Abstract
Morphological and functional features were compared along a developing third leaf and fully expanded leaf from high-light- and low-light-acclimated seedlings of Lolium multiflorum.
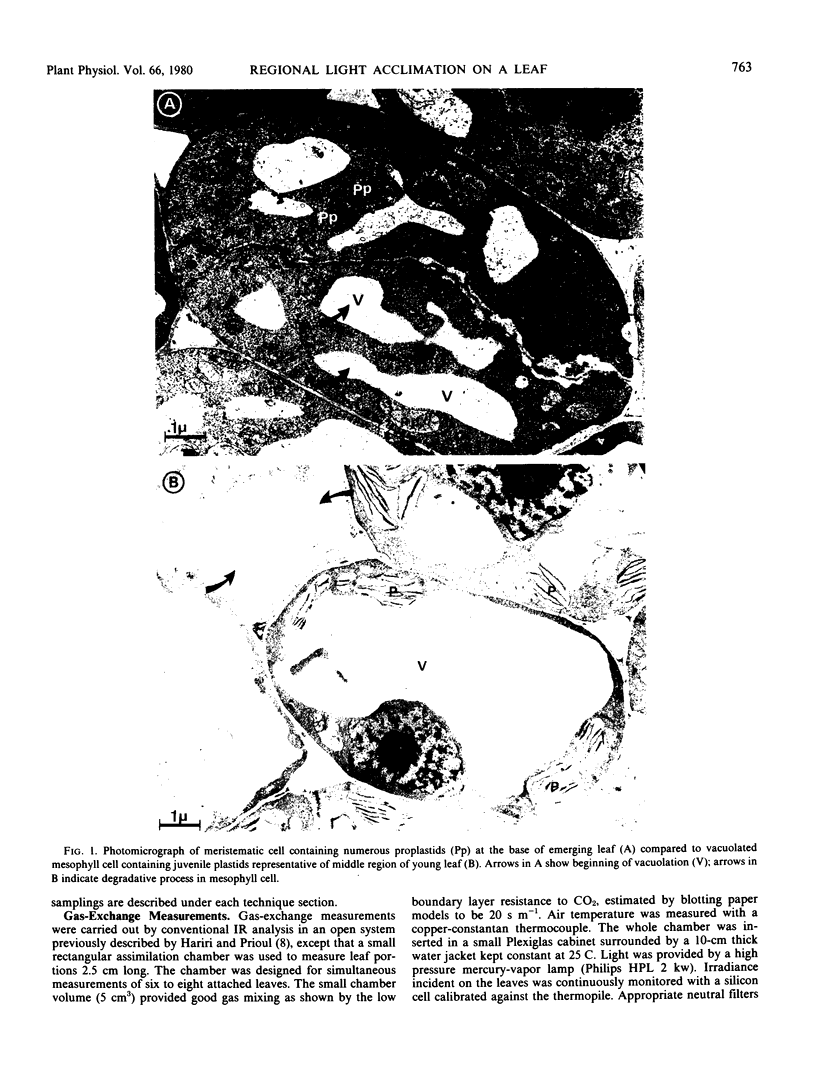
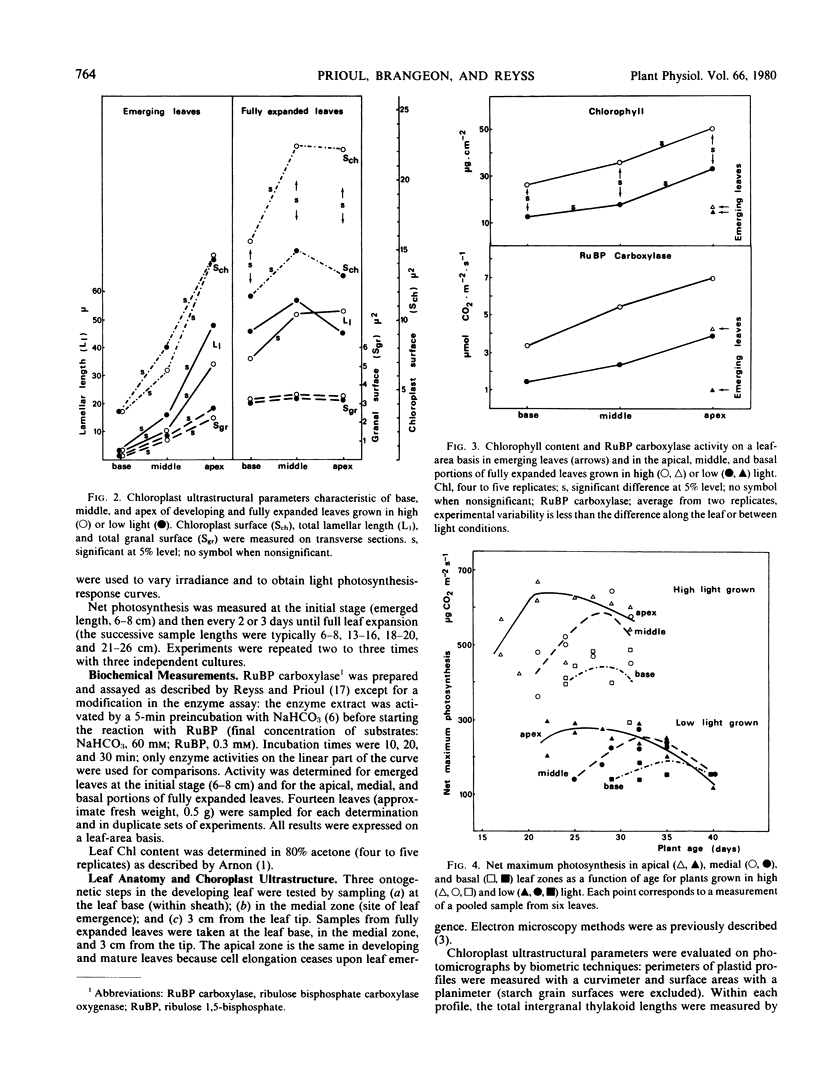
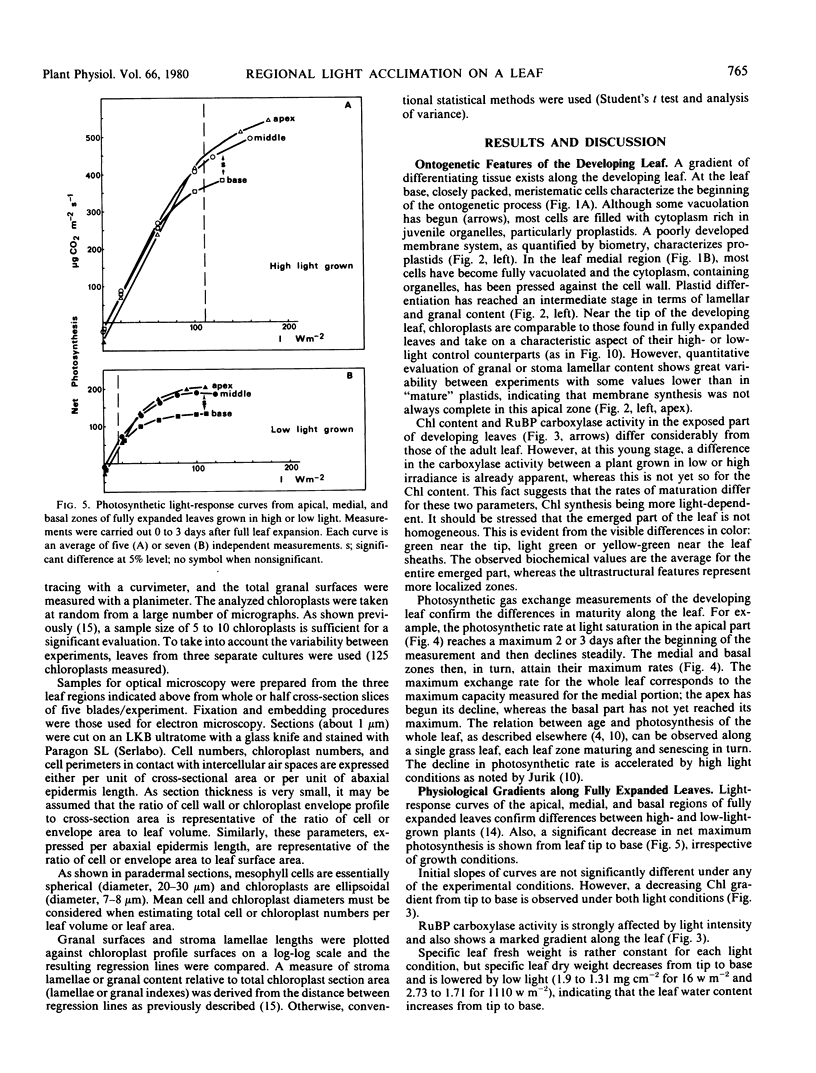
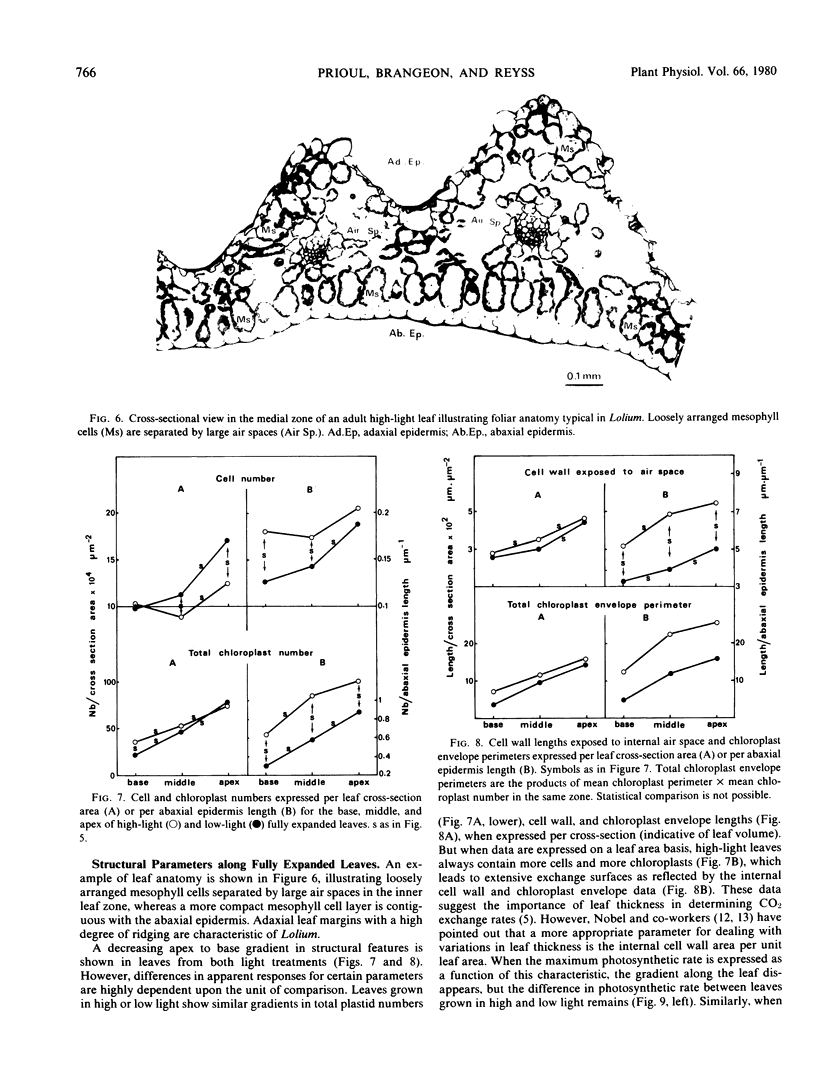
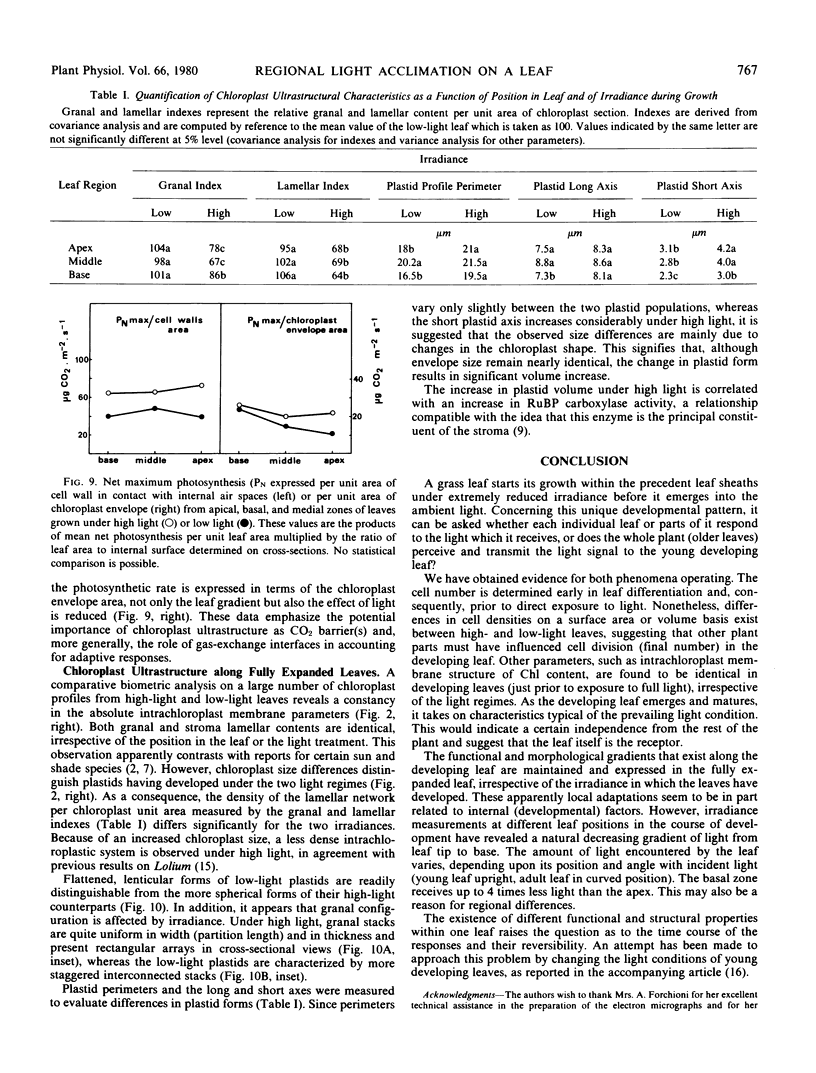
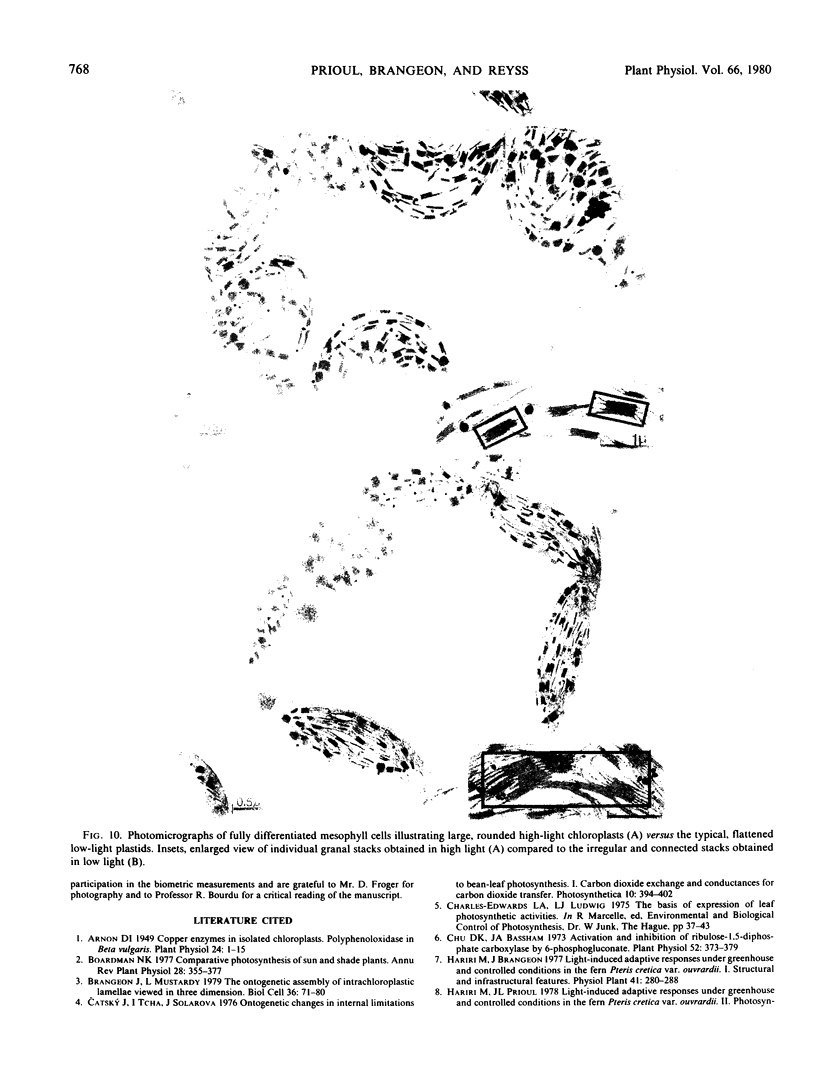
The young leaf contains a gradient of differentiating tissue, ranging from meristematic cells at the leaf base to mature tissue at the tip; this gradient can be related to the maturation of a functional photosynthetic apparatus. Along the fully expanded leaf, a decreasing gradient from tip to base is maintained for functional characteristics (net maximum photosynthesis, chlorophyll content, and ribulose bisphosphate carboxylase activity) and for a number of structural parameters (number of mesophyll cells and their external surface area, number of chloroplasts and their envelope area), irrespective of the light regime. In contrast, a constancy in the absolute intrachloroplastic lamellar content per plastid was revealed whatever the position in the leaf or irradiance received. However, the relative membrane content was lower in high-light chloroplasts due to their larger volume compared to low-light plastids (dilution effect).
The longitudinal differences in functional and morphological characteristics are interpreted as the result of interaction between the internal gradient of differentiating tissue along a developing young leaf and the external light conditions during development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Activation and inhibition of ribulose 1,5-diphosphate carboxylase by 6-phosphogluconate. Plant Physiol. 1973 Oct;52(4):373–379. doi: 10.1104/pp.52.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurik T. W., Chabot J. F., Chabot B. F. Ontogeny of Photosynthetic Performance in Fragaria virginiana under Changing Light Regimes. Plant Physiol. 1979 Mar;63(3):542–547. doi: 10.1104/pp.63.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S. Photosynthetic Rates of Sun versus Shade Leaves of Hyptis emoryi Torr. Plant Physiol. 1976 Aug;58(2):218–223. doi: 10.1104/pp.58.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S., Zaragoza L. J., Smith W. K. Relation between Mesophyll Surface Area, Photosynthetic Rate, and Illumination Level during Development for Leaves of Plectranthus parviflorus Henckel. Plant Physiol. 1975 Jun;55(6):1067–1070. doi: 10.1104/pp.55.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul J. L., Brangeon J., Reyss A. Interaction between External and Internal Conditions in the Development of Photosynthetic Features in a Grass Leaf: II. REVERSIBILITY OF LIGHT-INDUCED RESPONSES AS A FUNCTION OF DEVELOPMENTAL STAGES. Plant Physiol. 1980 Oct;66(4):770–774. doi: 10.1104/pp.66.4.770. [DOI] [PMC free article] [PubMed] [Google Scholar]