Abstract
Purpose of Review
Sepsis, defined by the presence of infection and host inflammation, is a lethal clinical syndrome with an increasing mortality rate worldwide. In severe disease, the coagulation system becomes diffusely activated, with consumption of multiple clotting factors resulting in Disseminated Intravascular Coagulation (DIC). When present, DIC portends a higher mortality rate. Understanding the mechanisms that tie inflammation and diffuse thrombosis will allow therapeutic interventions to be developed. The Coagulopathy of Acute Sepsis is a dynamic process that is time and disease burden specific. Whole blood testing of coagulation may provide more clinically useful information than classical tests. Natural anticoagulants that regulate thrombosis are down regulated in sepsis. Patients may benefit from modulation of the coagulation system when systemic inflammation and hypercoagulopathy exist. Proper timing of anticoagulant therapy may ultimately lead to decreased incidence of multisystem organ dysfunction (MODS).
Recent Findings
The pathogenesis of coagulopathy in sepsis is driven by an up-regulation of procoagulant mechanisms and simultaneous down-regulation of natural anticoagulants. Inflammation caused by the invading organism is a natural host defense than cannot be eliminated during treatment. Successful strategies to prevent MODS center on stratifying patients at high risk for DIC and restoring the balance of inflammation and coagulation.
Summary
The prevention of DIC in septic patients is a key therapeutic target in preventing death from multisystem organ failure. Stratifying patients for therapy using thromboelastometry, specific markers for DIC, and composite scoring systems is an area of growing research.
Keywords: Sepsis, Coagulopathy Thrombosis, and Inflammation
INTRODUCTION
Sepsis has been used to describe the dynamic and often life-threatening systemic host response to infection. For centuries, physicians have sought for clues to curb the burden of disease. In 1841, the Austrian physician Ignaz Semmelweiss observed, “The fingers and hands of students and doctors, soiled by recent dissections, carry those death-dealing cadaver’s poisons into the genital organs of women in childbirth.” From this astute observation, protocols for proper hand hygiene were developed in his local maternity ward and fetal deaths from sepsis dropped from 16 to 3%.[1]
Today, sepsis remains a leading cause of death worldwide and has an incidence between 75–300 per 100,000.[2, 3] In the United States, the economic burden of sepsis is astounding. Nearly $24 billion dollars are spent annually on septic patients with an increasing trend.[4] Sepsis alone carries a 25% mortality rate, but when combined with organ failure, this mortality rate doubles.[3]
Currently, much attention has been focused on the inflammatory host response in sepsis. Indeed, septic patients exhibit several biological markers for inflammation that often precede organ failure providing a causal relationship between the two.[5] The inflammatory response to infection may ultimately serve as a protective mechanism against microbial invasion, however when exaggerated due the severity of disease can ultimately lead to multisystem organ dysfunction (MODS). Inflammation and disturbances in coagulation are inseparably tied, with each acting as positive feedback for activation of the other.[6] Coagulation abnormalities are nearly universal in septic patients and likely play a key role in in MODS.[7] The Coagulopathy of Acute Sepsis (CAS) varies from overt thromboembolic disease to microvascular fibrin deposition. In severe cases, fulminant DIC presents with both thrombosis and diffuse hemorrhage.
CAS is likely driven by derangements of multiple pathways versus a single mediator which explains why many singe therapies have failed to improve outcomes.[4] This review will discuss the pathogenesis of coagulopathy in acute sepsis and how it relates to multisystem organ dysfunction. It will also focus on tools to measure coagulation status and possible therapeutic interventions.
MEASUREMENT OF COAGULATION IN SEPSIS
Measurement of the coagulation disturbances in acute sepsis is a complex and time sensitive endeavor that is best interpreted through serial measurements. Classical coagulation laboratory tests (CCT) such as prothrombin time, partial thromboplastin time, and fibrinogen have several limitations. First, plasma based testing of coagulation eliminates the platelet contribution to thrombosis. Platelets actively contribute to thrombosis by providing a surface for thrombin generation and recruiting clotting factors that propagate the coagulation system.[8] CCTs do not reflect in-vivo blood coagulation and do not provide qualitative or functional data. Alternatives to CCTs such as measurement of natural anticoagulants, markers of fibrinolytic activity, and molecular markers of DIC are not routinely available, are not validated to specific disease patterns, and may not be practical in the clinical setting. Classical laboratory tests generally suffer from the same downfall: high sensitivity with low specificity. Table 1.
Table 1.
Classical Coagulation Testing in DIC
| Platelet Count |
| PT/PTT/INR |
| Fibrinogen |
| Fibrinolysis Markers: D-dimer (Fibrin Degradation Products) |
| Anticoagulant Markers: Protein C, Antithrombin III |
| Fibrinolytic Activity: Plasminogen, alpha 2 antiplasmin |
| Antifibrinolytic Activity: Plasminogen Activator Inhibitor (PAI-1) |
| DIC Markers: Prothrombin Activation Fragment F1+2, FIX and FX activation peptides |
| Composite Scoring Systems |
Whole Blood Viscoelastic Testing
Theoretically, viscoelastic measurements of whole blood should provide clinicians with insight into in-vivo coagulation. Used in a serial fashion, the evolution of coagulopathy in septic patients could be identified and used to guide therapy. Ideally, it could provide prognostic value for patients with sepsis that are at risk for developing MODS. Unfortunately, the quality of evidence supporting thromboelastometry (TEM) in routine sepsis monitoring is low to moderate.[9] In addition, studies using TEM in sepsis to determine proper therapy institution are lacking. Also, definitions for hyper- and hypocoagulation are not standardized and internal validity of their use in clinical trials is often a concern.[9] Concerning detection of coagulopathy in sepsis, a variety of studies showed heterogeneous results. Often when compared to CCTs and measured within the first 48 hours, TEM measurements were within normal ranges. Of note, patients that were deemed hypocoagulable (prolonged reaction time, reduced alpha angle, or deceased maximum amplitude) had increased mortality and were associated with DIC more often.[9] In a study of 30 patients with severe sepsis followed over 2 days, patients with higher Sequential Organ Failure Assessment (SOFA) and APACHE II scores had reduced maximum clot firmness and prolonged clot formation time.[10] Thromboelastometry may serve as a beneficial negative predictor for developing coagulopathy.
Concerning the prognostic value of thromboelastometry in determining mortality in acute sepsis, early hypocoagulability was an independent risk factor for 28-day mortality in a series of severely septic patients.[9, 11] Adamzik compared Simplified Acute Physiology Score II (SAPS II) and SOFA scores to ROTEM values and found good correlation between these systems. In fact, pathologically altered values in ROTEM correlated with 58.7% 30-day survival versus 85.7% when all values were normal. In this study of 98 patients, ROTEM predicted survival better than the SAPS II and SOFA.[12] Ostrowski used TEG to monitor severely ill patients upon admission to the ICU. Patients were found to be hypocoagulable 22%, normal 48%, and hypercoagulable 30%. Patients that were hypocoagulable more often progressed to MODS and death. Patients that were normal upon admission and developed hypocoagulation had an 80% mortality rate.[13] A key finding in most studies was that patients that were hypercoagulable or normal upon admission progressed to MODS and death less. This finding may allow for stratification of patients that are high risk of progressing to organ failure.
Platelet aggregometry offers another important viscoelastic measurement in sepsis. The test employs multiple platelet agonist and electrical impedance across coils to determine platelet function in sampled whole blood. Brenner performed multiplate testing in 90 patients, comparing 30 severely septic patients to 30 post-surgical, and 30 healthy patients. Septic patients displayed markedly reduced function to standard agonists compared to post-surgical and healthy volunteers.[14] The effects of thrombocytopenia and dysfunction have been clearly illustrated in the critically ill population, significantly increasing mortality when dysfunction is prolonged over an ICU stay.[15]
Composite Screening for Coagulopathy in Sepsis
Using combined data points to predict patients at risk for developing MODS from sepsis-induced coagulopathy is an area of interest in research. Diagnostic algorithms such as ISTH DIC score, SAPS II, SOFA, and APACHE II combined with classical and viscoelastic measurements may provide the most accurate prognostic values.[16] A 2005 study using composite scoring for coagulopathy revealed an evolution of coagulation disturbances that occurs in the first twenty-four hours of severe sepsis. Worsening coagulopathy in the first day was associated with greater 28-day mortality.[5] Koyama evaluated multiple plasma markers already evident in sepsis such as antithrombin-thrombin complex (TAT), Protein C (PC), and Plasminogen Activator Inhibitor-1 (PAI-1) to estimate mortality and the development of overt DIC. When these plasma markers were combined, the area under the curve in selecting patients that would develop overt DIC was 0.95.[17]
PATHOGENESIS OF THROMBUS FORMATION IN SEPSIS
Post-mortem autopsies of patients with severe sepsis routinely show diffuse bleeding with microvascular thrombus formation and end organ damage.[18] Animal studies utilizing endotoxemia have shown that it causes vascular fibrin deposition resulting in organ failure. Blocking or reversing the coagulopathy in these animals has been shown to reverse organ dysfunction.[19, 20] Finally, clinical outcome studies with patients diagnosed with DIC reveal increased mortality suggesting that prevention of DIC is a key therapeutic target.[21]
Procoagulant Up-Regulation
The host inflammatory response to an invading organism rapidly initiates a procoagulant state in the septic patient. Thrombin generation is detectable within a few hours in models where tumor necrosis factor and endotoxin were infused into human subjects.[22, 23] Additionally, endothelial injury that impairs key anticoagulant mechanisms is observed within 15 minutes of lipopolysaccharide infusion in rabbits.[24]
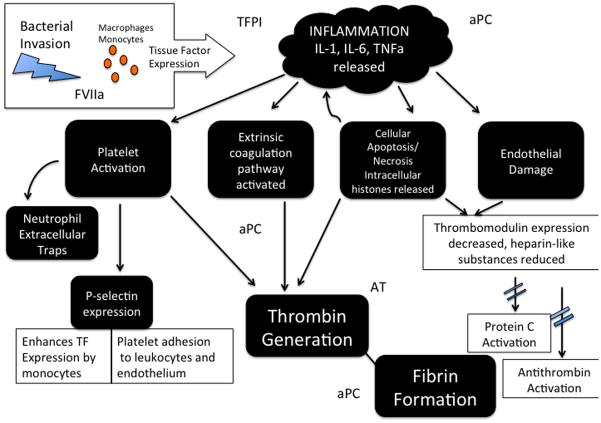
Key in the development of this procoagulant state is the interaction between tissue factor and inflammatory cytokine release. Tissue factor expression appears to be the initiating event in CAS. Tissue factor is responsible for binding and activating Factor VII on cell surfaces, thereby forming the enzyme-cofactor complex that results in amplified production of Factor Xa. Not only is tissue factor increased early in septic patients, but also impairment of the tissue factor pathway prevents coagulation abnormalities in animals.[25, 26] Debate over the primary source of tissue factor is ongoing as many cell types are capable of expressing tissue factor (TF). Endothelial cells and mononuclear phagocytes such as monocytes and macrophages can express TF, as can lung, kidney, and brain astrocytes.[27] Monocyte microparticles expressing TF in mice have also been shown to activate the coagulation system.[28] Proinflammatory cytokines such as TNF, IL-1, and IL-6 are up regulated after TF expression and play a major role in natural anticoagulant suppression and endothelial damage.[29]
Next, Platelet-Activating Factor (PAF) is directly released secondary to inflammation.[30] Platelet activation results in several accelerators of thrombosis. First, platelet p-selectin expression results in increased monocyte TF expression and platelet adhesion to leukocytes and endothelium.[31, 32] Once adhered to leukocytes and endothelium, platelets serve as a surface for thrombin generation and cellular signaling of other coagulation factors.[8]
Anticoagulant Impairment
The impairment of three endogenous anticoagulants is evident in severe sepsis and contributes to hypercoagulopathy in the early inflammatory stage. Figure 1.
Figure 1.
Tissue Factor Pathway Inhibitor (TFPI) is an early regulator of the coagulation pathway that is activated by tissue factor and FVIIa interaction. TFPI (previously known as Extrinsic Pathway Inhibitor) acts to prevent initial coagulation in two steps. First, TFPI binds to and inhibits FXa. Second, the TFPI-FXa complex binds to and inhibits TF-FVIIa thereby preventing early amplification of coagulation. TFPI is both consumed and degraded in sepsis leading to a procoagulant state.[33] TFPI is consumed quickly due to its relatively small concentration in plasma, approximately 1.0–2.5 nM.[34] Vascular endothelial cell expression of TFPI is also potentially degraded by plasmin, which is up regulated in early sepsis. This effect was demonstrated in baboons infused with E. Coli when TFPI activity was decreased coinciding with maximum TPA activity.[35]
Activated Protein C (APC) is a potent anticoagulant that also has profibrinolytic and antiinflammatory properties. Thus, APC derangement in sepsis significantly contributes to the early hypercoagulability. Protein C is activated by thrombin once bound to thrombomodulin. The Endothelial Protein C Receptor and Cofactor Protein S amplify its activation several fold.[36] Once activated, Protein C proteolytically cleaves Factor V and VII that are essential for the production of thrombin. Protein C synthesis is impaired while consumption and degradation by neutrophil elastase further diminishes its concentration in plasma.[29] Next, thrombomodulin expression is drastically reduced by inflammatory cytokines such as TNFα, IL-1, and IL-6.[37] Finally, EPCR is down regulated in severe sepsis thereby limiting activation of Protein C. Evidence also shows that due to endothelial damage, EPCR might be shed and not available for protein C augmentation. This effect occurs as early as day two in severe sepsis.[38]
The serine protease Antithrombin is a natural antagonist to thrombin that is activated several fold by circulating heparin-like substances. In severe sepsis, antithrombin synthesis is down regulated and consumption is markedly increased due to ongoing thrombin formation.[39] In addition, membrane bound heparin-like glycosaminoglycans on the endothelial surface are reduced by proinflammatory cytokines. This reduction further limits the bioactivity of antithrombin.[40]
Resistance To Fibrinolysis
In healthy human volunteers, the infusion of endotoxin produced a predictable and rapid change in the coagulation system. First, within 120 minutes inflammatory markers such as TNF and IL-6 rose with a concurrent rise in plasminogen activators indicating endothelial activation. Within 150 minutes, this was counteracted by an even greater and sustained rise in plasminogen activator inhibitor (PAI) thus supporting clot longevity.[23] It appears that both activated endothelial cells and platelets can express PAI.[41] Finally, the reduction in activated protein C (APC) due to reduced availability of thrombomodulin may also play a role in decreased fibrinolysis. Less APC is available to inhibit PAI thus augmenting clot stability.[42]
Thrombin induces the formation of thrombin-activatable fibrinolysis inhibitor (TAFI), a protease enzyme that reduces clot permeability and increases clot firmness.[43] In sepsis, tissue factor mediated thrombin production and inflammation produces dense clots that are resistant to fibrinolysis. This is thought to be mediated by both TAFI and platelet polyphosphate secretion that makes TPA less effective.[44, 45] In addition, the secretion of neutrophil elastase degrades fibrinolytic proteases contributing to clot persistence. The production of such a tightly aggregated clot could be a mechanism of defense against bacterial secreted proteases that break down clot integrity and allow for dissemination.[46] In patients with meningococcal infections TAFI levels are markedly increased, correlate with disease severity, and is associated with higher mortality.[33]
Endothelial Damage
The vascular endothelium is an important regulator of hemostasis and a site of cellular interaction for immune cells. Endothelial cells (EC) mediate pro- and anti-inflammatory mechanisms, regulate fibrinolysis, regulate vasomotor tone, and have immune cell signaling capabilities.[47] Thus, the endothelium acts as an important barrier for host defense in bacterial invasion. The surface layer of endothelial cells is a negatively charged, micro-thin layer of glycosaminoglycans and glycoproteins called the glycocalyx. The intact glycocalyx repels circulating platelets and acts as an anticoagulant layer due to its rich supply of heparin sulfates.[47] Ideally, the endothelium can balance procoagulant and anticoagulant mechanisms after injury thereby opposing thrombin generation when vascular repair is complete. However, when local injury becomes systemic, as in sepsis, the balance shifts towards a procoagulant state.[48]
Increased vascular permeability secondary to inflammation is a hallmark of sepsis and contributes significantly to organ dysfunction and possibly coagulation disturbances. Because inflammation and coagulation are closely tied, therapies involved in endothelial protection may benefit coagulation abnormalities. Table 2 lists the multiple mechanisms that are implicated in endothelial damage leading to increased permeability.[48–52]
Table 2.
Mechanisms of Vascular Damage in Sepsis
| Pathway | Cause |
|---|---|
| Endothelial Denudation | LPS induces detachment of ECs from the basal membrane. |
| VE-cadherin Dislocation | Inflammation induces internalization of vascular joining proteins |
| Catecholamine induced damage | Elevated noradrenaline levels associated with glycocalyx disruption |
| Inflammatory cytokine suppression of EC anticoagulant receptors | EPCR and thrombomodulin are down regulated |
| NET induced EC death | NET associated histones and proteases are directly cytotoxic |
| Angiopoietin-Tie2 | Angpt-2 sensitizes EC to inflammatory cytokines and promotes leakage |
| Endothelial apoptosis | LPS stimulates cell death |
The early shift towards a procoagulant state in sepsis is mediated by proinflammatory markers that result in decreased expression of membrane bound proteins such as thrombomodulin.[53] Endothelial injury also causes shedding and decreased expression of EPCR.[38, 54] The down regulating effect of this on the Protein C pathway has been described earlier. Endothelial cellular apoptosis resulting from LPS or endotoxin causes intracellular histone release that exacerbates inflammation and induces thrombosis. Endothelial disruption in inflammatory syndromes such as sepsis results in rapid platelet adhesion that can lead to microvascular thrombosis.[50] Cumulatively, destruction of the endothelial cell layer importantly contributes to early coagulopathy in sepsis.
DISSEMINATED INTRAVASCULAR COAGULATION
Clinically, DIC is defined by simultaneous diffuse thrombosis and bleeding. Consumption of clotting factors due to ongoing thrombosis eventually leads to a hypocoagulable state. Relevant coagulation abnormalities are present in 50 to 70% of patients with severe infection, whereas about 35% of patients will actually meet the criteria for DIC.[55] The hallmark for treating DIC remains to eradicate the underlying cause of disease and support coagulation derangements as they occur. The manifestations of DIC though must still be addressed quickly as the mortality associated with this grave diagnosis is high. Diagnostic criteria for DIC have been developed that employ common lab and clinical criteria. The criteria can be used clinically to distinguish overt DIC with diffuse bleeding and non-overt DIC where anticoagulation therapy could be useful.[4]
PATHOGENESIS OF MULTISYSTEM ORGAN DYSFUNCTION
Severe sepsis associated organ dysfunction occurs due to multiple interactions between the proinflammatory state and hypercoagulation. The role of DIC as a causative factor in MODS is well supported. Alternatively, recent work has led to our understanding of other mediators of MODS, namely the effect of necrotic and apoptotic cellular release of intracellular proteins. High mobility group box 1 (HMGB-1) proteins originate from dying cells, are released into the host circulation causing systemic inflammation, and propagate thrombosis.[56] Macrophages, endothelial cells, and monocytes are all capable of releasing HMGB-1 proteins. These cytokine-activating proteins are persistently elevated in septic patients and are associated with lethality in mice models.[56] Other intracellular released proteins such as histones associated with neutrophil extracellular traps (NETS) are highly toxic to organs, induce inflammation, and promote thrombosis.[32, 57, 58] NETS are cast by inflammatory cytokines and platelet activation and are comprised of histone rich DNA fibers and antimicrobial proteins.[58] Histones (H3 and H4) induced thrombin generation by multiple mechanisms. First, extracellular histones dose dependently impair thrombomodulin activation of Protein C thereby reducing the natural anticoagulant APC and eliminating its anti-inflammatory properties.[57] Secondly, thrombin production was increased by histone mediated platelet activation and p-selectin expression. P-selectin expression increases platelet adhesion to endothelial cells and leukocytes.[32] Thus, HMGB-1 and histone release in to the circulation augment inflammation and thrombosis, promote cellular death, and potentiate multisystem organ dysfunction.[33]
THROMBOSIS AS A PROTECTIVE MECHANISM IN SEPSIS
Our understanding of thrombosis and inflammation in sepsis has evolved over decades of animal and human research. A key to understanding our early misconception of sepsis lies in how early animal studies were conducted. Commonly, models of mice employed intravenous administration of endotoxin or lipopolysaccharide (LPS) or even live bacteria such as E. Coli. Models of LPS and endotoxin infusion tended to uniformly overestimate the proinflammatory response in the host.[59, 60] Thus, early efforts targeted inflammation alone as a mediator of sepsis and were unsuccessful in the clinical setting. Likewise, universally inhibiting the coagulation system had deleterious effects as evidenced by the higher mortality rate of patients that were hypocoagulable upon ICU admission and higher bleeding tendencies.[61] These strategies ignored the protective mechanism of compartmentalization. Compartmentalization involves the acute phase interaction of the proinflammatory response to elicit coagulation in attempt to sequester bacteria or invading organisms. Acute phase proteins such as fibrinogen and Factor V increase rapidly in acute sepsis augmenting the hypercoagulable response.[55, 62] Simultaneously, two powerful natural anticoagulants, Protein C and Antithrombin are down regulated. PC and AT could be viewed as negative acute phase proteins in this protective early mechanism.[63, 64] Today’s model of sepsis is viewed as both proinflammatory and antiinflammatory or MARS (mixed antiinflammatory response syndrome).
THERAPIES DIRECTED TOWARDS MEDIATORS OF SEPSIS
Therapies directed toward the Coagulopathy of Acute Sepsis should ideally restore the balance of inflammation and coagulation without negatively influencing the host’s response to infection. Several trials have failed to recognize inflammation as an important protective mechanism or used uniform therapy for patients in different stages of sepsis. Antibodies directed toward TNFα, IL-1 receptors, and endotoxin failed to demonstrate a reduction in mortality.[65–68] The failure of anticoagulant trials to show efficacy may be due to the inclusion of patients without DIC, uncertainty when to initiate treatment, and the tendency to underestimate the importance of bleeding.[69] This exemplifies the importance of developing specific diagnostic criteria for DIC that employs composite scoring systems, advanced markers for DIC, and thromboelastometry.
Tissue Factor Pathway Inhibitor
As described earlier, TFPI is rapidly consumed in sepsis. Earlier studies on the efficacy of TFPI replacement demonstrated that in patients with INR > 1.2, mortality was not changed and adverse bleeding events were increased.[70] However, subgroup analysis of this study did show that in patients with community acquired pneumonia (CAP) there was a trend toward survival. In 2011, Wunderink published results of a large placebo-controlled study in patients with CAP treated with recombinant TFPI and again found no survival benefit though biological activity in improving coagulation parameters was evident.[71]
Antithrombin
Antithrombin inhibits thrombin in a 1:1 manner and is maximally activated after interaction with receptors on the endothelial surface.[72] Antithrombin has antiinflammatory properties through its inhibition of thrombin-Factor X, a complex that stimulates IL-1 and IL-6. Recently, the Japanese Association of Acute Medicine DIC Committee evaluated antithrombin use in a prospective RCT. Patients with DIC treated with antithrombin over 3 days had faster recovery determined by DIC scores and did not have increased bleeding events.[73] In a nonrandomized larger study, Iba determined that in patients with low baseline antithrombin activity and sepsis, high dose antithrombin therapy 3000 IU/day (AT3000) was associated with improved survival (AT1500 65.2%, AT3000 74.7%).[74] Importantly, these studies used DIC scoring systems to determine overt DIC and proper timing of anticoagulation therapy.
Activated Protein C
It has been recognized for several years that a rapid decline in Protein C occurs in sepsis and that low levels of PC correlate with poor prognosis.[26] In 2001, the PROWESS study reported promising results of lower mortality with the use of recombinant APC (Xigris, Eli Lily).[75] However, it was later removed from the market when the PROWESS-SHOCK trial revealed no mortality benefit at 28 or 90 days.[61] Numerous clinical studies since then have looked at subsets of this population and determined that rAPC may have beneficial effects. Caserly evaluated 15022 patients registered with the Surviving Sepsis Campaign and found that groups treated with rAPC had reduced hospital mortality (OR 0.76, p<0.001). Also, hospital mortality was significantly reduced in patients that displayed multi-organ failure versus single organ failure (OR 0.82 versus 0.78).[76] A meta-analysis performed by Kalil in 2012 showed mortality benefit in patients with higher disease severity, but higher rates of adverse bleeding than were reported in the PROWESS trial. The results of this study indicated that rAPC could be beneficial in properly selected populations of septic patients.
Thrombomodulin
Thrombomodulin (CD141) is an endothelial transmembrane glycoprotein that has several regulatory functions in hemostasis. By binding thrombin, it prevents fibrinogen conversion to fibrin, and prevents thrombin from interacting with platelets. The thrombin-thrombomodulin complex activates Protein C resulting in a 100-fold increase in activity.[53] Recombinant thrombomodulin (rTM) has been studied in models of DIC and sepsis as well as clinical trials. Hoppensteadt’s study of rTM in DIC revealed that markers of thrombin production such as thrombin-antithrombin complex (TAT) and prothrombin fragment (F1.2) are sequentially reduced with rTM treatment.[77] In a study of 234 patients comparing soluble thrombomodulin (ART-123) to heparin in the treatment of DIC caused by malignancy or infection, the rate of DIC resolution was 66.1% with ART-123 versus 49.9% with heparin.[78] In this study, a statistically significant lower rate of adverse bleeding events with ART-123 was noticed. In another study of 86 patients with sepsis induced DIC, mortality was lower and improved sequential SOFA scores were associated with infusion of rTM versus those not treated with rTM (37% vs. 58%, p = 0.038).[79] This was confirmed later in a study of 750 patients with sepsis and DIC where patients treated with ART-123 were associated with improved 28-day mortality.[80] Determining the optimal timing for treatment will be important for this promising DIC treatment.
Apoptosis
Model studies of septic mice treated with IL-7 have demonstrated benefit in reducing apoptosis of important CD4 and CD8 T-Cells and improved immune function. IL-7 appears to have anti-apoptotic functions essential to leukocyte survival.[81, 82] Targeting programmed cell death may have an important role in decreasing inflammatory histones and HMGB-1 that are released in to the systemic vasculature of septic patients.[83, 84] Immunomodulation studies such as this reinforce the intimate tie between coagulation and inflammation.
CONCLUSIONS
The host defense to infectious invasion is a highly regulated process involving inflammatory and antiinflammatory processes. In an attempt to compartmentalize invasion, the host creates hemostatic thrombin barriers and dense fibrin networks. Pathologically, these fibrin depositions can result in microvascular thrombus and result in end organ ischemia. Establishing an operational definition of sepsis and DIC that is heralded by biological markers is the first step in constructing new therapeutic trials. Finally, we must improve patient stratification and staging to determine optimal timing of interventions.[85]
Key Points.
Inflammation and disturbances in coagulation are inseparably tied, with each acting as positive feedback for activation of the other.
Coagulation abnormalities are nearly universal in septic patients and likely play a key role in multisystem organ dysfunction.
Coagulopathy in sepsis is likely driven by derangements of multiple pathways versus a single mediator, which explains why many singe therapies have failed to improve outcomes.
Therapies directed toward the Coagulopathy of Acute Sepsis should ideally restore the balance of inflammation and coagulation without negatively influencing the host’s response to infection.
Therapeutic strategies are time sensitive and should target patients at high risk for developing DIC.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Disclosure of Funding For This Work: NIH R01 GM086416 (JFP)
Contributor Information
Jeff Simmons, Email: jwsimmons@uabmc.edu, Assistant Professor of Anesthesiology, Anesthesia Services Division, Trauma Section, UAB Department of Anesthesiology, 804 Jefferson Tower, 619 South 19th Street, Birmingham AL 35249, P: (205) 996-702.
Jean-Francois Pittet, Email: pittetj@uab.edu, David H. Chestnut Professor of Anesthesiology, Vice-Chair and Director, Critical Care Division, Department of Anesthesiology, Professor of Surgery and Cell Biology, Investigator, Center for Lung Injury and Repair, University of Alabama at Birmingham, Phone: 205-996-4755
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
◆ of special interest
◆◆ of outstanding interest
- 1.Funk DJ, Parrillo JE, Kumar A. Sepsis and septic shock: a history. Critical care clinics. 2009;25(1):83–101. viii. doi: 10.1016/j.ccc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Brun-Buisson C, Meshaka P, Pinton P, et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive care medicine. 2004;30(4):580–8. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- 3.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiological reviews. 2013;93(3):1247–88. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Critical care medicine. 2005;33(2):341–8. doi: 10.1097/01.ccm.0000153520.31562.48. [DOI] [PubMed] [Google Scholar]
- 6.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Critical care. 2006;10(4):222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ◆◆7.Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Seminars in thrombosis and hemostasis. 2013;39(5):559–66. doi: 10.1055/s-0033-1343894. This article expertly describes the relationship between inflammation and sepsis. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thrombosis and haemostasis. 2001;85(6):958–65. [PubMed] [Google Scholar]
- ◆9.Muller MC, Meijers JC, Vroom MB, et al. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Critical care. 2014;18(1):R30. doi: 10.1186/cc13721. This article reviews the utility of thromboelastometry in sepsis monitoring and the dynamic evolution of coagulopathy in severe disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daudel F, Kessler U, Folly H, et al. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Critical care. 2009;13(2):R42. doi: 10.1186/cc7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson PI, Stensballe J, Vindelov N, et al. Hypocoagulability, as evaluated by thrombelastography, at admission to the ICU is associated with increased 30-day mortality. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2010;21(2):168–74. doi: 10.1097/MBC.0b013e3283367882. [DOI] [PubMed] [Google Scholar]
- 12.Adamzik M, Langemeier T, Frey UH, et al. Comparison of thrombelastometry with simplified acute physiology score II and sequential organ failure assessment scores for the prediction of 30-day survival: a cohort study. Shock. 2011;35(4):339–42. doi: 10.1097/SHK.0b013e318204bff6. [DOI] [PubMed] [Google Scholar]
- ◆13.Ostrowski SR, Windelov NA, Ibsen M, et al. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: a prospective study. Journal of critical care. 2013;28(3):317, e1–11. doi: 10.1016/j.jcrc.2012.09.003. This article describes the prognostic utility of thromboelastomety in sepsis looking at 28 day mortality. [DOI] [PubMed] [Google Scholar]
- 14.Brenner T, Schmidt K, Delang M, et al. Viscoelastic and aggregometric point-of-care testing in patients with septic shock - cross-links between inflammation and haemostasis. Acta anaesthesiologica Scandinavica. 2012;56(10):1277–90. doi: 10.1111/j.1399-6576.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 15.Levi M, Lowenberg EC. Thrombocytopenia in critically ill patients. Seminars in thrombosis and hemostasis. 2008;34(5):417–24. doi: 10.1055/s-0028-1092871. [DOI] [PubMed] [Google Scholar]
- 16.Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: A useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Critical care medicine. 2006;34(2):314–20. doi: 10.1097/01.ccm.0000196832.27501.b2. quiz 28. [DOI] [PubMed] [Google Scholar]
- 17.Koyama K, Madoiwa S, Nunomiya S, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Critical care. 2014;18(1):R13. doi: 10.1186/cc13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimamura K, Oka K, Nakazawa M, et al. Distribution patterns of microthrombi in disseminated intravascular coagulation. Archives of pathology & laboratory medicine. 1983;107(10):543–7. [PubMed] [Google Scholar]
- 19.Creasey AA, Chang AC, Feigen L, et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. The Journal of clinical investigation. 1993;91(6):2850–60. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor FB, Jr, Chang A, Ruf W, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circulatory shock. 1991;33(3):127–34. [PubMed] [Google Scholar]
- 21.Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. Journal of thrombosis and haemostasis : JTH. 2004;2(11):1924–33. doi: 10.1111/j.1538-7836.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 22.van der Poll T, Buller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. The New England journal of medicine. 1990;322(23):1622–7. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 23.van Deventer SJ, Buller HR, ten Cate JW, et al. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76(12):2520–6. [PubMed] [Google Scholar]
- 24.Leclerc J, Pu Q, Corseaux D, et al. A single endotoxin injection in the rabbit causes prolonged blood vessel dysfunction and a procoagulant state. Critical care medicine. 2000;28(11):3672–8. doi: 10.1097/00003246-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Biemond BJ, Levi M, ten Cate H, et al. Complete inhibition of endotoxin-induced coagulation activation in chimpanzees with a monoclonal Fab fragment against factor VII/VIIa. Thrombosis and haemostasis. 1995;73(2):223–30. [PubMed] [Google Scholar]
- 26.Levi M. The coagulant response in sepsis. Clinics in chest medicine. 2008;29(4):627–42. viii. doi: 10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thrombosis research. 2010;125 (Suppl 1):S70–3. doi: 10.1016/j.thromres.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JG, Manly D, Kirchhofer D, et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. Journal of thrombosis and haemostasis : JTH. 2009;7(7):1092–8. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levi M. The coagulant response in sepsis and inflammation. Hamostaseologie. 2010;30(1):10–2. 4–6. [PubMed] [Google Scholar]
- 30.Zimmerman GA, McIntyre TM, Prescott SM, et al. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Critical care medicine. 2002;30(5 Suppl):S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 31.Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2011;17(1):80–7. doi: 10.1177/1076029609344981. [DOI] [PubMed] [Google Scholar]
- 32.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–61. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semeraro N, Ammollo CT, Semeraro F, et al. Sepsis, thrombosis and organ dysfunction. Thrombosis research. 2012;129(3):290–5. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. The Journal of pathology. 2006;208(3):327–39. doi: 10.1002/path.1871. [DOI] [PubMed] [Google Scholar]
- 35.Tang H, Ivanciu L, Popescu N, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. The American journal of pathology. 2007;171(3):1066–77. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esmon CT. The endothelial cell protein C receptor. Thrombosis and haemostasis. 2000;83(5):639–43. [PubMed] [Google Scholar]
- 37.Faust SN, Levin M, Harrison OB, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. The New England journal of medicine. 2001;345(6):408–16. doi: 10.1056/NEJM200108093450603. [DOI] [PubMed] [Google Scholar]
- 38.Guitton C, Gerard N, Sebille V, et al. Early rise in circulating endothelial protein C receptor correlates with poor outcome in severe sepsis. Intensive care medicine. 2011;37(6):950–6. doi: 10.1007/s00134-011-2171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Shimada K, Ozawa T. Human recombinant interleukin-1 beta- and tumor necrosis factor alpha-mediated suppression of heparin-like compounds on cultured porcine aortic endothelial cells. Journal of cellular physiology. 1990;144(3):383–90. doi: 10.1002/jcp.1041440304. [DOI] [PubMed] [Google Scholar]
- 41.Gando S. Role of fibrinolysis in sepsis. Seminars in thrombosis and hemostasis. 2013;39(4):392–9. doi: 10.1055/s-0033-1334140. [DOI] [PubMed] [Google Scholar]
- ◆42.Noel P, Cashen S, Patel B. Trauma-induced coagulopathy: from biology to therapy. Seminars in hematology. 2013;50(3):259–69. doi: 10.1053/j.seminhematol.2013.06.009. Well written, succient article detailing mechanisms involved in trauma coagulopathy as well as current therapies. [DOI] [PubMed] [Google Scholar]
- 43.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(11):2445–53. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 44.Mutch NJ, Engel R, Uitte de Willige S, et al. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115(19):3980–8. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- 45.Campbell RA, Overmyer KA, Selzman CH, et al. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114(23):4886–96. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ◆46.Dubin G, Koziel J, Pyrc K, et al. Bacterial proteases in disease - role in intracellular survival, evasion of coagulation/ fibrinolysis innate defenses, toxicoses and viral infections. Current pharmaceutical design. 2013;19(6):1090–113. doi: 10.2174/1381612811319060011. Interesting article describing bacterial survival tactics within the host. [DOI] [PubMed] [Google Scholar]
- 47.Ait-Oufella H, Maury E, Lehoux S, et al. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive care medicine. 2010;36(8):1286–98. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 48.Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. American journal of physiology Lung cellular and molecular physiology. 2012;303(5):L355–63. doi: 10.1152/ajplung.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee WL, Slutsky AS. Sepsis and endothelial permeability. The New England journal of medicine. 2010;363(7):689–91. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- ◆◆50.Levi M, van der Poll T. Endothelial injury in sepsis. Intensive care medicine. 2013;39(10):1839–42. doi: 10.1007/s00134-013-3054-1. This article describes the patterns of endothelial injury in sepsis and ensuing effects on coagulopathy. [DOI] [PubMed] [Google Scholar]
- 51.Ostrowski SR, Berg RM, Windelov NA, et al. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. Journal of critical care. 2013;28(5):586–96. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 52.David S, Kumpers P, van Slyke P, et al. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. The Journal of pharmacology and experimental therapeutics. 2013;345(1):2–6. doi: 10.1124/jpet.112.201061. [DOI] [PubMed] [Google Scholar]
- 53.Levi M, Van Der Poll T. Thrombomodulin in sepsis. Minerva anestesiologica. 2013;79(3):294–8. [PubMed] [Google Scholar]
- 54.Gleeson EM, O’Donnell JS, Preston RJ. The endothelial cell protein C receptor: cell surface conductor of cytoprotective coagulation factor signaling. Cellular and molecular life sciences : CMLS. 2012;69(5):717–26. doi: 10.1007/s00018-011-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schouten M, van ‘t Veer C, van der Poll T, et al. Effect of the factor V Leiden mutation on the incidence and outcome of severe infection and sepsis. The Netherlands journal of medicine. 2012;70(7):306–10. [PubMed] [Google Scholar]
- 56.Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ. The role of high mobility group box-1 protein in severe sepsis. Current opinion in infectious diseases. 2006;19(3):231–6. doi: 10.1097/01.qco.0000224816.96986.67. [DOI] [PubMed] [Google Scholar]
- 57.Ammollo CT, Semeraro F, Xu J, et al. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. Journal of thrombosis and haemostasis : JTH. 2011;9(9):1795–803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Copeland S, Warren HS, Lowry SF, et al. Acute inflammatory response to endotoxin in mice and humans. Clinical and diagnostic laboratory immunology. 2005;12(1):60–7. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24 (Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 61.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. The New England journal of medicine. 2012;366(22):2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 62.Levi M, Schouten M, van’t Veer C, et al. Factor v Leiden mutation in severe infection and sepsis. Seminars in thrombosis and hemostasis. 2011;37(8):955–60. doi: 10.1055/s-0031-1297374. [DOI] [PubMed] [Google Scholar]
- 63.Niessen RW, Lamping RJ, Jansen PM, et al. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thrombosis and haemostasis. 1997;78(3):1088–92. [PubMed] [Google Scholar]
- 64.Hayakawa M, Sawamura A, Yanagida Y, et al. The response of antithrombin III activity after supplementation decreases in proportion to the severity of sepsis and liver dysfunction. Shock. 2008;30(6):649–52. doi: 10.1097/SHK.0b013e318173e396. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler EJ, Fisher CJ, Jr, Sprung CL, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. The New England journal of medicine. 1991;324(7):429–36. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 66.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA : the journal of the American Medical Association. 1994;271(23):1836–43. [PubMed] [Google Scholar]
- 67.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA : the journal of the American Medical Association. 1995;273(12):934–41. [PubMed] [Google Scholar]
- 68.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. The New England journal of medicine. 1996;334(26):1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 69.Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thrombosis research. 2013;131(5):383–9. doi: 10.1016/j.thromres.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;290(2):238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 71.Wunderink RG, Laterre PF, Francois B, et al. Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. American journal of respiratory and critical care medicine. 2011;183(11):1561–8. doi: 10.1164/rccm.201007-1167OC. [DOI] [PubMed] [Google Scholar]
- 72.Roemisch J, Gray E, Hoffmann JN, et al. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2002;13(8):657–70. doi: 10.1097/00001721-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Critical care. 2013;17(6):R297. doi: 10.1186/cc13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iba T, Saito D, Wada H, et al. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a prospective multicenter survey. Thrombosis research. 2012;130(3):e129–33. doi: 10.1016/j.thromres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 75.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England journal of medicine. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 76.Casserly B, Gerlach H, Phillips GS, et al. Evaluating the use of recombinant human activated protein C in adult severe sepsis: results of the Surviving Sepsis Campaign. Critical care medicine. 2012;40(5):1417–26. doi: 10.1097/CCM.0b013e31823e9f45. [DOI] [PubMed] [Google Scholar]
- 77.Hoppensteadt D, Tsuruta K, Cunanan J, et al. Thrombin generation mediators and markers in sepsis-associated coagulopathy and their modulation by recombinant thrombomodulin. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2014;20(2):129–35. doi: 10.1177/1076029613492875. [DOI] [PubMed] [Google Scholar]
- 78.Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. Journal of thrombosis and haemostasis : JTH. 2007;5(1):31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 79.Ogawa Y, Yamakawa K, Ogura H, et al. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. The journal of trauma and acute care surgery. 2012;72(5):1150–7. doi: 10.1097/TA.0b013e3182516ab5. [DOI] [PubMed] [Google Scholar]
- 80.Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Critical care medicine. 2013;41(9):2069–79. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- 81.Kasten KR, Prakash PS, Unsinger J, et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infection and immunity. 2010;78(11):4714–22. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unsinger J, McGlynn M, Kasten KR, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. Journal of immunology. 2010;184(7):3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang K, Svabek C, Vazquez-Guillamet C, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Critical care. 2014;18(1):R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venet F, Foray AP, Villars-Mechin A, et al. IL-7 restores lymphocyte functions in septic patients. Journal of immunology. 2012;189(10):5073–81. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- ◆85.Marshall JC. Why have clinical trials in sepsis failed? Trends in molecular medicine. 2014;20(4):195–203. doi: 10.1016/j.molmed.2014.01.007. This editorial piece illustrates the multiple pathways involved in sepsis coagulopathy and emphasizes the importance of appropriate patient selection for interventions. [DOI] [PubMed] [Google Scholar]