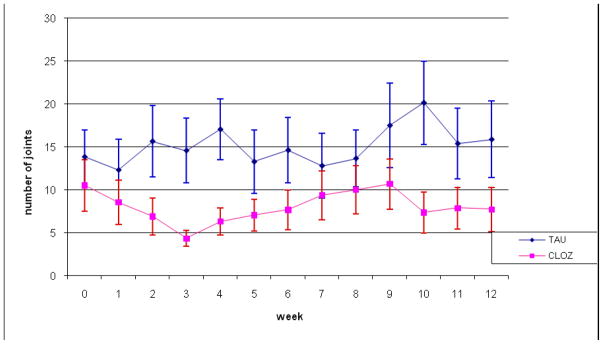
Intensity of cannabis use (joints per week) in patients on treatment as usual (TAU) and in patients switched to clozapine (CLOZ): explanatory analysis
Secondary outcomes included symptoms (negative, positive, total BPRS; composite, summary and total SANS; and SANS subscales for affective blunting, alogia, avolition, anhedonia, and disturbance of attention) and functioning (CGI). Data were censored for deviation from protocol (as above) for these secondary explanatory analyses; data were not censored for hospitalization/incarceration for either explanatory or intent-to-treat analyses. In all cases, the two groups did not differ in terms of symptoms or functioning. Negative linear trends (indicating improvement) over time were found for total and positive BPRS.
In a subsidiary analysis, the two groups were also compared for alcohol use, measured as heavy drinking days per week (with heavy drinking defined as five or more drinks per day). Censoring was carried out as for cannabis use, but in this case both linear and quadratic terms were included in LRE models to capture nonlinear trends over time. Interestingly, there were significant differences for both explanatory (t = 2.32, df = 27.4; p = .028) and intent-to-treat analyses (t = 2.4, df = 28.3; p = .023), with greater alcohol use in the clozapine group -- due primarily to one subject in that group who drank heavily throughout the study. When data for this subject were removed from the analyses, the differences were no longer significant (as shown in Figure 2 for explanatory analysis). Moreover, a comparison of Figure 2 and Figure 1 showed an important contrast between the cannabis and the alcohol results: the near significant results for cannabis use reflect systematic differences between the two treatment groups, while those for alcohol use do not (once outlying data are removed). Additionally, there was no correlation (longitudinally) between changes in drinking and changes in cannabis use over time, based on days of heavy drinking per week and days of cannabis use per week. Lastly, there was a floor effect for possible clozapine action on heavy drinking, given that drinking levels were very low throughout the study – on average less than one day per week when the one heavy drinker is omitted. (This is most clearly demonstrated by Figure 2, when the data for the outlying subject are removed from the analysis.)
Figure 2.

Mean heavy drinking days (per week) in patients on treatment as usual (TAU) and in patients on clozapine (CLOZ): explanatory analysis
Discussion
This study provides the first evidence from a randomized, controlled trial that clozapine may limit cannabis use more than continued treatment with other antipsychotics in patients with schizophrenia and co-occurring cannabis use disorders. Clozapine was well tolerated in this group of patients, with noted side effects of the types expected for this medication. Interestingly, we did not find differences in psychiatric symptoms between the two groups, despite the well-described beneficial effects of clozapine for people with treatment-resistant schizophrenia. This may reflect the overall moderate levels of psychiatric symptoms in this group of patients without treatment-refractory psychosis.
The magnitude of the effect of clozapine (effect size ~0.6) on decreasing cannabis use was moderate, suggesting a clinically meaningful role for this medication in this population. But the small size of the study sample limits the conclusions that can be drawn. Recruitment of patients with schizophrenia and co-occurring cannabis use disorders into randomized medication trials is challenging and requires a number of sites and a large recruitment team that were not available for this study. Given the relatively tenuous connection that these patients may have to treatment, and the reluctance that their clinicians have in referring them for research studies, the recruitment team needs to be carefully integrated within treatment systems.
A limitation of the study beyond sample size included the fact that the prescribing physicians were not blinded to study medication. Because clozapine causes characteristic side effects, blinding of the prescribers and patients in this type of study tends to be difficult and was not attempted here. Ratings of symptoms and substance use were, however, completed by blinded raters. Nonetheless, whether expectancy effects contributed to the apparent efficacy of clozapine in reducing cannabis use cannot be ascertained here.
In this study of patients with cannabis use disorders, the finding that clozapine was not associated with improvement in heavy alcohol use was not consistent with previous research (e.g., (M.F. Brunette et al., 2006; Drake et al., 2000)). Nevertheless, the lack of correlation between changes in alcohol and changes in cannabis use suggests that the decrease we noted in cannabis use was not related to an increase in alcohol use in these patients. Moreover, as noted above, the two groups did not differ significantly in alcohol use during treatment when data for a single subject with unusually heavy drinking were removed from the analysis. The undue influence of such a subject reflects both the small size of the sample and the generally low amount of drinking among these cannabis using patients. We note that by design this sample was recruited for level of cannabis, not alcohol use, and thus, it would not be unexpected to find an outlying heavy drinking subject in only one of the treatment groups (only half of participants were diagnosed with an alcohol disorder). Taken together, the alcohol data appear to support the robustness of the primary finding of clozapine’s effect on cannabis use, despite failing to replicate a similar result for alcohol use.
In summary, this small, randomized trial of clozapine compared to other antipsychotics suggests that clozapine may reduce cannabis use in patients with schizophrenia and cannabis use disorders independent of any effect on symptoms or overall functioning. Although effective treatments for cannabis use disorder in schizophrenia are sorely needed, at present, clozapine’s side effect profile severely restricts its use in clinics. Given that these findings are promising and consistent with previous research on the effect of clozapine on cannabis use, a fully powered, blinded clinical trial is warranted to definitively establish whether clozapine is effective for the treatment of cannabis use disorders in this population. If it can be demonstrated to definitely decrease cannabis use in patients with schizophrenia, it may be appropriate to expand the routine use of clozapine beyond that of those with treatment refractory psychosis – to include those stable outpatients who have a co-occurring cannabis use disorder.
Acknowledgments
This study was supported by grant # DA 13196 from the National Institute on Drug Abuse (NIDA). We would like to thank Draeger Medical Systems for loaning an ECG machine for this study.
Footnotes
Disclosures
Dr. Brunette has no known conflict of interest. She has received funding for research from Bristol Meyers Squib Foundation.
Dr. Green reports research grant support from National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, National Institute of Mental Health, Janssen and Eli Lilly, Forest Laboratories and AstraZeneca. He also serves on a Data Safety Monitoring Board reviewing Eli Lilly studies, owns shares of stock in Johnson & Johnson, Pfizer and Mylan, and has two pending patents on the treatment of substance abuse.
Dr. Dawson has no known conflict of interest.
Mr. O’Keefe has no known conflict of interest.
Dr. Narasiman has no known conflict of interest. She has received research funding from National Institute on Drug Abuse, National Institute of Mental Health, Janssen, Forest, and Pfizer.
Dr. Noordsy has no known conflict of interest. He has received research funding and/or consulting or speaking honoraria from the following companies that make psychotropic medications: Astra Zeneca, Bristol Meyers Squib, Janssen, Eli Lilly, Lundbeck, Novartis, Merck, and Sunovion.
Dr. Wojcik has no known conflict of interest.
References
- Akerele E, Levin FR. Comparison of olanzapine to risperidone in substance-abusing individuals with schizophrenia. American Journal on Addictions. 2007;16(4):260–268. doi: 10.1080/10550490701389658. [DOI] [PubMed] [Google Scholar]
- Albanese MJ, Khantzian EJ, Murphy SL, Green AI. Decreased substance use in chronically psychotic patients treated with clozapine. American Journal of Psychiatry. 1994;151:780–781. doi: 10.1176/ajp.151.5.780b. [DOI] [PubMed] [Google Scholar]
- Alterman AJ, Erdlen FR, Murphy E. Alcohol abuse in the psychiatric hospital population. Addictive Behaviors. 1981;6:69–73. doi: 10.1016/s0306-4603(81)80012-3. [DOI] [PubMed] [Google Scholar]
- Amodei N, Lamb RJ. Convergent and concurrent validity of the Contemplation Ladder and URICA scales. Drug and Alcohol Dependence. 2004;73(3):301–306. doi: 10.1016/j.drugalcdep.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ananth J, Vandewater, et al. Missed diagnosis of substance abuse in psychiatric patients. Hospital & Community Psychiatry. 1989;40:297–299. doi: 10.1176/ps.40.3.297. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195(4279):646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. The British Journal of Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Clapp L, Martin B, Wiberg JL, Alfers J, Beresford HF. Aripiprazole in schizophrenia with cocaine dependence A pilot study. Journal of Clinical Psychiatry. 2005;25(4):363–366. doi: 10.1097/01.jcp.0000169419.38899.5b. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Waltrip RW, 2nd, Listwak S, Holmes C, Goldstein DS. The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology. 1994;10(1):1–7. doi: 10.1038/npp.1994.1. [DOI] [PubMed] [Google Scholar]
- Brown ES, Jeffress J, Liggin JDM, Garza M, Beard L. Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipschotic to aripiprazole. Journal of Clinical Psychiatry. 2005;66(6):756–760. doi: 10.4088/jcp.v66n0613. [DOI] [PubMed] [Google Scholar]
- Brown ES, Nejtek VA, Perantie DC, Rajan Thomas N, Rush AJ. Cocaine and amphetamine use in patients with psychiatric illness: a randomized trial of typical antipsychotic continuation or discontinuation. Journal of Clinical Psychopharmacology. 2003;23(4):384–388. doi: 10.1097/01.jcp.0000085412.08426.08. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Drake RE, Xie H, McHugo GJ, Green AI. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophrenia Bulletin. 2006:32–38. doi: 10.1093/schbul/sbl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette MF, O’Keefe C, Dawson R, Buckley P, Green AI. An open label study of quetiapine in patients with schizophrenia and alcohol disorders. Journal of mental health and substance use disorders. 2009;2(3):203–211. [Google Scholar]
- Brunette MF, O’Keefe C, Zimmet S, Wojcik JD, Dawson R, Green AI. Olanzapine for Alcohol Use Disorder in Patients with Schizophrenia. Journal of Dual Diagnosis. 2008;4(4):344–354. doi: 10.1080/15504263.2011.570118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley P, McCarthy M, Chapman P, Richman C, Yamamoto B. Clozapine treatment of comorbid substance abuse in patients with schizophrenia. Schizophrenia Research. 1999;36:272. [Google Scholar]
- Byerly MJ, Thompson A, Carmody T, Bugno R, Ervin T, Kashner M, et al. Validity of electronicaly monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58:844–847. doi: 10.1176/ps.2007.58.6.844. [DOI] [PubMed] [Google Scholar]
- Clark R, Teague G, Ricketts S, Bush P, Zubkoff M, Keller A. Measuring resource use in economic evaluations: Determining the social costs of mental illness. Journal of Mental Health Administration. 1994;21:32–41. doi: 10.1007/BF02521343. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Carpenter CF, Tandon R. Patterns of substance abuse in schizophrenia: nature and significance. J Psychiatr Res. 1994;28(3):267–275. doi: 10.1016/0022-3956(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Dickey B, Azeni H. Persons with dual diagnoses of substance abuse and major mental illness: their excess costs of psychiatric care. American Journal of Public Health. 1996;86:973–977. doi: 10.2105/ajph.86.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ. Drug abuse in schizophrenic patients: Clinical correlates and reasons for use. American Journal of Psychiatry. 1991;148:224–230. doi: 10.1176/ajp.148.2.224. [DOI] [PubMed] [Google Scholar]
- Drake RE, Mueser KT, Brunette MF, McHugo GJ. A review of treatment for people with severe mental illness and co-occurring substance use disorders. Psychchiatricl Rehabilitation Journal. 2004;27(4):360–374. doi: 10.2975/27.2004.360.374. [DOI] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophrenia Bulletin. 2000;26(2):441–449. doi: 10.1093/oxfordjournals.schbul.a033464. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department; 1996. [Google Scholar]
- Freed EX. Alcoholism and schizophrenia: the search for perspectives. Journal of Studies on Alcohol. 1975;36(7):853–881. doi: 10.15288/jsa.1975.36.853. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. American Journal of Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- George TP, Sernyak MJ, Ziedonis D, Woods SM. Effects of clozapine on smoking in chronic schizophrenic outpatients. Journal of Clinical Psychiatry. 1995;56(8):344–346. [PubMed] [Google Scholar]
- Green AI, Alam MY, Sobieraj JT, Pappalardo KM, Waternaux C, Salzman C, et al. Clozapine response and plasma catecholamines and their metabolites. Psychiatry Res. 1993;46(2):139–149. doi: 10.1016/0165-1781(93)90016-a. [DOI] [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophrenia Research. 2003;60(1):81–85. doi: 10.1016/s0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, O’Keefe C. Substance abuse and schizophrenia: Pharmacotherapeutic intervention. Journal of Subance Abuse Treatment. 2008;34(1):61–71. doi: 10.1016/j.jsat.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harvard Review of Psychiatry. 1999;6(6):287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Deparment of Health, Education and Welfare; 1976. [Google Scholar]
- Hambrecht M, Hafner H. Substance abuse and the onset of schizophrenia. Biol Psychiatry. 1996;40(11):1155–1163. doi: 10.1016/S0006-3223(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Demler O, Falloon IRH, Gagnon E, Guyer M, et al. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R) Biological Psychiatry. 2005;58(8):668–676. doi: 10.1016/j.biopsych.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12 month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kivlahan DR, Heiman JR, Wright RC, Mundt JW, Shupe JA. Treatment cost and rehospitalization rate in schizophrenic outpatients with a history of substance abuse. Hosp Community Psychiatry. 1991;42(6):609–614. doi: 10.1176/ps.42.6.609. [DOI] [PubMed] [Google Scholar]
- Knudsen P, Vilmar T. Cannabis and neuroleptic agents in schizophrenia. Acta Psychiatrica Scandinavica. 1984;69:162–174. doi: 10.1111/j.1600-0447.1984.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients wtih schizophrenia: A meta-analysis. Schizophrenia Bulletin. 2010;36(6):1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lane RD, Glazer WM, Hansen TE, Berman WH, Kramer SI. Assessment of tardive dyskinesia using the Abnormal Involuntary Movement Scale. Journal of Nervous and Mental Disease. 1985;173(6):353–357. doi: 10.1097/00005053-198506000-00005. [DOI] [PubMed] [Google Scholar]
- Lee ML. Clozapine and substance abuse in patients with schizophrenia. Canadian Journal of Psychiatry. 1998;45:855–856. [PubMed] [Google Scholar]
- Lieberman J, Johns C, Pollack S, Masiar S, Bookstein P, Cooper T, et al. Biochemical effects of clozapine in cerebrospinal fluid of patients with schizophrenia. In: Tamminga C, Schulz S, editors. Advances in neuropsychiatry and psychopharmacology vol. 1: Schizophrenia research. Vol. 1. New York: Raven Press; 1991. pp. 341–349. [Google Scholar]
- Linszen D, Dingemans P, Lenior M. Cannabis abuse and the course of recent onset schizophrenic disorders. Archives of General Psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- Lukoff K, Liberman R, Nuechterlein K. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Marcus P, Snyder R. Reduction of comorbid substance abuse with clozapine (letter) American Journal of Psychiatry. 1995;152:959. doi: 10.1176/ajp.152.6.959a. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Levin E, Rose JE. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology. 1995;119:124–126. doi: 10.1007/BF02246063. [DOI] [PubMed] [Google Scholar]
- McHugo GJ, Drake RE, Burton HL, Ackerson TH. A scale for assessing the stage of substance abuse treatment in persons with severe mental illness. Journal of Nervous & Mental Disease. 1995;183(12):762–767. doi: 10.1097/00005053-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Bennett M, Kushner MG. Epidemiology of substance use disorders among persons with chronic mental illness. In: Lehman AF, Dixon LB, editors. Double jeopardy: chronic mental illness and substance use disorders. Netherlands: Harwood Academic Publishers; 1995. pp. 9–25. [Google Scholar]
- Mueser KT, Yarnold PR, Levinson DF, Singh H, ASB, Kee K, et al. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophrenia Bulletin. 1990;16:31–56. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychology Medicine. 1986;16:515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- Noordsy DL, O’Keefe C, Mueser KT, Xie H. Six-month outcomes for patients who switched to olanzapine treatment. Psychiatric Services. 2001;52(4):501–507. doi: 10.1176/appi.ps.52.4.501. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. Acta Psychiatr Scand. 1992;85(2):127–130. doi: 10.1111/j.1600-0447.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Leslie D, Finney JW, Rosenheck R. Atypical antipsychotic medication and substance use-related outcomes in the treatment of schizophrenia. The American Journal on Addictions. 2006;15(1):44–49. doi: 10.1080/10550490500419052. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Lipp O, Elie R, Mancinin-Marie A, Demers MF, et al. Quetiapine in patients with comorbid schizophrenia-spectrum and substance use disorders: an open-label trial. Current Medical Research and Opinion. 2006;22(7):1277–1285. doi: 10.1185/030079906X112561. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study.[comment] JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Rice HE, Smith CB, Silk KR, Rosen J. Platelet alpha 2-adrenergic receptors in schizophrenic patients before and after phenothiazine treatment. Psychiatry Res. 1984;12(1):69–77. doi: 10.1016/0165-1781(84)90139-2. [DOI] [PubMed] [Google Scholar]
- Sarafoff M, Davis L, Ruther E. Clozapine induced increase of human plasma norepinephrine. J Neural Transm. 1979;46(2):175–180. doi: 10.1007/BF01250337. [DOI] [PubMed] [Google Scholar]
- Simpson GN, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. 1970;212 (suppl 44):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Losonczy MF, Davis CW, Kaune M, Williams J, Ziedonis D. Risperidone decreases craving and relapses in individuals with schizophrenia and cocaine dependence. Can J Psychiatry. 2002;47(7):671–675. doi: 10.1177/070674370204700710. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Williams J, Ziedonis D, Sussner BD, Losonczy MF, Engelhart C, et al. A double-blind placebo-controlled pilot study of risperidone for decreasing cue-elicited craving in recently withdrawn cocaine dependent patients. Journal of Substance Abuse Treatment. 2004;27(1):45–49. doi: 10.1016/j.jsat.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Ziedonis D, Williams J, Losonczy MF, Steinberg ML, Kaune M. The efficacy of olanzapine for decreasing cue-elicited craving in individuals with schizophrenia and cocaine dependence: a preliminary report. Journal of Clinical Psychopharmacology. 2006;26(1):9–12. doi: 10.1097/01.jcp.0000194624.07611.5e. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Saungers B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating Alcohol and Drug Abuse Treatment Effectiveness: Recent Advances. New York: Pergamon; 1980. pp. 129–150. [Google Scholar]
- Svensson TH, Mathe JM, Andersson JL, Nomikos GG, Hildebrand BE, Marcus M. Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and alpha 1-adrenoceptor antagonism. J Clin Psychopharmacol. 1995;15(1 Suppl 1):11S–18S. doi: 10.1097/00004714-199502001-00003. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Godbout R, Mantz J, Glowinski J. Influence of the ascending monoaminergic systems on the activity of the rat prefrontal cortex. Prog Brain Res. 1990;85:357–364. doi: 10.1016/s0079-6123(08)62690-4. discussion 364–355. [DOI] [PubMed] [Google Scholar]
- Treffert DA. Marijuana use in schizophrenia: a clear hazard. American Journal of Psychiatry. 1978;135(10):1213–1215. doi: 10.1176/ajp.135.10.1213. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Peters JL, Van Kammen WB, Neylan T, Yao JK, Shaw D, et al. Noradrenaline, state dependency and relapse prediction in schizophrenia. In: Weller M, editor. International Perspectives in Schizophrenia. London: John Libbey; 1990. pp. 253–268. [Google Scholar]
- Warsi M, Sattar SP, Bhatia SC, Petty F. Aripiprazole reduces alcohol use. Canadian Journal of Psychiatry. 2005;50(4):244. doi: 10.1177/070674370505000415. [DOI] [PubMed] [Google Scholar]
- Xie H, McHugo GJ, Fox M, Drake RE. Substance abuse relapses in a 10-year prospecitve follow-up of clients with co-occurring severe mental illness and substance use disorders. Psychiatric Services. 2005;56:1282–1287. doi: 10.1176/appi.ps.56.10.1282. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Opler LA. Clozapine reverses cocaine craving in a treatment-resistant mentally ill chemical abuser: a case report and a hypothesis. Journal of Nervous Mental Disorders. 1994;182:591–592. doi: 10.1097/00005053-199410000-00017. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. Journal of Clinical Psychopharmacology. 2000;20:94–98. doi: 10.1097/00004714-200002000-00016. [DOI] [PubMed] [Google Scholar]


