Abstract
Schistosoma japonicum infection is believed to be endemic in 28 of the 80 provinces of The Philippines and the most recent data on schistosomiasis prevalence have shown considerable variability between provinces. In order to increase the efficient allocation of parasitic disease control resources in the country, we aimed to describe the small-scale spatial variation in S. japonicum prevalence across The Philippines, quantify the role of the physical environment in driving the spatial variation of S. japonicum, and develop a predictive risk map of S. japonicum infection. Data on S. japonicum infection from 35,754 individuals across the country were geolocated at the barangay level and included in the analysis. The analysis was then stratified geographically for the regions of Luzon, the Visayas and Mindanao. Zero-inflated binomial Bayesian geostatistical models of S. japonicum prevalence were developed and diagnostic uncertainty was incorporated. Results of the analysis show that in the three regions, males and individuals aged ≥ 20 years had significantly higher prevalence of S. japonicum compared with females and children < 5 years. The role of the environmental variables differed between regions of The Philippines. Schistosoma japonicum infection was widespread in the Visayas whereas it was much more focal in Luzon and Mindanao. This analysis revealed significant spatial variation in the prevalence of S. japonicum infection in The Philippines. This suggests that a spatially targeted approach to schistosomiasis interventions, including mass drug administration, is warranted. When financially possible, additional schistosomiasis surveys should be prioritized for areas identified to be at high risk but which were under-represented in our dataset.
Keywords: Schistosoma japonicum, Risk mapping, Philippines, Disease control, Disease elimination
1. Introduction
Zoonotic schistosomiasis, caused by Schistosoma japonicum, is endemic to areas of China, Indonesia and The Philippines, where it primarily affects children and adolescents as well as individuals in high-risk occupational groups such as rice farmers and fishermen (Leonardo et al., 2002; Zhou et al., 2010). The most recent national parasite survey in The Philippines concluded that S. japonicum infection was endemic in 28 of the 80 provinces of the country (Leonardo et al., 2008, 2012). An estimated 6.7 million people are considered at risk, with 1.8 million estimated to be infected (Leonardo et al., 2002). Schistosoma japonicum infection is a major cause of anaemia (Leenstra et al., 2006), stunted growth (Coutinho et al., 2005; Leenstra et al., 2006), and chronic abdominal organ pathology including portal vein distension, hepato- and splenomegaly and hepatic fibrosis (Li et al., 2000a, 2002, 2003; Balen et al., 2007). Studies from The Philippines have also suggested that S. japonicum and soil-transmitted helminth (STH) infections impair the cognitive development of school-aged children as measured by school performance (Ezeamama et al., 2005, 2012).
Treatment with praziquantel reverses the adverse health effects of S. japonicum, particularly in malnourished and anaemic individuals (Coutinho et al., 2005). Interventions using mass drug administration (MDA) of praziquantel in the late 1980s and 1990s successfully reduced the national prevalence of infection to a persistent 4–5%. However, recent experience in Western Samar has shown very low acceptance of MDA by the population, despite community mobilization activities, with an average coverage of 48.3% in 50 villages, and values as low as 15.8% (Tallo et al., 2008). This has likely contributed to the prevalence of infection still being reported in some communities to be as high as 65% (Leonardo et al., 2008, 2012).
The role that reservoir hosts play in the epidemiology of human S. japonicum in The Philippines remains uncertain. Observational studies in Western Samar have shown that the prevalence of infection and S. japonicum strains in dogs and humans are correlated (McGarvey et al., 2006; Rudge et al., 2008). A mathematical model of S. japonicum transmission has shown that most of the transmission to humans was attributed to contamination from humans, with perhaps a small role of infected rats (Riley et al., 2008). More recently it has been suggested that bovines, particularly carabao, may be more important in the transmission of S. japonicum in The Philippines than had been previously recognized (Gordon et al., 2012).
The most up-to-date schistosomiasis prevalence data from The Philippines, and data from Western Samar, have shown considerable variability between communities (Tarafder et al., 2006; Leonardo et al., 2008, 2012). Spatial clustering of schistosome infections in high-prevalence communities is a well-described phenomenon (Clements et al., 2006). The only application of spatial analysis to the study of parasitic diseases in The Philippines comes from a subnational study in the province of Mindanao, which focused on malaria and schistosomiasis (Leonardo et al., 2005). The identification of clusters of schistosomiasis risk in The Philippines will have important public health implications in that it will enable targeting of MDA, helping to increase the efficiency of parasite control in the country, and to identify areas where elimination could be achieved (Clements et al., 2006, 2008).
In this study we used data from the most recent national schistosomiasis prevalence survey and additional schistosomiasis survey data from Samar, with the aims of: i) describing the spatial variation in S. japonicum risk across regions of The Philippines; ii) quantifying the role of variables in the physical environment in the spatial variation of S. japonicum, using models that account for the highly clustered nature of infection and for diagnostic uncertainty; and iii) developing predictive risk maps of S. japonicum infection to better position health authorities to target interventions to control the disease in The Philippines.
2. Materials and methods
2.1. Ethics statement
Ethical clearance for this analytical study was provided by the University of Queensland Human Research Ethics Committee, Australia (Project Number 2011000692).
2.2. Infection data
We used S. japonicum infection data collected during the most recent national schistosomiasis surveys conducted in 2008 (Leonardo et al., 2008, 2012) (Supplementary Data S1). The surveys were divided into four phases: phase 1 for Mindanao (excluding Maguindanao), phase 2 for the Visayas, phase 3 for Luzon and phase 4 for Maguindanao. By the time of the survey in Maguindanao, several rounds of mass treatment had been conducted, but information on time since the last MDA was not available for each barangay (the smallest administrative division in The Philippines equivalent to a village, district or ward) included in the analysis. The proportion of eligible people who participated in the national schistosomiasis survey varied from 73% in the Luzon survey to 45% in the Maguindanao survey. To improve the geographical coverage of our data, we also included parasitological data from a previous survey in 50 barangays of Western Samar (Tarafder et al., 2006) (Supplementary Data S1). In brief, villages in this study were largely rice farming communities; 70% of the villagers identified farming as their primary occupation, with 15% declaring themselves as not being farmers (mostly females staying at home). While in the national survey, S. japonicum infections were diagnosed by the detection of eggs in two stool samples collected on two separate days from each individual, using a Kato–Katz thick smear examination, in the Western Samar study, samples were collected for up to three consecutive days and analysed with two slides per sample. In the national survey however, the submission of the second stool sample was not consistent (Leonardo et al., 2008, 2012). Therefore, for the purpose of maintaining consistency between the datasets, we used the results of only the first stool sample available in both the national survey and in the Western Samar study.
2.3. Geolocation of barangays
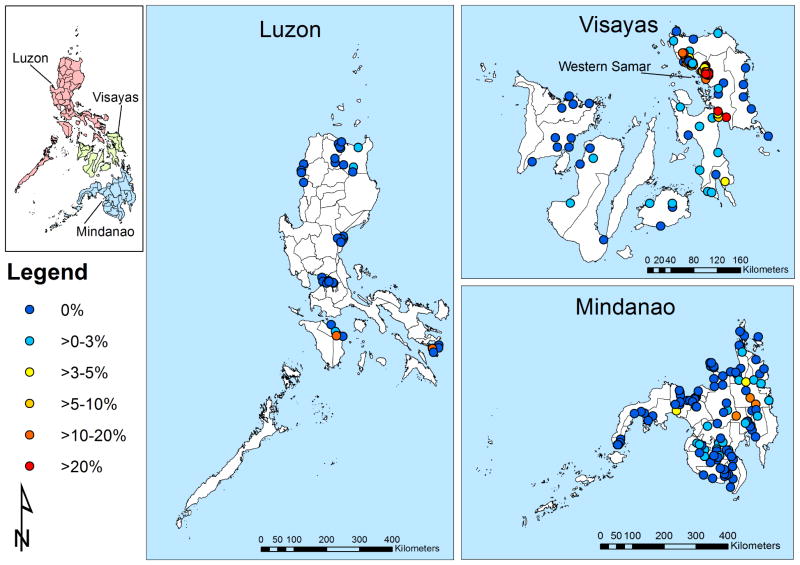
The barangay, which is an administrative subdivision in The Philippines, was used as the geographical unit for the analysis. The mean length of the longest axis of barangays was 11 km (S.D. = 10.3). Barangay coordinates were available for the Samar study but not for the national schistosomiasis survey. To geolocate barangays, we used the centroid of the barangays, obtained from an up-to-date barangay shapefile downloaded from the DIVA geographic information system (GIS) website (http://www.diva-gis.org/Data). Using this approach, data from 36,828 individuals (91.3%; n = 40,357) aged 1–96 years from the national survey were geo-referenced. There was a total of 37 barangays in Luzon, 93 barangays in the Visayas and 108 barangays in Mindanao (Fig. 1). The prevalence of schistosomiasis in locations for which no geographical information was available (corresponding to a total of 8.7% of individuals) was not systematically different from those included in the analysis.
Fig. 1.
Geographical distribution of schistosomiasis survey locations and observed prevalence of infection in The Philippines
2.4. Data on the physical environment
Studies have consistently shown the importance of the physical environment, such as topographical features and climate variables, as drivers of S. japonicum infection (Raso et al., 2009; Schrader et al., 2013). The most important driver for the exposure of humans to the infective larval stages is the existence of water bodies contaminated with the intermediate snail hosts. A shapefile of large perennial inland water bodies was obtained from the Food and Agriculture Organization of the United Nations (http://www.fao.org/geonetwork/srv/en/main.home) and the distance to large perennial water bodies (DPWB) was estimated in the GIS. Climatic factors such as land surface temperature and rainfall have been shown to be important to schistosomiasis transmission because these determine the existence of water bodies and the survival of the larval stages and the snail intermediate hosts (Prah and James, 1977; Woolhouse and Chandiwana, 1990; Pietrock and Marcogliese, 2003). It has been established that the optimum temperature range for schistosomiasis transmission is water temperatures of 22–27°C (Shiff et al., 1979). Land surface temperature can be a good approximation of the water temperature of perennial water bodies because the thermal conditions of shallow waters usually reflect the ambient temperature of the air. Electronic data for land surface temperature (LST) and rainfall for a 1 km × 1 km grid cell resolution were also obtained from the WorldClim data warehouse. Land cover, particularly the presence of flooded agricultural land (such as paddy fields), is also an important environmental factor for Asian schistosomiasis (Zhou et al., 2012). Electronic data for the normalised difference vegetation index (NDVI, which quantifies the greenness of vegetation, and is influenced by elevation, temperature, rainfall and other factors such as urbanisation)) for a 1 km × 1 km grid cell resolution were obtained from the National Oceanographic and Atmospheric Administration’s Advanced Very High Radiometer (http://www.ciesin.org/TG/RS/noaaavhr.html). Values of the environmental variables were extracted for each barangay using the spatial overlay procedure in the GIS.
2.5. Variable selection and residual spatial variation
For the purpose of the analysis, the presence of S. japonicum eggs in stool identified by the Kato-Katz method was used as the outcome variable, and thus all individuals were categorized into infected or uninfected based on the presence of at least one parasite egg. The individual level variables (age, sex) and variables of the physical environment (DPWB, LST, rainfall and NDVI) were considered in the initial variable screening stage. Correlations between environmental variables were investigated and scatter plots constructed to assess the linearity between prevalence of infection and environmental variables. Multivariable logistic regression models for a Bernoulli-distributed outcome, with cluster correction by barangay using robust standard errors, were built using the statistical software Stata version 10.1 (Stata corporation, College Station, TX, USA). Spatial dependence in the residuals of this model was investigated using a semivariogram in the statistical software R, using the geoR package version 2.14.1 (The R foundation for statistical computing, Vienna, Austria, http://www.R-project.org). A semivariogram is a graphical representation of the spatial variation left unexplained by the covariates included in the model. Semivariograms allow for the quantification of spatial cluster size and the tendency for geographical clustering within a region. The semivariogram is characterized by three parameters: the sill, which is the spatially structured component of the semivariance (indicative of the tendency for geographical clustering), the nugget, which is the spatially unstructured component of the semivariance (representing random variation, very small-scale spatial variability or measurement error) and the range, which is the distance at which locations can be considered independent (indicative of the size of geographical clusters).
2.6. Spatial risk prediction and model validation
Three separate zero-inflated binomial (ZIB) Bayesian geostatistical models of S. japonicum prevalence, one each for Luzon, the Visayas and Mindanao, were developed in WinBUGS 1.4 (Medical Research Council, Cambridge, UK and Imperial College London, UK) (see Supplementary Data S1). ZIB models were selected because prevalence in most survey locations was zero (Fig. 1). The models incorporated adjustment for diagnostic uncertainty due to the low sensitivity of the Kato-Katz method. Adjustment for diagnostic uncertainty was included in the model formulation to account for the fact that most S. japonicum infections were of low intensity and that only one stool sample was considered in the analysis. A ZIB model assumes two sources for the zero-prevalence: some zeros are structural and not random, and the remainder arise with a probability defined by a Binomial distribution. The model includes an intercept, the individual level variables age and sex, the environmental variables LST, rainfall, DPWB, and a geostatistical random effect. The geostatistical random effect modelled spatial correlation as a function of the separating distance between pairs of barangays. The covariate effects were summarized using the mean and 95% credible intervals (CrI, representing the range of values that contains the true value with a probability of 95%). In addition, the model includes adjustment for diagnostic uncertainty by modelling sensitivity and specificity as random variables (Supplementary Table S1). True prevalence was modelled as a function of the observed prevalence and test sensitivity and specificity, with the generalised linear model fit to the true prevalence parameter. Priors for the sensitivity and specificity were specified as beta distributions parameterized by two parameters (alpha and beta); we used alpha and beta parameter values reported in previous studies (Supplementary Data S1). Competing models with and without the ZIB formulation, and with and without diagnostic uncertainty, were also tested and the model fit was assessed using the deviance information criterion (DIC).
The prediction model included the individual level variables age and sex and the variables of the physical environment temperature, rainfall and distance to large perennial water bodies. While using age and sex allowed prediction of the subgroup most at risk of S. japonicum infection in the study area (i.e. males aged at least 20 years old), the use of the environmental variables allowed prediction across a continuous landscape. The predictive spatial distribution for each age and sex class is identical but the overall mean varies.
To determine the discriminatory performance of the model predictions in the validation subset of the data relative to observed prevalence thresholds (1% and 10%) in that subset, the area under the curve (AUC) of the receiver operating characteristic (ROC) was used. The prevalence threshold of 10% was used because it is the lowest prevalence threshold recommended to trigger schistosomiasis control using MDA and 1% was used because it approximated the median prevalence for the regions. An AUC value of more than 70% was taken to indicate acceptable predictive performance (Brooker et al., 2002).
3. Results
3.1. Data analysis
For the purpose of spatial modelling, 2,696 individuals in Luzon, 13,295 individuals in the Visayas and 19,763 individuals in Mindanao, with complete information regarding S. japonicum infection status, barangay geolocation and demographics (i.e. age and sex), were included in the analysis (Table 1). The mean observed prevalence of S. japonicum infection in the combined datasets was 1.6% in Luzon, 4.1% in the Visayas and 0.6% in Mindanao. The majority of S. japonicum infections were of low intensity; none of the individuals included in the analysis from Luzon had egg counts greater than 50 eggs/g, and only eight individuals from Visayas and 60 individuals from Mindanao had egg counts greater than 50 eggs/g.
Table 1.
Characteristics of 35,754 individuals and properties of the physical environment of survey locations in The Philippines included in the analysis.
| Region
|
|||
|---|---|---|---|
| Variable | Luzon | Visayas | Mindanao |
| Number of survey locations | 36 | 93 | 108 |
| Total number of individuals | 2,696 | 13,295 | 19,763 |
| Number with Schistosoma japonicum infection | 43 | 549 | 126 |
| Age in years | |||
| <5 | 274 | 1,635 | 2,733 |
| 5–20 | 462 | 5,257 | 7,433 |
| >20 | 1,960 | 6,403 | 9,597 |
| Sex | |||
| Male | 1,269 | 6,650 | 9,648 |
| Female | 1,427 | 6,645 | 10,115 |
| Rainfall (mm) | |||
| Mean | 314.22 | 242.51 | 213.78 |
| S.D. | 127.53 | 45.52 | 55.43 |
| Land surface temperature (C°) | |||
| Mean | 27.51 | 27.42 | 26.07 |
| S.D. | 1.03 | 0.44 | 1.46 |
| Normalised Difference Vegetation Index | |||
| Mean | 1,493.45 | 1,568.97 | 1,545.80 |
| S.D. | 148.97 | 73.30 | 72.16 |
| Distance to perennial water bodies (km) | |||
| Mean | 16.7 | 39.8 | 38.9 |
| S.D. | 15.5 | 18.5 | 33.3 |
3.2. Residual spatial dependence
Our results indicate that the spatial process of S. japonicum infection in tThe Philippines is non-stationary (i.e. it varies between the three regions of tThe Philippines). While the residual semivariogram for Luzon did not reveal significant small-scale spatial variation unaccounted for by the variables in the final (non-spatial) multivariable model, the residual semivariogram of the final multivariable model for the Visayas and Mindanao showed considerable residual spatial variation (Fig. 2).
Fig. 2.
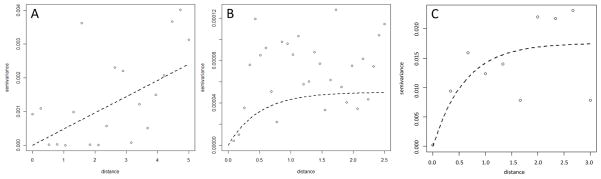
Residual semivariograms for Schistosoma japonicum infection in the regions of Luzon (A), Visayas (B) and Mindanao (C) in The Philippines.
3.3. Spatial risk prediction
The effect of the different factors on the prevalence level varied from region to region (Table 2). For factors associated with the prevalence level, the magnitudes of the effects were generally larger for the Visayas than for Luzon and Mindanao.
Table 2.
Mean regression coefficient estimates (95% Bayesian Credible Interval, CrI) for variables included in a spatial random-effect zero-inflated binomial Bayesian model. Results are reported separately for the regions of Luzon, Visayas and Mindanao, in The Philippines.
| Variable | Luzon Posterior mean (95% CrI) | Visayas Posterior mean (95% CrI) | Mindanao Posterior mean (95% CrI) |
|---|---|---|---|
| Age 5–19 (vs Age <5) | 1.66 (−1.37, 5.17) | 9.305 (1.67, 20.54) | 2.70 (−5.08, 13.57) |
| Age ≥20 (vs Age <5) | 2.97 (0.42, 6.48) | 10.23 (2.66, 21.39) | 6.04 (1.86, 13.92) |
| Male (vs Female) | 1.19 (0.03, 2.21) | 2.92 (0.69, 7.82) | 10.03 (1.94, 21.67) |
| Distance to water bodiesa | −6.68 (−14.04, −0.66) | −21.81 (−43.20, −3.53) | 1.23 (−5.40, 5.21) |
| Land surface temperaturea | −4.75 (−8.81, −1.69) | −1.73 (−15.88, 10.10) | 5.79 (−3.76, 18.2) |
| NDVIa | 4.13 (0.86, 8.25) | −13.78 (−30.55, −2.41) | −2.49 (−16.54, 17.60) |
| Rainfalla | 1.04 (−8.52, 10.93) | 13.9 (5.93, 20.92) | −7.53 (−14.78, −1.06) |
| Intercept | −8.08 (−12.88, −4.56) | −19.5 (−33.90, −5.20) | 2.94 (−6.84, 14.15) |
| Spatial parameters | |||
| Phi (ϕ) | 11.41 (2.263, 19.59) | 10.56 (1.32, 29.15) | 12.12 (3.00, 19.6) |
| Variance of spatial random effect | 17.56 (0.04, 97.3) | 20.72 (2.40, 191.10) | 38.63 (11.55, 113.6) |
Variables were standardized to have mean = 0 and S.D. = 1.
Statistically significant effect sizes are presented in bold. NDVI, normalised difference vegetation index.
Model results (Table 2) indicated that the prevalence of infection was increased in adults aged 20 years or older in all three regions and in 5–19 year olds in the Visayas compared with children aged 5 years old or less. In addition, the prevalence of infection was higher in males than females in all three regions.
In Luzon and the Visayas, the prevalence of infection decreased as the DPWB increased. As LST increased, the prevalence of infection decreased in Luzon. While NDVI was associated with a decrease in prevalence in the Visayas, it was associated with an increase in prevalence in Luzon. Increased rainfall was associated with increased prevalence in the Visayas but with decreased prevalence in Mindanao.
Phi (ϕ) indicates the rate of decay of spatial autocorrelation and varied from 10.6, 11.4 and 12.1 in the Visayas, Luzon and Mindanao, respectively (Table 2). This indicates that, after accounting for the effect of covariates, the radii of the clusters were approximately 33.3 km, 29.2 km and 27.5 km in the Visayas, Luzon and Mindanao, respectively (note, ϕ is measured in decimal degrees and 3/ϕ determines the cluster size; one decimal degree is approximately 111 km at the Equator). The tendency for spatial clustering was the weakest for Luzon and the strongest in Mindanao (note, the higher the value of the spatial variance parameter, the higher the tendency for spatial clustering) (Table 2).
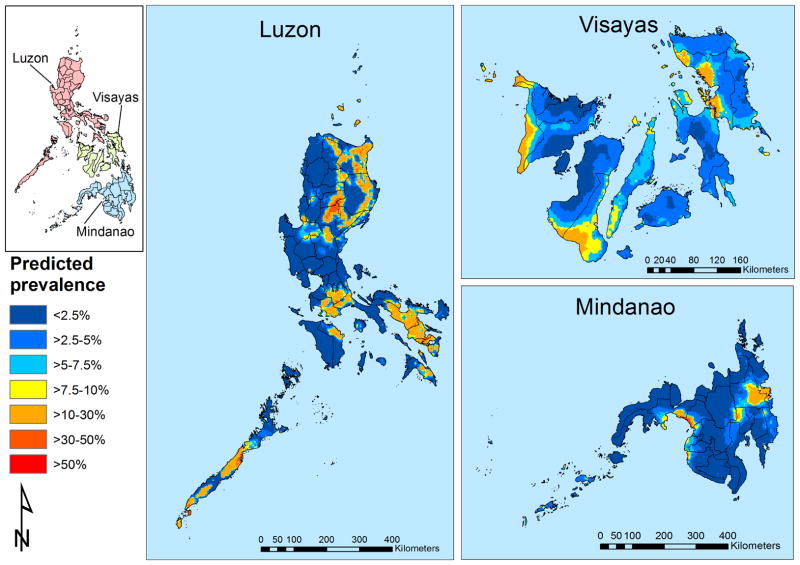
The geographical distribution of the prevalence of S. japonicum was plotted for males 20 years and older (the highest risk group; Fig. 3). Infection was widespread in the Visayas whereas in Luzon and Mindanao it was much more focal. After accounting for the effect of diagnostic uncertainty, the minimum predicted prevalences were 0.7%, 1.6% and 0.03% in Luzon, the Visayas and Mindanao, respectively. In Fig. 3, the prevalence of S. japonicum infection in the Visayas was predicted to be highest (>20%) in large clusters in Western Samar and northeastern Leyte, Biliran, Cebu, Antique and Negros Oriental. In Luzon, areas of high prevalence of S. japonicum infection were predicted in very small clusters in the northern tip of Cagayan, coastal and central Isabela, the northern border between Quirino in the Cagayan Valley and Nueva Viscaya in the Cordillera Administrative Region, between Laguna and Batangas in the Calabarzon Region, in the northern tip of Mindoro Oriental and a very small area in central Palawan in the Mimaropa Region, and to Albay and Sorgon in the Bicol Region. In Mindanao, areas of high prevalence of S. japonicum infection were predicted in small clusters in coastal areas of Maguindanao and in an elongated cluster between Agusan del Sur and Davao in the Caraga Region. Elongated clusters of moderate risk (<10–20%) for S. japonicum infection were predicted to occur in the Cordillera Administrative Region and between Isabela and Quirino in Luzon.
Fig. 3.
Predicted spatial distribution of Schistosoma japonicum for males aged ≥20 years for The Philippines.
Model validation results showed that spatial models for the Visayas and Mindanao had acceptable predictive ability in that these were able to discriminate prevalence thresholds of more than 1% and more than 10% with an AUC of more than 70% (Table 3). The model for Luzon demonstrated a poor mean discriminatory ability (AUC <70%) but the 95% confidence interval included values of acceptable discriminatory ability.
Table 3.
Summary of model validation results for predictive models of Schistosoma japonicum infection in the regions of Luzon, Visayas and Mindanao, in The Philippines.
| Spatial model | Area under the ROC curve (95% CI) | Area under the ROC curve (95% CI) |
|---|---|---|
| Prevalence threshold of 1% | Prevalence threshold of 10% | |
| Luzon | 0.68 (0.59, 0.75) | 0.62 (0.55, 0.73) |
| Visayas | 0.71 (0.65, 0.81) | 0.79 (0.73, 0.82) |
| Mindanao | 0.70 (0.66, 0.79) | 0.74 (0.68, 0.81) |
4. Discussion
The findings of the study show important spatial variation in the prevalence of schistosomiasis in The Philippines, which previous aggregated mapping studies failed to convey (Leonardo et al., 2008, 2012). The findings of this study have operational value because they assist in identifying communities where interventions should be prioritised to achieve schistosomiasis control and eventual elimination.
This study confirms previous reports that the prevalence of S. japonicum in The Philippines differs with age and sex (Kurtis et al., 2006; Leonardo et al., 2008, 2012). In line with the known age-prevalence profile of schistosomiasis, our study showed that the probability of the presence of S. japonicum infection was highest in individuals aged greater than 20 years compared with children. This finding may be partially explained by occupational exposure to water contaminated with schistosome cercariae, presumably due to agriculture activities such as farming and fishing (Li et al., 2000b; Leonardo et al., 2008). This postulation is corroborated by our finding that infection prevalence is highest in communities in close proximity to the water bodies. In addition, the higher effect size for the proximity to water bodies for the Visayas compared with Luzon suggests that, in addition to occupational exposure, the lower socio-economic status and low acceptance of MDA known to occur in the Visayas may also be an important predictor of S. japonicum infection in this region (Tallo et al., 2008).
This study also showed that the physical environment, including factors such as NDVI and rainfall, had different associations with S. japonicum infection among different regions of The Philippines. The differences in effects for vegetation index and rainfall between Luzon and Visayas may reflect differences between both regions with respect to farm land for rice crops, which are predominant in Luzon. In the Visayas, the increased prevalence associated with increased rainfall supports the view that continuous rainfall and the subsequent flooding that is known to occur may facilitate the establishment of snail colonies on vegetation, which leads to S. japonicum exposure. However, in Mindanao, prevalence of infection is highest in areas with lower rainfall, indicating that topography may be an important unmeasured factor which can affect stream flow and establishment of snail colonies.
The finding that considerable S. japonicum clustering was left unaccounted for by variables included in the non-spatial multivariable model for the Visayas and Mindanao (as assessed by the residual semivariogram) justified the need for formally modelling the second-order spatial variation using model-based geostatistics (MBG). While nationwide prevalence surveys used standard diagnostic testing, most survey locations were found to be without infection (Leonardo et al., 2008, 2012). The low prevalence estimated in previous analysis of this dataset may partly have been due to the day-to-day variation in egg output of the adult worms and the poor sensitivity of the Kato–Katz thick smear examination, which is especially low in infections of low intensity (Wang et al., 1998, 2005; Yu et al., 1998, 2007; Booth et al., 2003). A major advantage of our approach is that it accounted for the excess of zero-prevalence obtained in the prevalence survey.
Using MBG models of S. japonicum prevalence, the results provide new insight with regard to the small-scale spatial distribution of S. japonicum risk, adding value to previous work (Leonardo et al., 2008,.2012). Overall, the results of our analysis show that schistosomiasis in The Philippines is highly clustered, showing remarkable spatial variation even within known endemic areas. For example, recently the Cagayan Valley in the northern tip of Luzon was reported to be a new endemic focus (Leonardo et al., 2013) and our predictive map for this area shows remarkable spatial variation with some areas having a predicted prevalence greater than 20%. To address the low response (proportion = 32.2%) to the national schistosomiasis control survey in the Visayas (Leonardo et al., 2008), we included further data from Western Samar. This resulted in a more detailed geographical distribution of S. japonicum risk across that region which is in contrast with previous reports that had suggested that schistosomiasis in the Visayas is not as widespread as in Mindanao. Furthermore, the Caraga region in eastern Mindanao has long been considered the area with the highest prevalence of the disease, with Agusan del Sur as number one on the list of Schistosoma-endemic provinces, and our maps show considerable spatial variation within the Caraga Region.
Our models also predicted the presence of S. japonicum infection in areas not known to be endemic, highlighting regions where cases may have remained unreported or that may become future transmission sites due to their environmental suitability. Indeed one of the challenges for the control of S. japonicum in The Philippines is the ease with which people move between islands, the movement of infected live animals, fomites (e.g. trading of fertilizer and adherence of intermediate hosts to the skin and feet of animals) and humans (e.g. infected agricultural labourers (Gurarie and Seto, 2009; Leonardo, L., 2010. Environmental issues and health literacy in the control of schistosomiasis in the Philippines. In, Proceedings of the Obihiro Asia and the Pacific Seminar on Education for Rural Development (OASERD), Obihiro University of Agriculture and Veterinary Sciences, Japan, pp. 13–20)) and the networks of irrigation and rivers coursing through the landscape of The Philippines. For example, clusters of moderate infection risk (20–30%) occur to the southwest of the Cagayan Valley in central Luzon, which should be further investigated. In addition, our predictive maps for Luzon show an extensive area of high risk for infection in most of the Bicol Region and a smaller sized cluster in the northern tip of Mindoro Oriental. The moderate risk areas noted in Mindoro Oriental can be attributed to the environmental suitability brought about by heavy rainfalls which frequently cause the Naujan Lake there to overflow, exposing people to potentially contaminated waters. Our map also indicates that the southern half of Palawan is environmentally suitable for S. japonicum transmission.
The MDA coverage in The Philippines is known to be low and, for that reason, prevalence of S. japonicum infection is feared to rise (Leonardo et al., 2002; Tallo et al., 2008). The maps we have generated can be used as decision support tools for improving the efficiency of MDA coverage by targeting MDA to the communities most at risk. Areas of priority include high risk areas defined by a predicted prevalence >20% in Western Samar in the Visayas and Lanao del Sur, Maguindanao and south of the Caraga region in Mindanao. While MDA has been repeatedly delivered in these endemic areas of Mindanao, the level of endemicity and the geographical clustering of infection presented by our results suggest that, when financially possible, interventions that include snail control, environmental sanitation, health education and water sanitation and hygiene (WASH) programs, in addition to MDA, could be targeted to the areas identified to achieve sustainable control and possible disease elimination.
The findings reported in this study need to be interpreted in light of the study limitations. First, the response proportion in the Luzon survey was 73% and we were not able to obtain more data to improve the geographical coverage of the surveys. Second, comparison of the prevalence of the disease in Maguindanao with that of the other provinces in Mindanao should be interpreted with caution for a number of reasons: the Maguindanao response proportion was low (45.2%); the Maguindanao survey was conducted 3 years after that of Mindanao and during this interval, several rounds of mass treatment had already been conducted. Additionally, Mindanao has been the beneficiary of numerous local and international projects aimed at improving the health and economic situation on the island. Unfortunately this information (i.e. time since the last MDA) was not available for each barangay included in the analysis. Third, the data from Western Samar were collected as part of a cohort study and the sampling method was not designed to be representative of all residents of the selected villages, but rather of rice farmers working in either irrigated or rain-fed rice farms. However, a large proportion of the village households were sampled in most participating villages, decreasing the impact of the sampling method. Finally, the loss of statistical support for environmental covariates may reflect the cross-sectional nature of our data and the absence of small-scale confounders (such as household socioeconomic indicators and individual behaviour) in our regional models. Due to the extreme focality of infection, it is very likely that household socioeconomic and individual behaviour indicators may play an important role in the spatial variation of S. japonicum risk. Unfortunately we did not have data on these factors available for analysis and further studies should investigate their contribution to the spatial distribution of S. japonicum in different regions of The Philippines.
This study revealed significant spatial variation in S. japonicum infection risk, suggesting that spatially targeted interventions could result in efficiency gains for schistosomiasis control in The Philippines. The findings also show the value of updating the current schistosomiasis database for The Philippines with new data. Further, additional data for Samar has allowed the identification of high risk areas which could not have been detected had the analysis been carried out using national survey data alone. Additional surveys should be prioritized to areas in Luzon, which are currently under-represented in our database.
Highlights.
Our results demonstrate significant spatial clustering of Schistosoma japonicum within different regions of The Philippines.
This paper contributes evidence for a targeted delivery of mass drug administration for S. japonicum control.
Candidate areas in The Philippines where S. japonicum elimination efforts could be focused were predicted.
We predicted areas in The Philippines where the environment is suitable for S. japonicum transmission.
Acknowledgments
The authors thank the regional coordinators, regional directors, provincial health officers, provincial health team leaders, municipal health officers, medical technologists, midwives and barangay health workers for their commitment which was key to completing all phases of this national prevalence survey. We would like to thank Prof. May Lebanan (University of The Philippines, Manila, The Philippines) for assisting us with preparation of the data sets. The research team acknowledges the World Health Organization, Switzerland, for providing funds to conduct the surveys. The Samar study was funded by the Ecology of Infectious Disease program jointly funded by the United States National Institutes of Health and National Science Foundation grant # TW01582. This work was sponsored by a research project grant (APP1006254) from the Australian National Health and Medical Research Council (NHMRC). RJSM is supported by a University of Queensland Postdoctoral Research Fellowship (Australia), DJG is an Australian Research Council Fellow (DECRA), ACAC is supported by a NHMRC Senior Research Fellowship (Australia) and DPM is a NHMRC Senior Principal Research Fellow (Australia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balen J, Zhao ZY, Williams GM, McManus DP, Raso G, Utzinger J, Zhou J, Li YS. Prevalence, intensity and associated morbidity of Schistosoma japonicum infection in the Dongting Lake region, China. Bull World Health Organ. 2007;85:519–526. doi: 10.2471/BLT.06.034033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Cote d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]
- Brooker S, Hay SI, Bundy DA. Tools from ecology: useful for evaluating infection risk models? Trends Parasitol. 2002;18:70–74. doi: 10.1016/s1471-4922(01)02223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AC, Lwambo NJ, Blair L, Nyandindi U, Kaatano G, Kinung'hi S, Webster JP, Fenwick A, Brooker S. Bayesian spatial analysis and disease mapping: tools to enhance planning and implementation of a schistosomiasis control programme in Tanzania. Trop Med Int Health. 2006;11:490–503. doi: 10.1111/j.1365-3156.2006.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AC, Garba A, Sacko M, Toure S, Dembele R, Landoure A, Bosque-Oliva E, Gabrielli AF, Fenwick A. Mapping the probability of schistosomiasis and associated uncertainty, West Africa. Emerg Infect Dis. 2008;14:1629–1632. doi: 10.3201/eid1410.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho HM, McGarvey ST, Acosta LP, Manalo DL, Langdon GC, Leenstra T, Kanzaria HK, Solomon J, Wu H, Olveda RM, Kurtis JD, Friedman JF. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis. 2005;192:528–536. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72:540–548. [PMC free article] [PubMed] [Google Scholar]
- Ezeamama AE, McGarvey ST, Hogan J, Lapane KL, Bellinger DC, Acosta LP, Leenstra T, Olveda RM, Kurtis JD, Friedman JF. Treatment for Schistosoma japonicum, reduction of intestinal parasite load, and cognitive test score improvements in school-aged children. PLoS Negl Trop Dis. 2012;6:e1634. doi: 10.1371/journal.pntd.0001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CA, Acosta LP, Gray DJ, Olveda RM, Jarilla B, Gobert GN, Ross AG, McManus DP. High Prevalence of Schistosoma japonicum Infection in Carabao from Samar Province, the Philippines: Implications for Transmission and Control. PLoS Negl Trop Dis. 2012;6:e1778. doi: 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2009;6:495–508. doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtis JD, Friedman JF, Leenstra T, Langdon GC, Wu HW, Manalo DL, Su L, Jiz M, Jarilla B, Pablo AO, McGarvey ST, Olveda RM, Acosta LP. Pubertal development predicts resistance to infection and reinfection with Schistosoma japonicum. Clin Infect Dis. 2006;42:1692–1698. doi: 10.1086/504326. [DOI] [PubMed] [Google Scholar]
- Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines 1. Am J Clin Nutr. 2006;83:371–379. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- Leonardo L, Rivera P, Saniel O, Villacorte E, Lebanan MA, Crisostomo B, Hernandez L, Baquilod M, Erce E, Martinez R, Velayudhan R. A national baseline prevalence survey of schistosomiasis in the Philippines using stratified two-step systematic cluster sampling design. J Trop Med. 2012;2012:936128. doi: 10.1155/2012/936128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo L, Rivera P, Saniel O, Antonio SJ, Chigusa Y, Villacorte E, Christoper CJ, Moendeg K, Manalo D, Crisostomo B, Sunico L, Boldero N, Payne L, Hernandez L, Velayudhan R. New endemic foci of schistosomiasis infections in the Philippines. Acta Trop. 2013 doi: 10.1016/j.actatropica.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Leonardo LR, Acosta LP, Olveda RM, Aligui GD. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Trop. 2002;82:295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Leonardo LR, Rivera PT, Crisostomo BA, Sarol JN, Bantayan NC, Tiu WU, Bergquist NR. A study of the environmental determinants of malaria and schistosomiasis in the Philippines using Remote Sensing and Geographic Information Systems. Parassitologia. 2005;47:105–114. [PubMed] [Google Scholar]
- Leonardo LR, Rivera P, Saniel O, Villacorte E, Crisostomo B, Hernandez L, Baquilod M, Erce E, Martinez R, Velayudhan R. Prevalence survey of schistosomiasis in Mindanao and the Visayas, The Philippines. Parasitol Int. 2008;57:246–251. doi: 10.1016/j.parint.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Li YS, Sleigh AC, Ross AG, Li Y, Williams GM, Tanner M, McManus DP. Two-year impact of praziquantel treatment for Schistosoma japonicum infection in China: re-infection, subclinical disease and fibrosis marker measurements. Trans R Soc Trop Med Hyg. 2000a;94:191–197. doi: 10.1016/s0035-9203(00)90274-8. [DOI] [PubMed] [Google Scholar]
- Li YS, Sleigh AC, Ross AG, Williams GM, Tanner M, McManus DP. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int J Parasitol. 2000b;30:273–281. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- Li YS, Sleigh AC, Li Y, Tanner M, Dessein A, Williams GM, McManus DP. Five-year impact of repeated praziquantel treatment on subclinical morbidity due to Schistosoma japonicum in China. Trans R Soc Trop Med Hyg. 2002;96:438–443. doi: 10.1016/s0035-9203(02)90386-x. [DOI] [PubMed] [Google Scholar]
- Li YS, He YK, Zeng QR, McManus DP. Epidemiological and morbidity assessment of Schistosoma japonicum infection in a migrant fisherman community, the Dongting Lake region, China. Trans R Soc Trop Med Hyg. 2003;97:177–181. doi: 10.1016/s0035-9203(03)90112-x. [DOI] [PubMed] [Google Scholar]
- McGarvey ST, Carabin H, Balolong E, Jr, Belisle P, Fernandez T, Joseph L, Tallo V, Gonzales R, Tarafder MR, Alday P, Willingham AL, Olveda R. Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar province, Philippines. Bull World Health Org. 2006;84:446–452. doi: 10.2471/blt.05.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrock M, Marcogliese DJ. Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol. 2003;19:293–299. doi: 10.1016/s1471-4922(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Prah SK, James C. The influence of physical factors on the survival and infectivity of miracidia of Schistosoma mansoni and S. haematobium I. Effect of temperature and ultra-violet light. J Helminthol. 1977;51:73–85. doi: 10.1017/s0022149x00007288. [DOI] [PubMed] [Google Scholar]
- Raso G, Li Y, Zhao Z, Balen J, Williams GM, McManus DP. Spatial distribution of human Schistosoma japonicum infections in the Dongting Lake Region, China. PLoS One. 2009;4:e6947. doi: 10.1371/journal.pone.0006947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S, Carabin H, Belisle P, Joseph L, Tallo V, Balolong E, Willingham AL, Fernandez TJ, Gonzales RO, Olveda R, McGarvey ST. Multi-host transmission dynamics of Schistosoma japonicum in Samar province, the Philippines. PLoS Med. 2008;5:e18. doi: 10.1371/journal.pmed.0050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JW, Carabin H, Balolong E, Tallo V, Shrivastava J, Lu DB, Basanez MG, Olveda R, McGarvey ST, Webster JP. Population genetics of Schistosoma japonicum within the Philippines suggest high levels of transmission between humans and dogs. PLoS Negl Trop Dis. 2008;2:e340. doi: 10.1371/journal.pntd.0000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Hauffe T, Zhang Z, Davis GM, Jopp F, Remais JV, Wilke T. Spatially explicit modeling of schistosomiasis risk in eastern China based on a synthesis of epidemiological, environmental and intermediate host genetic data. PLoS Negl Trop Dis. 2013;7:e2327. doi: 10.1371/journal.pntd.0002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff CJ, Coutts WC, Yiannakis C, Holmes RW. Seasonal patterns in the transmission of Schistosoma haematobium in Rhodesia, and its control by winter application of molluscicide. Trans R Soc Trop Med Hyg. 1979;73:375–380. doi: 10.1016/0035-9203(79)90157-3. [DOI] [PubMed] [Google Scholar]
- Tallo VL, Carabin H, Alday PP, Balolong E, Jr, Olveda RM, McGarvey ST. Is mass treatment the appropriate schistosomiasis elimination strategy? Bull World Health Organ. 2008;86:765–771. doi: 10.2471/BLT.07.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafder MR, Balolong E, Jr, Carabin H, Belisle P, Tallo V, Joseph L, Alday P, Gonzales RO, Riley S, Olveda R, McGarvey ST. A cross-sectional study of the prevalence of intensity of infection with Schistosoma japonicum in 50 irrigated and rain-fed villages in Samar Province, the Philippines. BMC Public Health. 2006;6:61. doi: 10.1186/1471-2458-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hua Z, Wu W, Guo J, Wu X, Zheng J. Exploration on statistical method for calculating intensity of the infection in residents in nationwide sampling survey of schistosomiasis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1998;16:457–459. [PubMed] [Google Scholar]
- Wang Y, Yu JM, Zhang QL. Relationship of the intensity of Schistosoma japonicum infection to the variation in individual egg count. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005;23:270–273. [PubMed] [Google Scholar]
- Woolhouse ME, Chandiwana SK. Population dynamics model for Bulinus globosus, intermediate host for Schistosoma haematobium, in river habitats. Acta Trop. 1990;47:151–160. doi: 10.1016/0001-706x(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Yu JM, de VSJ, Yuan HC, Gryseels B. Variations in fecal Schistosoma japonicum egg counts. Am J Trop Med Hyg. 1998;59:370–375. doi: 10.4269/ajtmh.1998.59.370. [DOI] [PubMed] [Google Scholar]
- Yu JM, de VSJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, Olveda R. Schistosomiasis japonica control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- Zhou YB, Liang S, Jiang QW. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2012;5:275. doi: 10.1186/1756-3305-5-275. [DOI] [PMC free article] [PubMed] [Google Scholar]