Abstract
The atypical antipsychotic clozapine reduces alcohol drinking in patients with schizophrenia. We have proposed that clozapine’s ability to decrease alcohol drinking relates to its weak blockade of the dopamine D2 receptor and potent blockade of the norepinephrine α-2 receptor, as well as its ability to elevate plasma and brain norepinephrine. Another atypical antipsychotic, risperidone, which is a potent blocker of both the dopamine D2 receptor and norepinephrine α-2 receptor, does not decrease alcohol drinking. In this study, we used the Syrian golden hamster to test whether the ability of risperidone to reduce alcohol drinking would be enhanced if it were used in combination with the norepinephrine reuptake inhibitor desipramine. Hamsters were given free access to water and alcohol (15% v/v) until they reached a steady drinking baseline. They were then treated daily with each drug or drug combination for 20 days. Risperidone (0.2 mg/kg) only transiently decreased alcohol drinking. However, 5.0 mg/kg, and possibly 1.0 mg/kg, desipramine added to 0.2 mg/kg risperidone appeared to produce a more substantial and relatively sustained effect than risperidone alone. Data from this study provides leads toward the development of new treatments for patients with schizophrenia and alcoholism, and also for those with alcoholism alone.
Keywords: Alcoholism, addiction, schizophrenia, antipsychotic, norepinephrine, antipsychotic, norepinephrine reuptake inhibitor
1. INTRODUCTION
More than a third of patients with schizophrenia have co-occurring alcohol use disorder, a rate three times higher than in the general population (Drake et al., 1989; Regier et al., 1990). Although their level of alcohol use tends to be moderate, even moderate use of alcohol is known to worsen the clinical course of patients with schizophrenia (Alterman et al., 1981; Drake and Mueser, 1996; Gupta et al., 1996; Owen et al., 1996). Treatment options for patients with these co-occurring disorders are limited, and most of the antipsychotic medications used in this population have little impact on their alcohol use. We and others, however, have reported data suggesting that patients with schizophrenia treated with the atypical antipsychotic clozapine (CLOZ) have strikingly higher rates of remission from alcohol abuse, compared to those treated with other antipsychotic medications (Lee et al., 1998; Green et al., 1999; Zimmet et al., 2000; Drake et al., 2000; Green et al., 2008). Despite these promising data, however, the side effects produced by CLOZ have restricted its use to a narrow group of patients – those with treatment-refractory psychosis. Thus, it remains important to develop other CLOZ-like medications that are safer than CLOZ but share the ability to suppress alcohol drinking in patients with schizophrenia.
In previous and ongoing studies, we have used the Syrian golden hamster to elucidate the action of CLOZ on alcohol drinking. Unlike other rodent models of primary alcoholism that exhibit features resembling heavy alcohol drinking in humans (e.g., high blood alcohol levels, binge-like drinking) and signs of physical withdrawal, the golden hamster (an outbred animal) achieves only moderate blood alcohol levels and is, thus, a model of moderate alcohol drinking (Arvola and Forsander, 1961; Arvola and Forsander, 1963; Kulkosky and Cornell, 1979; McCoy et al., 1981; Piercy and Myers, 1995; Keung et al., 2000; Green et al., 2004; Chau et al., 2011). We have proposed that the golden hamster can serve as an animal model to screen medications that may ameliorate alcohol use in patients with schizophrenia for two reasons: (1) its pattern of alcohol drinking resembles the moderate alcohol use commonly seen in patients with schizophrenia (Test et al., 1989; Lehman et al., 1996); and (2) CLOZ decreases alcohol drinking in the hamster and in patients with schizophrenia, while the typical antipsychotic haloperidol (HAL), which does not limit alcohol drinking in patients with schizophrenia, also has no effect on alcohol drinking in the hamster (Green et al., 2004; Green et al., 2008; Chau et al., 2010).
We have presented a novel hypothesis related to the neurobiological mechanisms underlying the high prevalence of alcohol use disorder in schizophrenia, as well as the strikingly different effects of HAL and CLOZ on their alcohol use (Green et al., 1999). In essence, we have proposed that a dysfunction in brain reward circuitry underlies alcohol use in this population, and (a) that most antipsychotic drugs (e.g., HAL) do not decrease alcohol use in this population because they fail to restore the normal function of the reward circuitry (in part because of their potent dopamine [DA] D2 receptor blocking effect); but (b) that CLOZ, through its weak blockade of DA D2 receptors and its potent blockade of norepinephrine (NE) α2 receptors, as well as its ability to dramatically increase NE levels in patients with schizophrenia, may tend to have a normalizing effect on this dysfunctional reward circuitry (Green et al., 1999). Consistent with our hypothesis, we have shown that if the weak DA D2 blockade by CLOZ is strengthened by adding raclopride, a potent DA D2 antagonist, to CLOZ, CLOZ’s ability to reduce alcohol intake is significantly reduced (Chau et al., 2011).
Risperidone (RISP) is an atypical antipsychotic with pharmacologic properties overlapping those of CLOZ. Most notably, it is an antagonist of both DA D2 receptors and NE α 2 receptors (Richelson and Souder, 2000). However, reports from our group have suggested that RISP does not limit alcohol drinking in patients with schizophrenia (Green et al., 2003; Green et al., 2008). We have suggested that the inability of RISP to decrease alcohol drinking in these patients relates to the fact that, unlike CLOZ, a weak DA D2 receptor antagonist, RISP is a potent antagonist of the DA D2 receptor (Green et al., 1999; Chau et al., 2011), and despite its alpha 2 receptor antagonism, it does not elevate plasma NE levels to the extent of CLOZ (See et al., 1999; Elman et al., 2002). While previous studies have assessed the effects of RISP and other atypical antipsychotics on alcohol intake in rat models, with conflicting results, (e.g., (Silvestre et al., 1996; Ingman et al., 2003), we have described how these other alcohol-preferring rodents, in contrast to the Syrian golden hamster, do not adequately model alcohol drinking in patients with schizophrenia (Chau et al., 2013).
We speculated that while RISP would likely have minimal ability to decrease alcohol intake in the Syrian golden hamster, potentiation of the noradrenergic activity of RISP (especially a low dose of RISP, one producing only a modest D2 blockade) might limit alcohol drinking in rodents. Thus, we studied the dose-dependent effects of RISP on alcohol drinking in the hamster, as well as the effects of RISP in combination with desipramine (DMI), a potent NE reuptake inhibitor known to elevate plasma NE levels (Aberg-Wistedt et al., 1981).
2. METHODS
2.1. Subjects
Adult, male Syrian golden hamsters (Mesocricetus Auratus) (100–130g) were acquired from Harlan Inc. (Indianapolis, IN), maintained on a normal 12 h/12 h light/dark cycle, and individually housed in standard home cages with ad libitum access to food and water. For each experiment, hamsters were given free access to a water bottle, a bottle containing 15% alcohol (v/v), and food. Within each experiment, the positions of the two drinking bottles were rotated on a daily basis to prevent positional preference. A technician, blinded to the experimental conditions, measured fluid intake every 24 hours, food intake every 48 hours, and body weight every 3–4 days. Once alcohol intake reached a steady baseline (which required, on average, two weeks of alcohol access), drug treatment began. Alcohol drinking was measured every 24 hours, in line with our previous studies (Gulick and Green, 2010; Chau et al., 2011) suggesting that more frequent measures are not informative in the hamster, and that the highest blood alcohol levels occur 8–10 hours into the dark cycle (Chau et al., 2010). All injections were performed 1–2 hours prior to the start of the dark cycle to avoid any immediate locomotor effects of the drugs on alcohol intake. All experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory hamsters (NIH Publications No. 8023, revised 1978) and were approved by the Dartmouth Institutional Animal Care and Use Committee.
2.2. Procedures
2.2.1. Experiment 1: risperidone dose-response
To evaluate whether RISP decreases alcohol intake, we tested its effects on free access, chronic alcohol drinking in the hamster. Fifty hamsters were given access to separate bottles of water and 15% v/v alcohol for 12 days prior to randomization into 7 groups based on baseline alcohol intake (g/kg) (n=7–8 per group); (baseline alcohol intake was calculated using the last 4 days of the initial 12-day period of access to alcohol). The groups were subsequently treated daily for 20 days with either: vehicle (VEH) or a daily dose of RISP (0.05, 0.1, 0.2, 0.5, 1.0, or 2.0 mg/kg). This range of doses of RISP was chosen to provide a wide array of DA D2 receptor occupancy (Wadenberg et al., 2001). All hamsters continued to receive free access to food, water, and alcohol during the treatment period.
2.2.2. Experiment 2: low dose risperidone plus desipramine
We next evaluated whether adding DMI to the 0.2 mg/kg dose of RISP, which was shown to be most effective in reducing alcohol drinking in the first experiment and which approximates the DA D2 receptor binding potential of CLOZ (Schotte et al., 1989; Schotte et al., 1993; Nordstrom et al., 1995; Kapur et al., 2003), would enhance the ability of RISP to decrease alcohol intake. Sixty-two hamsters were given access to separate bottles of water and 15% v/v alcohol for 11 days prior to randomization into 6 groups (n=8–9 per group) with similar baseline alcohol intake values (g/kg). The groups were subsequently treated daily for 20 days with either: VEH; RISP (0.2 mg/kg); DMI (0.2, 1.0, or 5.0 mg/kg); or combinations of one dose of RISP and these doses of DMI. All hamsters continued to receive free access to food, water, and alcohol during the treatment period.
2.3. Drugs
RISP was generously provided by Janssen Pharmaceuticals (Titusville, NJ) and DMI was purchased from Sigma Aldrich (St. Louis, MO). All solutions were prepared for injection by first dissolving each drug in 0.5 N acetic acid, and then adjusting the volume to the desired concentration using a VEH solution (0.5 M sodium acetate, pH 5.5). The pH of each drug solution was adjusted to match the pH of VEH using 5 N NaOH. All drug and VEH solutions were injected subcutaneously in volumes of 2 ml/kg body weight. All injections were given 1 hour before dark to prevent potential acute side effects (e.g., decreased activity) from the drug from interfering with drinking and eating (which normally begin at the onset of the dark cycle).
2.4. Data analysis
Alcohol intake (g/kg), food intake (g/kg), and body weight (g) data were analyzed using two-way repeated measures analysis of variance (RMANOVA), using time (measured in days) and drug treatment as independent variables. When the analysis indicated that significant differences existed between treatments, post hoc pairwise comparisons between groups were made using the Bonferroni adjustment, which is more robust to departures from sphericity (underlying assumption of RMANOVA) than other multiple comparison adjustments (Maxwell, 1980). The pairwise comparisons were tested at each day to help interpret group×time interactions from the RMANOVAs; adjustment to p-values was carried out separately at each day. Fisher’s least significant difference (LSD) test was also used in Experiment 2 to explore differences between RISP alone (0.2 mg/kg) and each combination of RISP with DMI. Significance was determined at p<0.05; for multiple pairwise comparisons, the largest p-value is presented whenever more than one post hoc comparison is significant. Data are expressed as mean (M) ± standard error of the mean (SEM).
3. RESULTS
3.1. Experiment 1: risperidone dose-response
3.1.1. Alcohol intake
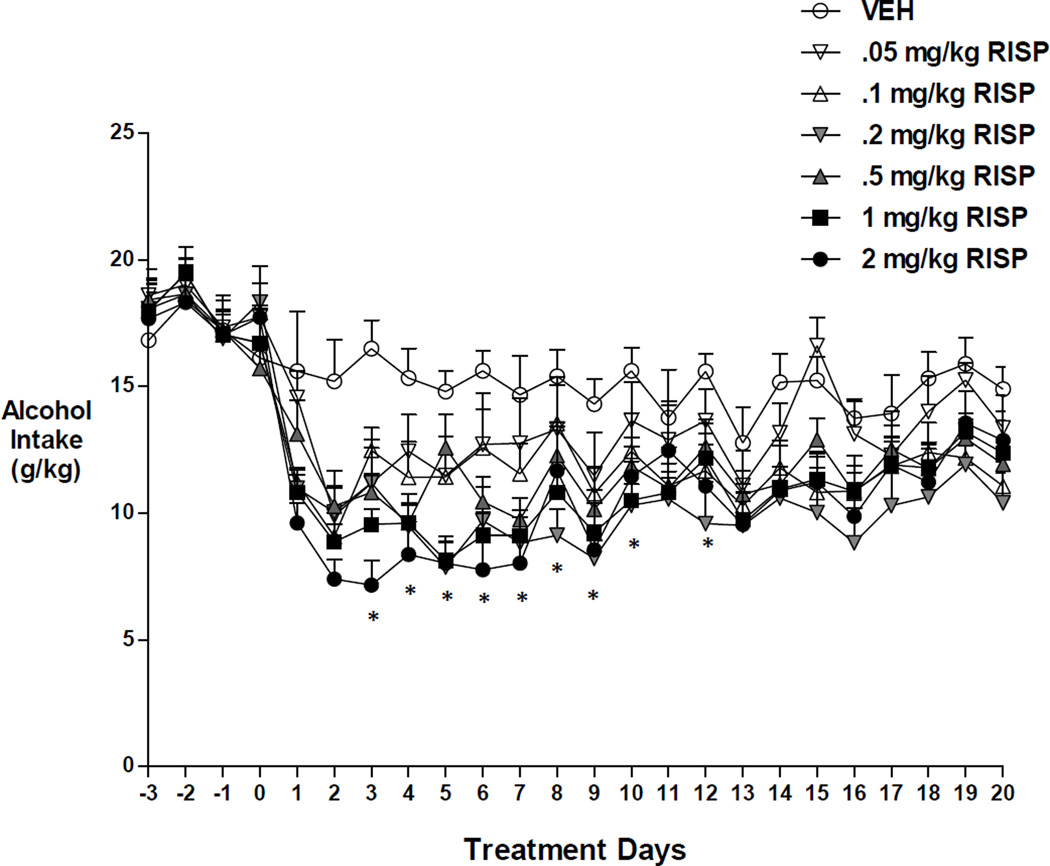
Two-way repeated measures ANOVA revealed a significant effect of time, F(23,989)=53.225, p<0.001, a significant effect of group F(6,43)=2.940, p<0.017, and a significant time by group interaction, F(138,989)=1.877, p<0.001, on alcohol intake in the hamster (Figure 1). Post hoc (Bonferroni) tests demonstrated that 0.2 mg/kg RISP was most effective in reduction of alcohol drinking relative to VEH, but the effects did not persist (days 3–10, 12, p < 0.05). The highest doses showed a similar pattern (1.0 mg/kg RISP: days 2–7, p < 0.05; 2.0 mg/kg RISP: days 1–7, 9, p< 0.05). Other doses differed significantly from VEH only for one or two days during early follow-up.
Figure 1.
Effects of RISP alone on alcohol intake in the hamster. RISP decreased alcohol drinking (g/kg) initially, but this effect diminished over time. *: p<0.05 represents significantly lower drinking in the 0.2 mg/kg RISP group compared to vehicle.
3.1.2. Water and food intake
The two-way ANOVA for water intake indicated a significant effect of time, F(30,1260)=8.45, p<0.001, but no effect of group and no group by time interaction. By contrast, there was a significant effect of time, F(30,1260)=13.05, p<0.001, no effect of group, and a time by group interaction, F(180,1110)=2.51, p<0.05, on food intake in the hamster. Food intake was lower in VEH relative to baseline during most of follow-up, while RISP-treated groups showed some gains in food intake during the first week of the trial but these gains were not maintained. Post hoc RISP-VEH differences were significant at day 6 (0.2 mg/ kg, p < 0.001) and day 8 (0.2 mg/kg, 0.5 mg/kg, 1.0 mg/kg, p < 0.05).
3.2. Experiment 2: low dose risperidone plus desipramine
3.2.1. Alcohol intake
In Experiment 2, the RISP 0.2 mg/kg group was prone to considerable variation, both over time and on any given day (data not shown). The high variance observed in the 0.2 mg/kg RISP (alone) group was due to one outlying animal that drank excessively or modestly on alternate days after day 7 of the treatment period. Moreover, another animal in the group did not drink at all (or only negligibly) during days 4–8, 10–11, and 18–20.
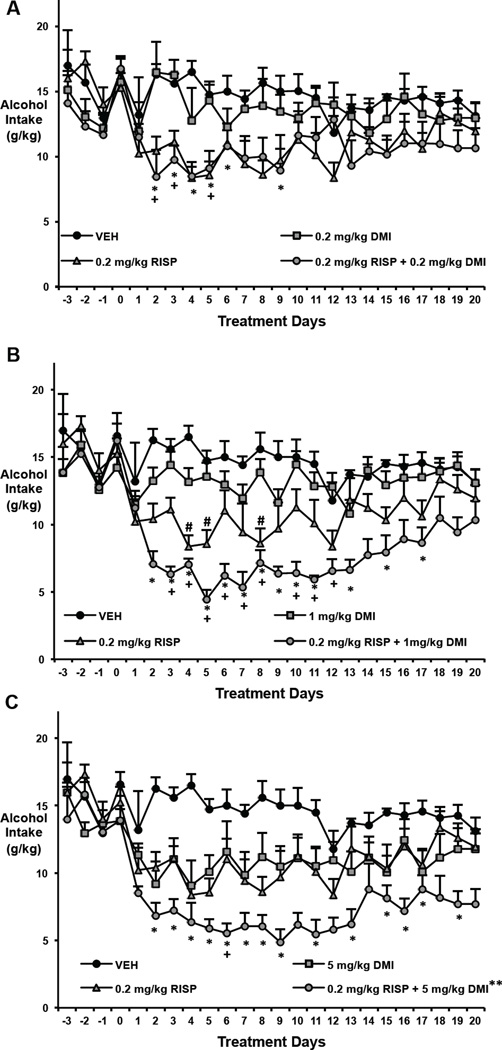
Because no other animal in any group in either Experiment 1 or Experiment 2 exhibited a sustained drop in drinking, we also deemed that animal to also be an outlier; Figure 2 shows the data without the outliers. When both animals were removed from the analysis, two-way repeated measures ANOVA indicated a significant effect of time, F(23,1196)=23.210, p<0.001, and of group, F(7,52)=6.833, p<0.001, as well as a time by group interaction, F(161, 1196)=17.488, p<0.001, on alcohol intake in the hamster.
Figure 2.
Effects of RISP (0.2 mg/kg) plus a DMI dose-range on alcohol intake in the hamster. (A-C) For visual clarity, different doses of DMI are depicted on separate graphs. Two outlying animals in the 0.2 mg/kg RISP were removed from these illustrations (see Section 3.2.1). Both RISP (0.2 mg/kg). and DMI (5 mg/kg) alone decreased alcohol drinking (g/kg). As predicted, DMI (1 mg/kg and 5 mg/kg) appears to enhance the ability of RISP to decrease alcohol drinking. *: p<0.05 represents significantly lower drinking in the combination groups compared to vehicle; +: p<0.05 represents significantly lower drinking in the combination groups compared to the corresponding DMI doses alone; #: p<0.05 represents significantly lower drinking in the 0.2 mg/kg RISP group compared to vehicle (significance only shown in Figure 2B for clarity); **: P<0.05 compared to 0.2 mg/kg RISP using Fisher's LSD.
Post hoc (Bonferroni) tests showed that the combination of RISP with the 1.0 and 5.0 mg/kg doses of DMI provided a more consistent difference from VEH than 0.2 mg/kg RISP alone (0.2 mg/kg RISP vs. VEH: days 4, 5, 8, p < 0.05; 0.2 mg/kg RISP with 1.0 mg/kg DMI vs. VEH: days 2–7, 11, 13, 15 and 17, p< 0.05; 0.2 mg/kg RISP with 5.0 mg/kg DMI vs. VEH: days 2–9, 11, 13, 15–17 and 19, p<0.05; Figures 2B and 2C). Fisher’s LSD test further showed that the combination with 5.0 mg/kg DMI was significantly different from 0.2 mg/kg RISP alone (p<0.05), and the combination with 1 mg/kg DMI trended toward being significantly different from 0,2 mg/kg RISP (p=0.058). By contrast, the combination of RISP with 0.2 mg/kg DMI only differed significantly from VEH on days 2–5 and 9 (p < 0.05) (Figure 2A) and did not differ significantly from RISP alone (using Fisher’s LSD).
Post hoc tests also showed that the combination of RISP with 1.0 mg/mg of DMI provided the most consistent difference from DMI alone (0.2 mg/kg RISP with 1.0 mg/kg DMI vs. 1.0 mg/kg DMI: days 3–8 and 10–12, p< 0.05) (Figure 2B). The other two doses of DMI differed on far fewer days than their corresponding combination with RISP (0.2 mg/kg RISP with 0.2 mg/kg DMI vs. 0.2 mg/kg DMI: days 2–3 and 5, p< 0.05); 0.2 mg/kg RISP with 5.0 mg/kg DMI vs. 5.0 mg/kg DMI: days 6, p< 0.05) (Figures 2A and 2C).
3.2.2. Water and food intake and body weight
The RMANOVA for water intake revealed a significant effect of time, F(27,1431)=10.34, p<0.001, no effect of group (p=0.063), and a time by group interaction, F(189, 1269)=1.23, p<0.05. Bonferroni post hoc tests demonstrated that the RISP alone group drank significantly more water than VEH on days 3 and 17, while 1.0 mg/kg and 0.2 mg/kg of DMI differed from VEH at day 13 and day 7, respectively. We also considered the impact of the two animals with unusual alcohol intake on the results for water intake. Removing the two animals with outlying alcohol drinking behavior from the analysis produced a more straight-forward result: there was a significant effect of time, F(23, 1196)=6.665, p<0.001, an effect of group, F(7,52)=3.607, p<0.005, but no time by group interaction. Post hoc results showed that the RISP alone group drank significantly more water than all groups except for the VEH group.
Two-way repeated measures ANOVA indicated a significant effect of time, F(27,1431)=33.70, p<0.001, an effect of group, F(7,54)=3.09, p<0.01, but no time by group interaction, on food intake in the hamster. Food intake in all groups increased after baseline but declined after mid-study. Similar results were obtained when the two outlying animals were removed.
The results for body weight were similar to those for water intake: a significant effect of time, F(27,1431)=73.32, p<0.001, no effect of group, and a time by group interaction, F(189, 1269)=7.4, p<0.001. Body weight increased significantly between baseline and each successive measurement period (every 3–4 days) in the controls and in the DMI alone groups (p<0.05), but there were not any significant changes in body weight in the groups treated with RISP. Post hoc tests did not reveal significant differences among groups at any measurement period. The ANOVA results were essentially unchanged when the two animals with outlying alcohol drinking behavior were removed from the analysis.
4. DISCUSSION
The data presented here indicate that the atypical antipsychotic RISP has only a transient ability to decrease chronic alcohol drinking in the Syrian golden hamster on its own. Our data are consistent with the previous reports of Panocka and colleagues indicating that a low dose of RISP can decrease alcohol drinking in non-selected Wistar rats, but not in alcohol preferring Sardinian rats (1993a; 1993b). These findings with RISP, together with our previously published data indicating that CLOZ dramatically decreases alcohol drinking in the hamster (Green et al., 2004; Chau et al., 2010), are also consistent with reports from our group and others suggesting that CLOZ limits alcohol use in patients with schizophrenia, whereas RISP does not appear to do so (e.g., Green et al., 2004)
Our data and the data of Panocka and colleagues (Panocka et al., 1993b) further demonstrate that a low dose of RISP (one with a DA D2 receptor blockade of approximately 40% -- similar to the D2 receptor blockade produced by clinically relevant doses of CLOZ (Schotte et al., 1989; Nordstrom et al., 1995; Kapur et al., 2003)) can significantly decrease alcohol drinking for the short-term, but that higher doses of RISP, with a DA D2 receptor blockade of 80% or higher, have no greater effect than the lower doses of RISP. A new finding in this study is that adjunctive use of DMI appears to enhance the ability of RISP to decrease alcohol drinking.
Previous literature has indicated that DMI can reduce alcohol drinking (McBride et al., 1988; Mason et al., 1996; Goldstein et al., 2004; Simon O'Brien et al., 2011), but at doses that may confer increased risk of cardiovascular toxicity (Pacher and Kecskemeti, 2004). The dose range for DMI treatment in humans is generally 150–200 mg/day (which converts to approximately 15–18 mg/kg in the hamster (Reagan-Shaw et al., 2008)). The apparent ability of low to moderate doses (1.0 and 5.0 mg/kg) of DMI to enhance the effects of a low dose of RISP in our study may provide an important lead toward the development of new medications that may potentially limit alcohol use in patients with schizophrenia without the adverse effects associated with high doses of DMI. The 1.0 mg/kg dose of DMI may, in fact, be optimal for combining with RISP as it has no ability to reduce alcohol drinking on its own, but can seemingly amplify the ability of RISP to do so. Furthermore, the combination of 1.0 mg/kg DMI with RISP was most consistent in being significantly different from its corresponding DMI dose (1.0 mg/kg DMI) alone from the three DMI dose combinations with RISP tested here, further supporting its potential use as an adjunct therapy to RISP for reducing alcohol drinking.
Since both RISP and DMI are metabolized by CYP2D, possible pharmacokinetic interactions should be considered. However, it seems unlikely that this would confound our data since the parent compounds and the CYP2D-mediated metabolites of RISP and DMI (9-hydroxy risperidone and 2-hydroxydesipramine) are all biologically active. Moreover, even if DMI caused an increased in RISP levels, we would not expect it to further reduce alcohol intake, since increasing the RISP dose in this study did not further reduce alcohol drinking (Figure 1).
Our study appears to provide support for our neurobiologic hypothesis regarding the effects of CLOZ – that strong noradrenergic activity in combination with a relatively weak DA D2 receptor blockade may be important components underlying CLOZ’s ability to decrease alcohol drinking. While our hypothesis suggests that blockade of NE α2 receptors may be important for CLOZ’s action, our finding that RISP alone, which is a potent NE alpha 2 antagonist (Richelson and Souder, 2000), does not decrease alcohol drinking, suggests that the alpha 2 antagonism itself may not be sufficient to explain CLOZ’s action. Importantly, in vitro studies suggest that CLOZ has NE reuptake blocking ability (Yoshimura et al., 2000).
Some limitations in this study include the inability of this study to assess whether DMI would have a beneficial effect in combination with RISP if RISP were not also a potent NE α2 receptor antagonist. Furthermore, we recognize that the presence of the two outlying animals in the RISP group tempers the conclusions we can draw from the present experiment and that our results require replication with a larger number of animals.
In summary, the results of this research appear to suggest that, similar to its effects in patients with schizophrenia, RISP alone does not persistently decrease alcohol drinking in the Syrian golden hamster. However, when a low dose of RISP is combined with the NE reuptake inhibitor DMI, the combination seems to demonstrate greater efficacy in reducing alcohol drinking in the hamster. This finding may provide a clue toward the development of new medications useful for the treatment of patients with schizophrenia and co-occurring alcohol use disorder, and potentially for those with alcoholism alone as well.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Alcohol Abuse and Alcoholism (AIG; 1R03AA014644 and 1R01AA018151-02), and from the National Center for Advancing Translational Science (AIG; NCATS UL1TR001086), and by an investigator-initiated contract from Janssen Pharmaceuticals (AIG). The authors have maintained full control of all primary data and they agree to allow the journal to review their data if requested.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aberg-Wistedt A, Jostell KG, Ross SB, Westerlund D. Effects of zimelidine and desipramine on serotonin and noradrenaline uptake mechanisms in relation to plasma concentrations and to therapeutic effects during treatment of depression. Psychopharmacology (Berl) 1981;74:297–305. doi: 10.1007/BF00432735. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Erdlen FR, Murphy E. Alcohol abuse in the psychiatric hospital population. Addictive Behaviors. 1981;6:69–73. doi: 10.1016/s0306-4603(81)80012-3. [DOI] [PubMed] [Google Scholar]
- Arvola A, Forsander A. Hamsters in experiments of free choice between alcohol and aater. 1963;24:591–597. [PubMed] [Google Scholar]
- Arvola A, Forsander O. Comparison between water and alcohol consumption in six animal species in free choice experiments. Nature. 1961;191:819–820. doi: 10.1038/191819a0. [DOI] [PubMed] [Google Scholar]
- Chau DT, Ahmed J, Wang TT, Xie H, Dawson R, Green AI. Raclopride lessens the ability of clozapine to suppress alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2011;61:646–652. doi: 10.1016/j.neuropharm.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Chau DT, Gulick D, Xie H, Dawson R, Green AI. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58:351–356. doi: 10.1016/j.neuropharm.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Khokhar JY, Dawson R, Ahmed J, Xie H, Green AI. The comparative effects of clozapine versus haloperidol on initiation and maintenance of alcohol drinking in male alcohol-preferring P rat. Alcohol. 2013;47:611–618. doi: 10.1016/j.alcohol.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser KT. Alcohol-use disorder and severe mental illness. Alcohol Health & Research World. 1996;20:87–93. [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Osher FC, Wallach MA. Alcohol use and abuse in schizophrenia. A prospective community study. The Journal of Nervous and Mental Disease. 1989;177:408–414. doi: 10.1097/00005053-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophrenia Bulletin. 2000;26:441–449. doi: 10.1093/oxfordjournals.schbul.a033464. [DOI] [PubMed] [Google Scholar]
- Elman I, Goldstein DS, Green AI, Eisenhofer G, Folio CJ, Holmes CS, Pickar D, Breier A. Effects of risperidone on the peripheral noradrenegic system in patients with schizophrenia: a comparison with clozapine and placebo. Neuropsychopharmacology. 2002;27:293–300. doi: 10.1016/S0893-133X(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Schaffer A, Levitt A, Zaretsky A, Joffe RT, Wesson V, Bagby RM. Depressive symptoms and alcohol consumption among nonalcoholic depression patients treated with desipramine. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2004;49:859–862. doi: 10.1177/070674370404901210. [DOI] [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophrenia Researcb. 2003;60:81–85. doi: 10.1016/s0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Green AI, Chau DT, Keung WM, Dawson R, Mesholam RI, Schildkraut JJ. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Research. 2004;128:9–20. doi: 10.1016/j.psychres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, O'Keefe C. Substance abuse and schizophrenia: pharmacotherapeutic intervention. Journal of Substance Abuse Treatment. 2008;34:61–71. doi: 10.1016/j.jsat.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a rewarddeficiency syndrome that can be ameliorated by clozapine? Harvard Review of Psychiatry. 1999;6:287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Gulick D, Green AI. Role of caloric homeostasis and reward in alcohol intake in Syrian golden hamsters. Physiology & Behavior. 2010;101:518–526. doi: 10.1016/j.physbeh.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Hendricks S, Kenkel AM, Bhatia SC, Haffke EA. Relapse in schizophrenia: is there a relationship to substance abuse? Schizophrenia Research. 1996;20:153–156. doi: 10.1016/0920-9964(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Ingman K, Honkanen A, Hyytia P, Huttunen MO, Korpi ER. Risperidone reduces limited access alcohol drinking in alcohol-preferring rats. European Journal of Pharmacology. 2003;468:121–127. doi: 10.1016/s0014-2999(03)01669-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. Journal of Pharmacology and Experimental Therapeutics. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Keung WM, Kunze L, Li DJ, Lazo O. Volitional ethanol consumption affects overall serotonin metabolism in Syrian golden hamsters (Mesocricetus auratus) Biochemical and Biophysical Research Communications. 2000;271:823–830. doi: 10.1006/bbrc.2000.2718. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Cornell NW. Free-choice ethanol intake and ethanol metabolism in the hamster and rat. Pharmacology Biochemistry and Behavior. 1979;11:439–444. doi: 10.1016/0091-3057(79)90121-7. [DOI] [PubMed] [Google Scholar]
- Lee ML, Dickson RA, Campbell M, Oliphant J, Gretton H, Dalby JT. Clozapine and substance abuse in patients with schizophrenia. Canadian Journal of Psychiatry. 1998;43:855–856. [PubMed] [Google Scholar]
- Lehman AF, Myers CP, Dixon LB, Johnson JL. Detection of substance use disorders among psychiatric inpatients. The Journal of Nervous and Mental Disease. 1996;184:228–233. doi: 10.1097/00005053-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Kocsis JH, Ritvo EC, Cutler RB. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA: the Journal of the American Medical Association. 1996;275:761–767. [PubMed] [Google Scholar]
- Maxwell S. Pairwise multiple comparisons in repeated measures designs. Journal of Educational Statistics. 1980;5:269–287. [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Effects of Ro 15-4513, fluoxetine and desipramine on the intake of ethanol, water and food by the alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacology, Biochemistry, and Behavior. 1988;30:1045–1050. doi: 10.1016/0091-3057(88)90137-2. [DOI] [PubMed] [Google Scholar]
- McCoy GD, Haisley AD, Powchik P, Tambone PC. Ethanol consumption by Syrian golden hamsters. Food intake and blood ethanol levels. J Stud Alcohol. 1981;42:508–513. doi: 10.15288/jsa.1981.42.508. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. American Journal of Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatric Services. 1996;47:853–858. doi: 10.1176/ps.47.8.853. [DOI] [PubMed] [Google Scholar]
- Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Current Pharmaceutical Design. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panocka I, Ciccocioppo R, Pompei P, Massii M. 5-HT2 receptor antagonists do not reduce ethanol preference in Sardinian alcohol-preferring (sP) rats. Pharmacology Biochemistry and Behavior. 1993a;46:853–856. doi: 10.1016/0091-3057(93)90212-c. [DOI] [PubMed] [Google Scholar]
- Panocka I, Pompei P, Massi M. Suppression of alcohol preference in rats induced by risperidone, a serotonin 5-HT2 and dopamine D2 receptor antagonist. Brain Research Bulletin. 1993b;31:595–599. doi: 10.1016/0361-9230(93)90128-x. [DOI] [PubMed] [Google Scholar]
- Piercy KT, Myers RD. Female Syrian golden hamster: drinking of high concentrations of ethanol aversive to other mammals. Alcohol. 1995;12:207–211. doi: 10.1016/0741-8329(94)00084-q. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. The FASEB Journal. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA: the Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sciences. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Schotte A, de Bruyckere K, Janssen PF, Leysen JE. Receptor occupancy by ritanserin and risperidone measured using ex vivo autoradiography. Brain Research. 1989;500:295–301. doi: 10.1016/0006-8993(89)90325-9. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Megens AA, Leysen JE. Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Research. 1993;631:191–202. doi: 10.1016/0006-8993(93)91535-z. [DOI] [PubMed] [Google Scholar]
- See RE, Fido AA, Maurice M, Ibrahim MM, Salama GM. Risperidone-induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Biological Psychiatry. 1999;45:1653–1656. doi: 10.1016/s0006-3223(98)00199-1. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, O'Neill MF, Fernandez AG, Palacios JM. Effects of a range of dopamine receptor agonists and antagonists on ethanol intake in the rat. European Journal of Pharmacology. 1996;318:257–265. doi: 10.1016/s0014-2999(96)00821-7. [DOI] [PubMed] [Google Scholar]
- Simon O'Brien E, Legastelois R, Houchi H, Vilpoux C, Alaux-Cantin S, Pierrefiche O, Andre E, Naassila M. Fluoxetine, desipramine, and the dual antidepressant milnacipran reduce alcohol self-administration and/or relapse in dependent rats. Neuropsychopharmacology. 2011;36:1518–1530. doi: 10.1038/npp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test MA, Wallisch LS, Allness DJ, Ripp K. Substance use in young adults with schizophrenic disorders. Schizophrenia Bulletin. 1989;15:465–476. doi: 10.1093/schbul/15.3.465. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Yanagihara N, Hara K, Terao T, Nakamura J, Ueno S, Toyohira Y, Uezono Y, Kaneko S, Kawamura M, Abe K, Izumi F. Inhibitory effects of clozapine and other antipsychotic drugs on noradrenaline transporter in cultured bovine adrenal medullary cells. Psychopharmacology (Berl) 2000;149:17–23. doi: 10.1007/s002139900339. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. Journal of Clinical Psychopharmacology. 2000;20:94–98. doi: 10.1097/00004714-200002000-00016. [DOI] [PubMed] [Google Scholar]